Nanosheets with High-Performance Electrochemical Oxygen Reduction Reaction Revived from Green Walnut Peel
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Materials Synthesis
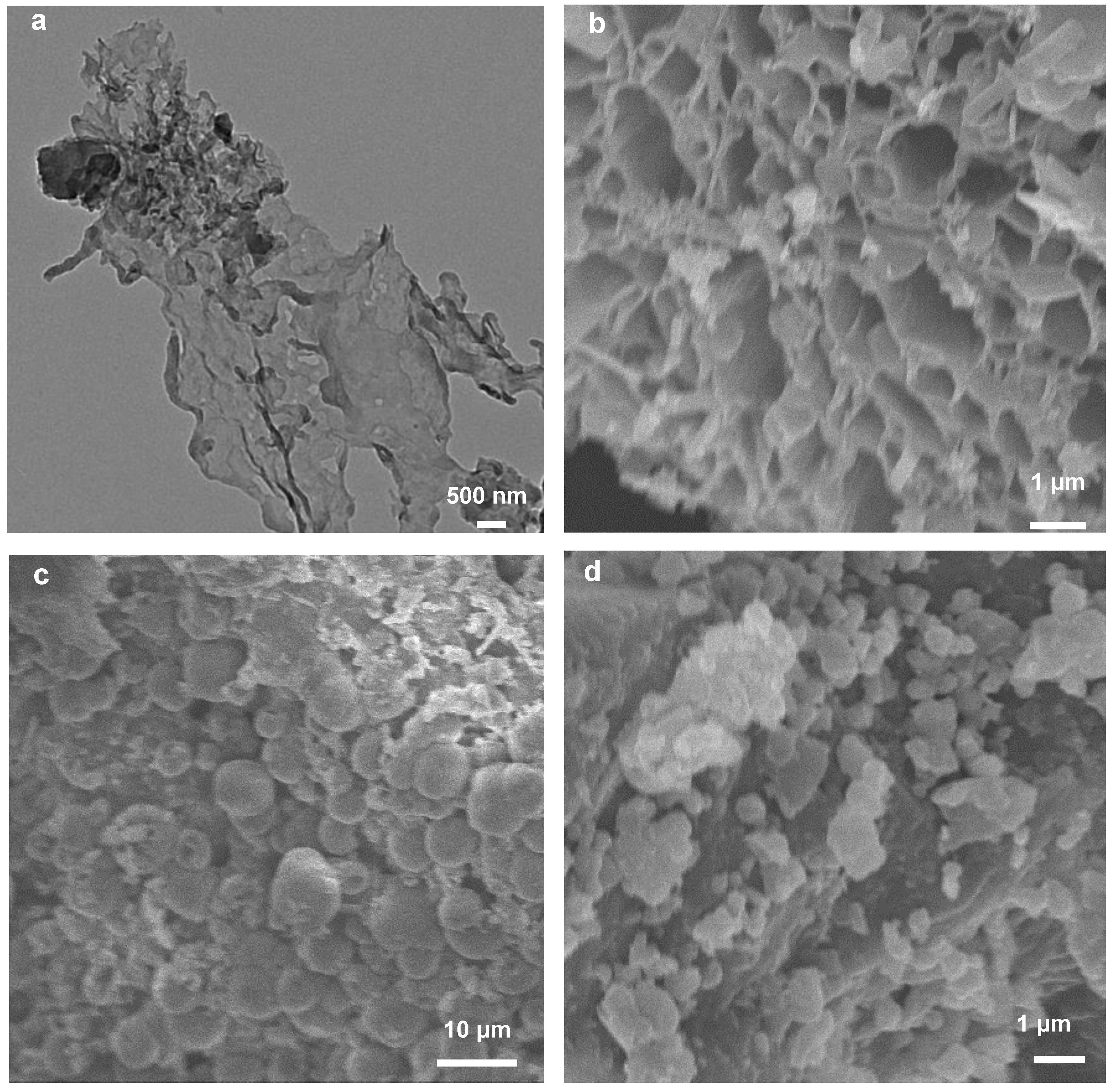
3.3. Structural Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Katsounaros, I.; Cherevko, S.; Zeradjanin, A.R.; Mayrhofer, K.J.J. Oxygen electrochemistry as a cornerstone for sustainable energy conversion. Angew. Chem. Int. Ed. 2014, 53, 102. [Google Scholar] [CrossRef]
- Wu, K.H.; Wang, D.W.; Zong, X.; Zhang, B.S.; Liu, Y.F.; Gentle, I.R.; Su, D.S. Functions in cooperation for enhanced oxygen reduction reaction: The independent roles of oxygen and nitrogen sites in metal-free nanocarbon and their functional synergy. J. Mater. Chem. A 2017, 5, 3239. [Google Scholar] [CrossRef]
- Service, R.F. Cellulosic ethanol: Biofuel researchers prepare to reap a new harvest. Science 2007, 315, 1488. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Zhou, L.H.; Sun, L.H.; Huang, B.H.; Yuan, Y. Nitrogen-doped porous activated carbon derived from cocoon silk as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. Rsc Adv. 2017, 7, 13383–13389. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.C.; Yan, C.; Robertson, A.W.; Gao, Y.G.; Ding, J.J.; Zhang, Y.Q.; Ma, T.; Sun, Z.Y. N-Doping of graphene oxide at low temperature for the oxygen reduction reaction. Chem. Commun. 2017, 53, 873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.; Jeon, E.K.; Kim, B.S. Mussel-inspired nitrogen-doped graphene nanosheet supported manganese oxide nanowires as highly efficient electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 6167. [Google Scholar] [CrossRef] [Green Version]
- Arenz, M.; Mayrhofer, K.J.J.; Stamenkovic, V.; Blizanac, B.B.; Tomoyuki, T.; Ross, P.N.; Markovic, N.M. The effect of the particle size on the kinetics of CO electrooxidation on high surface area Pt catalysts. J. Am. Chem. Soc. 2005, 127, 6819. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Najam, T.; Molochas, C.; Nazir, M.A.; Brouzgou, A.; Javed, M.S.; Rehman, A.u.; Tsiakaras, P. Nanostructure Engineering of Metal-Organic Derived Frameworks: Cobalt Phosphide Embedded in Carbon Nanotubes as an Efficient ORR Catalyst. Molecules 2021, 26, 6672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, Q.Q.; Zhang, C.L.; Xu, J.T.; Zhai, B.; Zhang, B. Adsorption of neutral red onto mn-impregnated activated carbons prepared from typha orientalis. Bioresour. Technol. 2008, 99, 8974. [Google Scholar] [CrossRef]
- Zhou, Y.; Umasankar, Y.; Ramasamy, R.P. Laccase-TiO2 Nanoconjugates as Catalysts for Oxygen Reduction Reaction in Biocathodes. J. Electrochem. Soc. 2015, 162, H911–H917. [Google Scholar] [CrossRef]
- Hudak, N.S.; Gallaway, J.W.; Barton, S.C. Mediated Biocatalytic Cathodes Operating on Gas-Phase Air and Oxygen in Fuel Cells. J. Electrochem. Soc. 2009, 156, B9–B15. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically active graphene-porphyrin MOF composite for oxygen reduction reaction. J. Am. Chem Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef]
- Sravani, B.; Raghavendra, P.; Chandrasekhar, Y.; Reddy, Y.V.M.; Sivasubramanian, R.; Venkateswarlu, K.; Madhavi, G.; Sarma, L.S. Immobilization of platinum-cobalt and platinum-nickel bimetallic nanoparticles on pomegranate peel extract-treated reduced graphene oxide as electrocatalysts for oxygen reduction reaction. Int. J. Hydrog. Energy 2020, 45, 7680–7690. [Google Scholar] [CrossRef]
- Venkateswarlu, K. Ashes from organic waste as reagents in synthetic chemistry: A review. Environ. Chem. Lett. 2021, 19, 3887–3950. [Google Scholar] [CrossRef]
- Naidu, B.R.; Lakshmidevi, J.; Naik, B.S.S.; Venkateswarlu, K. Water extract of pomegranate ash as waste-originated biorenewable catalyst for the novel synthesis of chiral tert-butanesulfinyl aldimines in water. Mol. Catal. 2021, 511, 111719. [Google Scholar] [CrossRef]
- Boruah, P.R.; Ali, A.A.; Saikia, B.; Sarma, D. A novel green protocol for ligand free Suzuki-Miyaura cross-coupling reactions in WEB at room temperature. Green Chem. 2015, 17, 1442–1445. [Google Scholar] [CrossRef]
- Godoi, M.; Leitemberger, A.; Correa Bohs, L.M.; Silveira, M.V.; Rafiq, L.; Montes D’Oca, M.G. Rice straw ash extract, an efficient solvent for regioselective hydrothiolation of alkynes. Environ. Chem. Lett. 2019, 17, 1441–1446. [Google Scholar] [CrossRef]
- Su, D.S.; Zhang, J.; Frank, B.; Thomas, A.; Wang, X.C.; Paraknowitsch, J.; Schlogl, R. Metal-Free Heterogeneous Catalysis for Sustainable Chemistry. ChemSusChem 2010, 3, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.H.; Fu, P.; Cai, X.X.; Zhou, S.G.; Yuan, Y. Naturally derived carbon nanofibers as sustainable electrocatalysts for microbial energy harvesting: A new application of spider silk. Appl. Catal. B 2016, 188, 31. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Li, M.T.; Zhang, L.P.; Dai, L.M.; Xia, Z.H. Design principles for heteroatom-doped carbon nanomaterials as highly efficient catalysts for fuel cells and metal-air batteries. Adv. Mater. 2015, 27, 6834. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Xu, X.X.; Feng, Z.M.; Huo, Y.Q.; Bian, L.J. Loading of cluster-based coordination compound on biomass derived N-doped mesoporous carbon matrix: A bifunctional electrocatalyst for overall water splitting. ACS Sustain. Chem. Eng. 2018, 6, 10282. [Google Scholar] [CrossRef]
- Liang, C.; Liang, S.; Xia, Y.; Gan, Y.P.; Fang, L.B.; Jiang, Y.Z.; Tao, X.Y.; Huang, H.; Zhang, J.; Zhang, W.K. Synthesis of hierarchical porous carbon from metal carbonates towards high-performance lithium storage. Green Chem. 2018, 20, 1484. [Google Scholar] [CrossRef]
- Han, C.; Chen, Z. The mechanism study of oxygen reduction reaction (ORR) on non-equivalent P, N co-doped graphene. Appl. Surf. Sci. 2020, 511, 145382. [Google Scholar] [CrossRef]
- Morozan, A.; Jousselme, B.; Palacin, S. Low-platinum and platinum-free catalysts for the oxygen reduction reaction at fuel cell cathodes. Energy Environ. Sci. 2011, 4, 1238–1254. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Emerging applications of biochar-based materials for energy storage and conversion. Energy Environ. Sci. 2019, 12, 1751–1779. [Google Scholar] [CrossRef]
- Paulus, U.A.; Schmidt, T.J.; Gasteiger, H.A.; Behm, R.J. Oxygen reduction on a high-surface area Pt/Vulcan carbon catalyst: A thin-film rotating ring-disk electrode study. J. Electroanal. Chem. 2001, 495, 134. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Sun, H.; Indrawirawan, S.; Wang, Y.; Kang, J.; Liang, F.; Zhu, Z.H.; Wang, S. Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis. ACS Appl. Mater. Interfaces 2015, 7, 4169–4178. [Google Scholar] [CrossRef]
- Unni, S.M.; Devulapally, S.; Karjule, N.; Kurungot, S. Graphene enriched with pyrrolic coordination of the doped nitrogen as an efficient metal-free electrocatalyst for oxygen reduction. J. Mater. Chem. 2012, 22, 23506–23513. [Google Scholar] [CrossRef]
- Mulyadi, A.; Zhang, Z.; Dutzer, M.; Liu, W.; Deng, Y.L. Facile approach for synthesis of doped carbon electrocatalyst from cellulose nanofibrils toward high-performance metal-free oxygen reduction and hydrogen evolution. Nano Energy 2017, 32, 336–346. [Google Scholar] [CrossRef]
- Konwar, L.J.; Sugano, Y.; Chutia, R.S.; Shchukarev, A.; Maki-Arvela, P.; Kataki, R.; Mikkola, J.P. Sustainable synthesis of N and P co-doped porous amorphous carbon using oil seed processing wastes. Mater. Lett. 2016, 173, 145–148. [Google Scholar] [CrossRef]
- Guo, C.; Liao, W.; Li, Z.; Sun, L.; Chen, C. Easy conversion of protein-rich enoki mushroom biomass to a nitrogen-doped carbon nanomaterial as a promising metal-free catalyst for oxygen reduction reaction. Nanoscale 2015, 7, 15990–15998. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, J.; Zhu, J.W.; Liu, Z.Y.; Zhang, C.T.; Mu, S.C. A self-template and KOH activation co-coupling strategy to synthesize ultrahigh surface area nitrogen-doped porous graphene for oxygen reduction. RSC Adv. 2016, 6, 73292–73300. [Google Scholar] [CrossRef]
- Lai, L.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.; Gong, H.; Shen, Z.; Lin, J.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Li, J.M.; Wang, W.; Wang, F.X.; Kang, Y.M.; Tan, T.; Lei, Z.Q. Astragali Radix-derived nitrogen-doped porous carbon: An efficient electrocatalyst for the oxygen reduction reaction. Int. J. Hydrog. Energy 2018, 43, 551–561. [Google Scholar] [CrossRef]
- Shen, W.Z.; Fan, W.B. Nitrogen-containing porous carbons: Synthesis and application. J. Mater. Chem. A 2013, 1, 999. [Google Scholar] [CrossRef]
- Rao, C.V.; Cabrera, C.R.; Ishikawa, Y. In Search of the Active Site in Nitrogen-Doped Carbon Nanotube Electrodes for the Oxygen Reduction Reaction. J. Phys. Chem. Lett. 2010, 1, 2622–2627. [Google Scholar] [CrossRef]
- Favaro, M.; Carraro, F.; Cattelan, M.; Colazzo, L.; Durante, C.; Sambi, M. Multiple doping of graphene oxide foams and quantum dots: New switchable systems for oxygen reduction and water remediation. J. Mater. Chem. A 2015, 3, 14334–14347. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, M.; Xu, W.; Wu, X.; Jiang, J. Catalytically Active Carbon From Cattail Fibers for Electrochemical Reduction Reaction. Front. Chem. 2019, 7, 786. [Google Scholar] [CrossRef]
- Huang, B.B.; Liu, Y.C.; Guo, Q.; Fang, Y.X.; Titirici, M.M.; Wang, X.C.; Xie, Z.L. Porous carbon nanosheets from biological nucleobase precursor as efficient pH-independent oxygen reduction electrocatalyst. Carbon 2020, 156, 179–186. [Google Scholar] [CrossRef]
- Tang, Z.J.; Pei, Z.X.; Wang, Z.F.; Li, H.F.; Zeng, J.; Ruan, Z.H.; Huang, Y.; Zhu, M.S.; Xue, Q.; Yu, J.; et al. Highly anisotropic, multichannel wood carbon with optimized heteroatom doping for supercapacitor and oxygen reduction reaction. Carbon 2018, 130, 532–543. [Google Scholar] [CrossRef]
- Ye, W.Y.; Tang, J.H.; Wang, Y.J.; Cai, X.X.; Liu, H.W.; Lin, J.Y.; Van der Bruggen, B.; Zhou, S.G. Hierarchically structured carbon materials derived from lotus leaves as efficient electrocatalyst for microbial energy harvesting. Sci. Total Environ. 2019, 666, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, L.K.; Wang, G.; Gao, M.R.; Ge, J.; Yuan, W.J.; Shen, Y.H.; Xie, A.J.; Yu, S.H. Nitrogen-doped nanoporous carbon nanosheets derived from plant biomass: An efficient catalyst for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 4095–4103. [Google Scholar] [CrossRef]


| Catalysts | References | ||
|---|---|---|---|
| PAC-800 | 0.99 V | 0.82 V | 4 |
| TARC-N | 0.98 V | 0.86 V | 34 |
| G800-ZC-2.0 | 0.98 V | 0.81 V | 33 |
| HC-900 | 0.95 V | 0.80 V | / |
| N-CSs | 0.85 V | 0.81 V | / |
| GWS180M800 | 1.01 V | 0.82 V | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yan, L.; Hou, J. Nanosheets with High-Performance Electrochemical Oxygen Reduction Reaction Revived from Green Walnut Peel. Molecules 2022, 27, 328. https://doi.org/10.3390/molecules27010328
Zhou Y, Yan L, Hou J. Nanosheets with High-Performance Electrochemical Oxygen Reduction Reaction Revived from Green Walnut Peel. Molecules. 2022; 27(1):328. https://doi.org/10.3390/molecules27010328
Chicago/Turabian StyleZhou, Yifei, Lei Yan, and Junhua Hou. 2022. "Nanosheets with High-Performance Electrochemical Oxygen Reduction Reaction Revived from Green Walnut Peel" Molecules 27, no. 1: 328. https://doi.org/10.3390/molecules27010328






