Unpredicted Concentration-Dependent Sensory Properties of Pyrene-Containing NBN-Doped Polycyclic Aromatic Hydrocarbons
Abstract
:1. Introduction
2. Results and Discussion
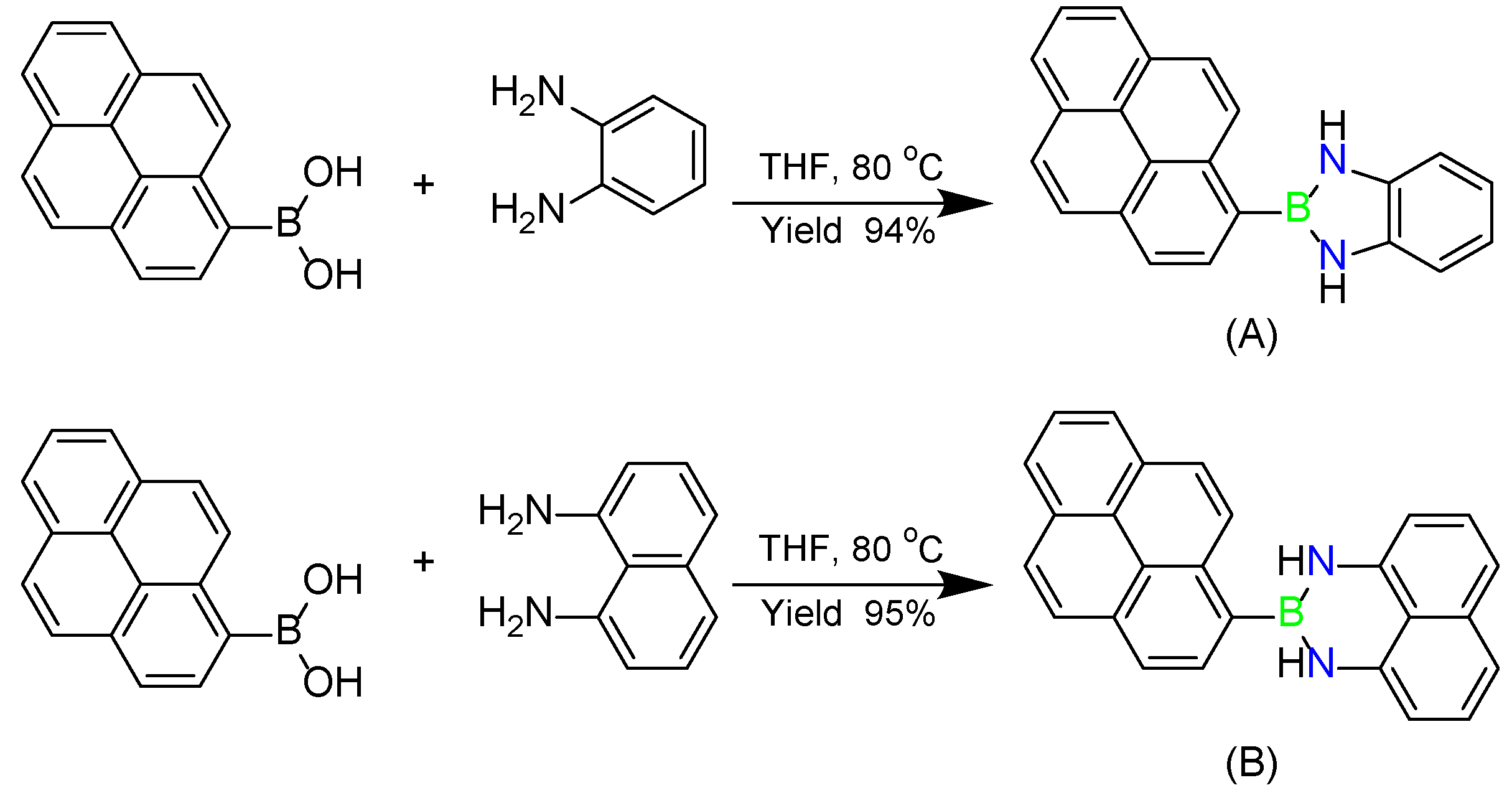
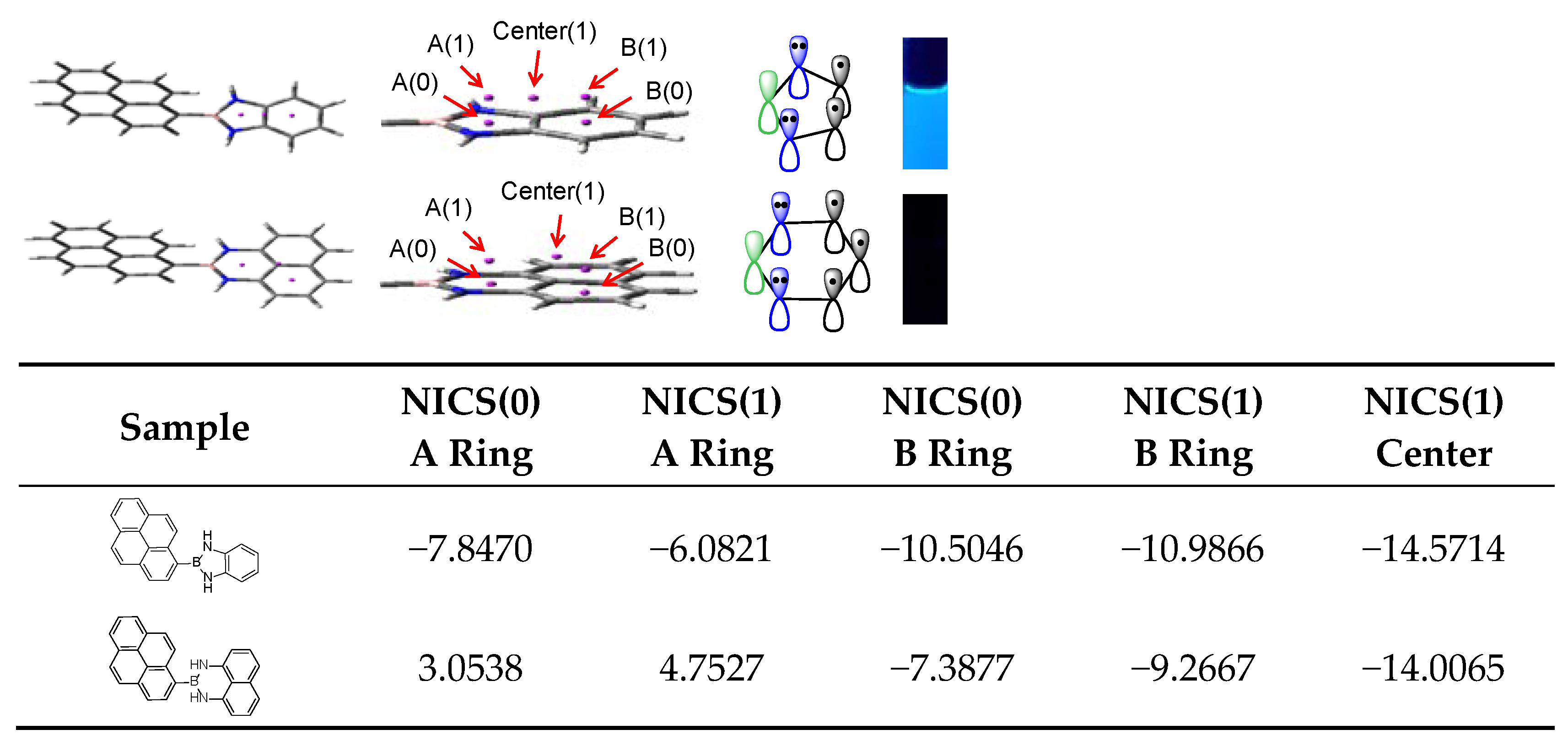
2.1. Fabrication and Aromaticity of NBN-Doped PAHs
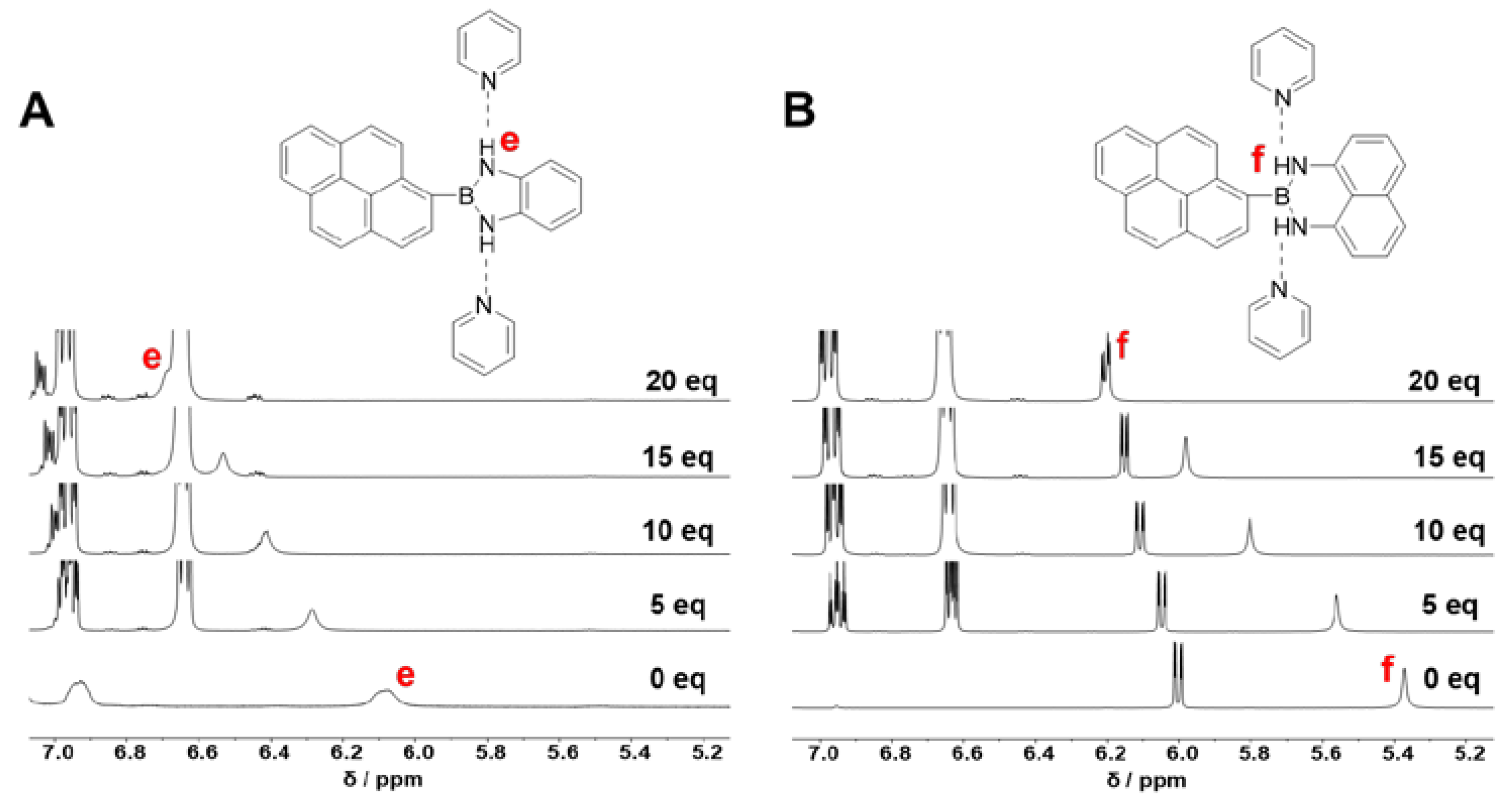
2.2. Luminescent Properties of NBN-Doped PAHs
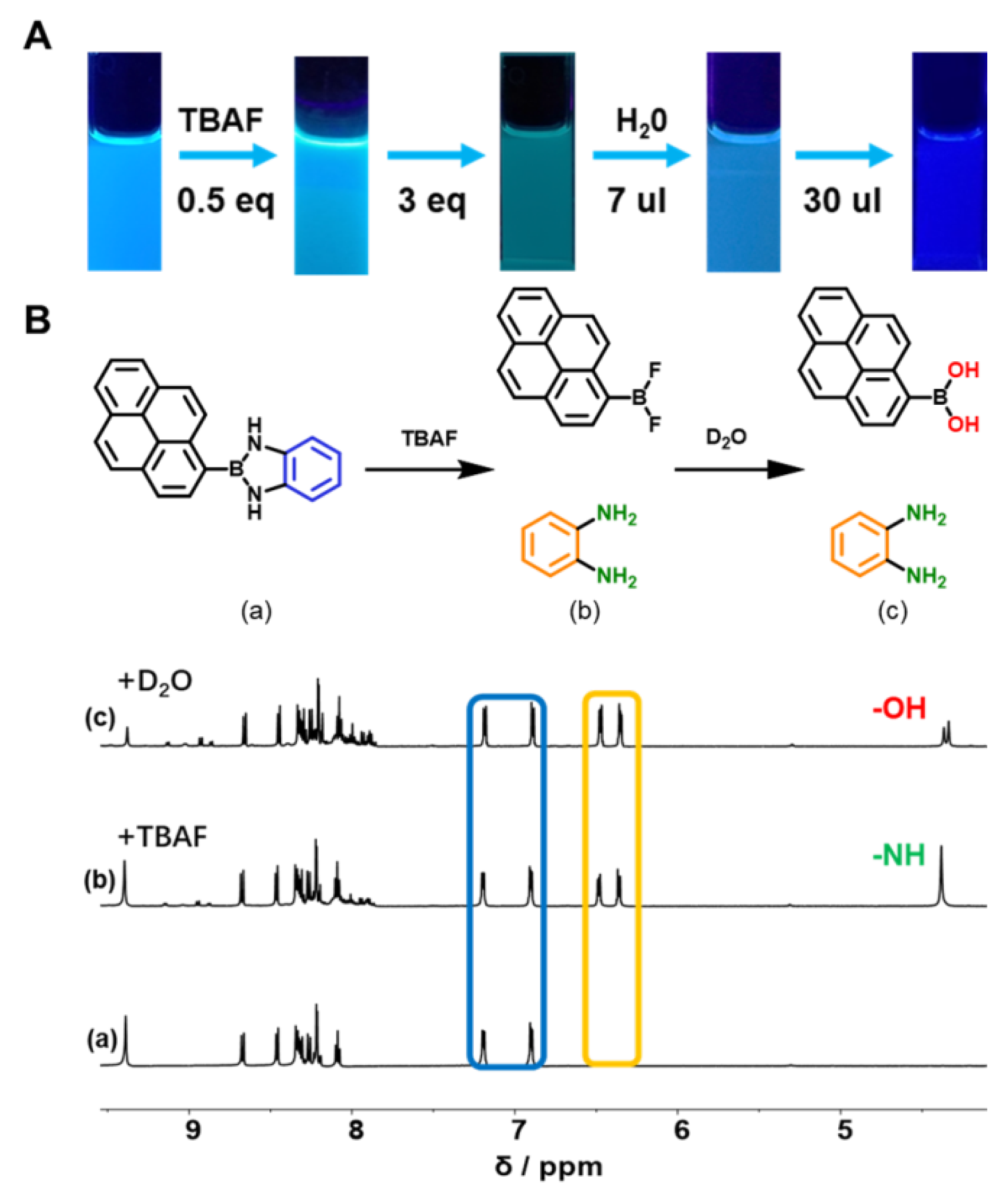
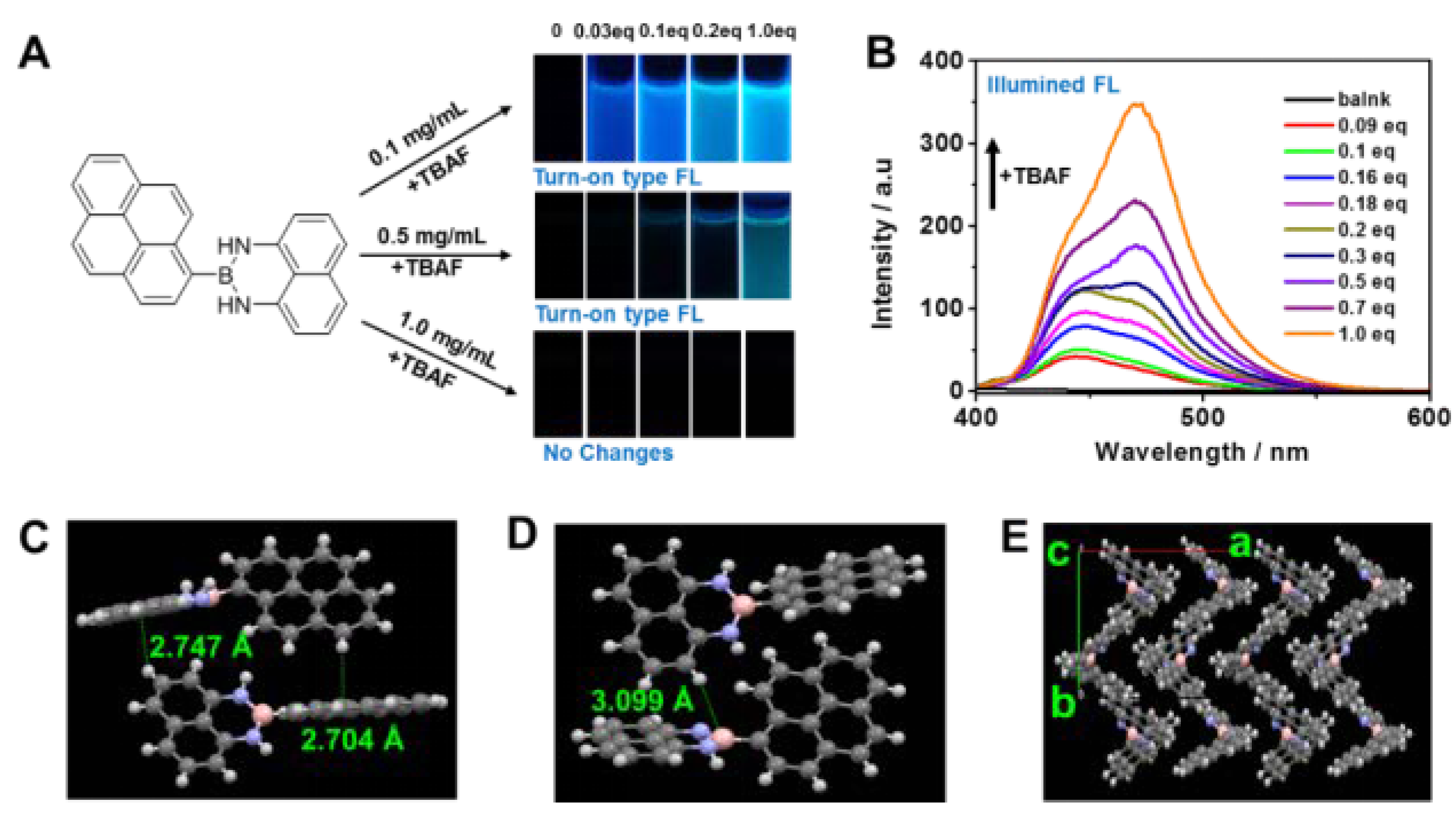
2.3. Fluoride Ion Detection
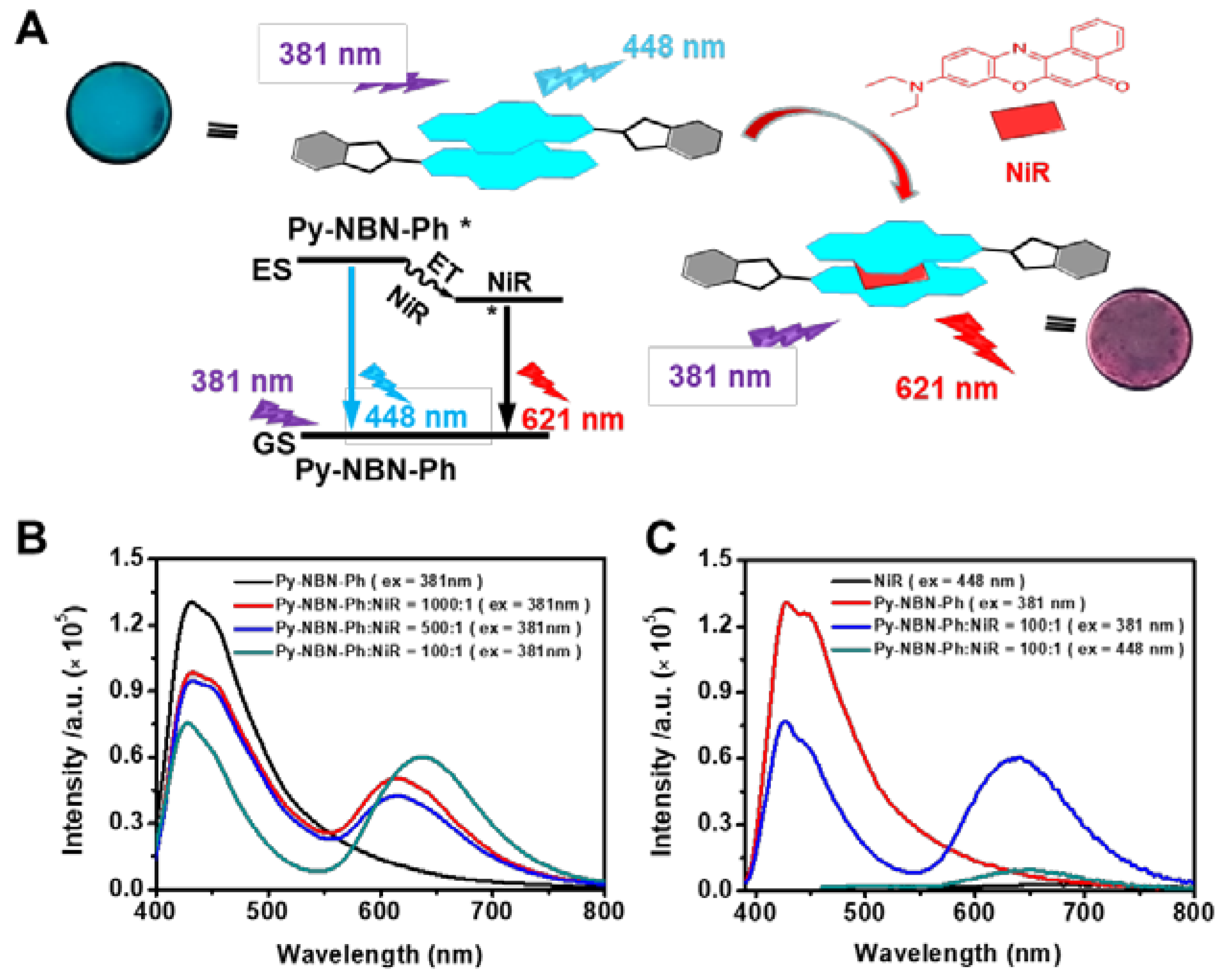
2.4. Artificial Light-Harvesting Film
3. Materials and Methods
3.1. Materials
3.2. Synthetic Procedures
3.2.1. Synthesis of Py-NBN-Ph
3.2.2. Synthesis of Py-NBN-Naphth
3.2.3. Ab Initio Calculations
3.2.4. The Detection of TBAF
3.2.5. Thin-Film Fabrication
3.3. Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vidal, F.; Lin, H.; Morales, C.; Jakle, F. Polysiloxane/Polystyrene Thermo-Responsive and Self-Healing Polymer Network via Lewis Acid-Lewis Base Pair Formation. Molecules 2018, 23, 405. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.-H.; Li, S.-S.; Tian, C.-Y.; Yang, M.-M.; Li, C.; Zhou, Y.; Sun, X.-L.; Zhang, J.; Wan, W.-M. Borinic Acid Polymer: Simplified Synthesis and Enzymatic Biofuel Cell Application. Macromol. Rapid Commun. 2017, 38, 1600687. [Google Scholar] [CrossRef]
- Lv, X.-H.; Wang, X.-Y.; Zhou, Y.; Xu, H.; Wan, W.-M. Promoting water dissociation performance by borinic acid for the strong-acid/base-free hydrogen evolution reaction. Chem. Commun. 2019, 55, 9821–9824. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, J.S.A.; Marshall, J.L.; Mazière, A.; Lovinger, G.J.; Li, B.; Zakharov, L.N.; Dargelos, A.; Graciaa, A.; Chrostowska, A.; Liu, S.-Y. Two BN Isosteres of Anthracene: Synthesis and Characterization. J. Am. Chem. Soc. 2014, 136, 15414–15421. [Google Scholar] [CrossRef] [PubMed]
- Mellerup, S.K.; Wang, S. Boron-Based Stimuli Responsive Materials. Chem. Soc. Rev. 2019, 48, 3537–3549. [Google Scholar] [CrossRef] [PubMed]
- Neena, K.K.; Thilagar, P. Replacing the non-polarized C=C bond with an isoelectronic polarized B–N unit for the design and development of smart materials. J. Mater. Chem. C 2016, 4, 11465–11473. [Google Scholar] [CrossRef]
- Ren, Y.; Sezen, M.; Guo, F.; Jäkle, F.; Loo, Y.-L. [d]-Carbon-Carbon Double Bond Engineering in Diazaphos-phepines: A Pathway to Modulate the Chemical and Electronic Structures of Heteropines. Chem. Sci. 2016, 7, 4211–4219. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.L.; Liu, D.M.; Lv, X.H.; Zhou, P.; Sun, M.; Wan, W.M. Thermo-responsive rheological behavior of borinic acid polymer in dilute solution. RSC Adv. 2016, 6, 83393–83398. [Google Scholar] [CrossRef]
- Wan, W.-M.; Li, S.-S.; Liu, D.-M.; Lv, X.-H.; Sun, X.-L. Synthesis of Electron-Deficient Borinic Acid Polymers with Multiresponsive Properties and Their Application in the Fluorescence Detection of Alizarin Red S and Electron-Rich 8-Hydroxyquinoline and Fluoride Ion: Substituent Effects. Macromolecules 2017, 50, 6872–6879. [Google Scholar] [CrossRef]
- Ayhan, O.; Eckert, T.; Plamper, F.A.; Helten, H. Poly(iminoborane)s: An Elusive Class of Main-Group Polymers? Angew. Chem. Int. Edit. 2016, 55, 13321–13325. [Google Scholar] [CrossRef]
- Lorenz, T.; Crumbach, M.; Eckert, T.; Lik, A.; Helten, H. Poly(p -phenylene iminoborane): A Boron-Nitrogen Analogue of Poly(p -phenylene vinylene). Angew. Chem. Int. Ed. 2017, 56, 2780–2784. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, J.-Y.; Pei, J. BN Heterosuperbenzenes: Synthesis and Properties. Chem. Eur. J. 2015, 21, 3528–3539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Nettleton, D.O.; Karki, R.G.; Zécri, F.J.; Liu, S.-Y. Medicinal Chemistry Profiling of Monocyclic 1,2-Azaborines. ChemMedChem 2017, 12, 358–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudebusch, G.; Zakharov, L.N.; Liu, S. Rhodium-Catalyzed Boron Arylation of 1,2-Azaborines. Angew. Chem. Int. Ed. 2013, 52, 9316–9319. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, A.D.O.; Escobedo-Morales, A.; Skakerzadeh, E.; Anota, E.C. Effect of homonuclear boron bonds in the adsorption of DNA nucleobases on boron nitride nanosheets. J. Mol. Liq. 2021, 322, 114951. [Google Scholar] [CrossRef]
- Juárez, A.R.; Ortiz-Chi, F.; Pino-Ríos, R.; Cárdenas-Jirón, G.; Villanueva, M.S.; Anota, E.C. The boron nitride (B116N124) fullerene: Stability and electronic properties from DFT simulations. Chem. Phys. Lett. 2020, 741, 137097. [Google Scholar] [CrossRef]
- Escobedo-Morales, A.; Tepech-Carrillo, L.; Bautista-Hernández, A.; Camacho-García, J.H.; Cortés-Arriagada, D.; Chigo-Anota, E. Effect of Chemical Order in the Structural Stability and Physicochemical Properties of B12N12 Fullerenes. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Mellerup, S.; Li, C.; Peng, T.; Wang, S. Regioselective Photoisomerization/C−C Bond Formation of Asymmetric B(ppy)(Mes)(Ar): The Role of the Aryl Groups on Boron. Angew. Chem. Int. Ed. 2017, 56, 6093–6097. [Google Scholar] [CrossRef]
- Shi, Y.-G.; Wang, J.-W.; Li, H.; Hu, G.-F.; Li, X.; Mellerup, S.K.; Wang, N.; Peng, T.; Wang, S. A simple multi-responsive system based on aldehyde functionalized amino-boranes. Chem. Sci. 2018, 9, 1902–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.P.; Liu, Y.L.; Gopalakrishna, T.Y.; Phan, H.; Huang, X.B.; Bao, L.P.; Guo, J.; Zhou, J.; Luo, S.L.; Wu, J.S.; et al. B-N-B Bond Embedded Phenalenyl and Its Anions. J. Am. Chem. Soc. 2017, 139, 15760–15767. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.M.; Mikulas, T.C.; Zakharov, L.N.; Dixon, D.A.; Liu, S.Y. Boron-Substituted 1,3-Dihydro-1,3-azaborines: Synthesis, Structure, and Evaluation of Aromaticity. Angew. Chem. Int. Edit. 2013, 52, 7527–7531. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.G.; Zhang, Y.Z.; Li, B.; Zakharov, L.N.; Vasiliu, M.; Dixon, D.A.; Liu, S.Y. A Modular Synthetic Approach to Monocyclic 1,4-Azaborines. Angew. Chem. Int. Edit. 2016, 55, 8333–8337. [Google Scholar] [CrossRef] [PubMed]
- Braunschweig, H.; Damme, A.; Jimenez-Halla, J.O.C.; Pfaffinger, B.; Radacki, K.; Wolf, J. Metal-Mediated Synthesis of 1,4-Di-tert-butyl-1,4-azaborine. Angew. Chem. Int. Edit. 2012, 51, 10034–10037. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.G.; Marwitz, A.J.V.; Liu, S.-Y. Recent Advances in Azaborine Chemistry. Angew. Chem. Int. Edit. 2012, 51, 6074–6092. [Google Scholar] [CrossRef] [PubMed]
- Tasseroul, J.; Lorenzo-Garcia, M.M.; Dosso, J.; Simon, F.; Velari, S.; De Vita, A.; Tecilla, P.; Bonifazi, D. Probing Peripheral H-Bonding Functionalities in BN-Doped Polycyclic Aromatic Hydrocarbons. J. Org. Chem. 2020, 85, 3454–3464. [Google Scholar] [CrossRef]
- Liu, K.; Lalancette, R.A.; Jäkle, F. Tuning the Structure and Electronic Properties of B–N Fused Dipyri-dylanthracene and Implications on the Self-Sensitized Reactivity with Singlet Oxygen. J. Am. Chem. Soc. 2019, 141, 7453–7462. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Marder, T.B. B-N versus C-C: How Similar are They? Angew. Chem. Int. Edit. 2008, 47, 242–244. [Google Scholar] [CrossRef]
- Wan, W.M.; Baggett, A.W.; Cheng, F.; Lin, H.; Liu, S.Y.; Jakle, F. Synthesis by Free Radical Polymerization and Properties of BN-polystyrene and BN-poly(vinylbiphenyl). Chem. Commun. 2016, 52, 13616–13619. [Google Scholar] [CrossRef]
- Xu, S.; Zakharov, L.N.; Liu, S.-Y. A 1,3-Dihydro-1,3-azaborine Debuts. J. Am. Chem. Soc. 2011, 133, 20152–20155. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, G.; Xu, L.; Zhuo, Y.; Wan, W.; Yan, N.; He, G. 9,10-Azaboraphenanthrene-containing small molecules and conjugated polymers: Synthesis and their application in chemodosimeters for the ratiometric detection of fluoride ions. Chem. Sci. 2018, 9, 4444–4450. [Google Scholar] [CrossRef] [Green Version]
- Alahmadi, A.F.; Lalancette, R.A.; Jäkle, F. Highly Luminescent Ladderized Fluorene Copolymers Based on B–N Lewis Pair Functionalization. Macromol. Rapid Commun. 2018, 39, e1800456. [Google Scholar] [CrossRef]
- Liu, K.; Lalancette, R.A.; Jäkle, F. B–N Lewis Pair Functionalization of Anthracene: Structural Dynamics, Optoelectronic Properties, and O2 Sensitization. J. Am. Chem. Soc. 2017, 139, 18170–18173. [Google Scholar] [CrossRef] [PubMed]
- Araneda, J.F.; Neue, B.; Piers, W.E. Enforced Planarity: A Strategy for Stable Boron-Containing π-Conjugated Materials. Angew. Chem. Int. Edit. 2012, 51, 9977–9979. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y. BN Embedded Polycyclic π-Conjugated Systems: Synthesis, Optoelectronic Properties, and Photovoltaic Applications. Front. Chem. 2018, 6, 341. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, Y.; Li, J.; Cao, J.; Zhu, J.; Sun, X.W.; Zhang, Q. Synthesis, Characterization, Physical Properties, and OLED Application of Single BN-Fused Perylene Diimide. J. Org. Chem. 2015, 80, 196–203. [Google Scholar] [CrossRef]
- Lu, J.S.; Ko, S.B.; Walters, N.R.; Kang, Y.; Sauriol, F.; Wang, S.N. Formation of Azaborines by Photoelimi-nation of B,N-Heterocyclic Compounds. Angew. Chem. Int. Edit. 2013, 52, 4544–4548. [Google Scholar] [CrossRef]
- Wang, S.N.; Yuan, K.; Hu, M.F.; Wang, X.; Peng, T.; Wang, N.; Li, Q.S. Cleavage of Unstrained C-C Bonds in Acenes by Boron and Light: Transformation of Naphthalene into Benzoborepin. Angew. Chem. Int. Edit. 2018, 57, 1073–1077. [Google Scholar] [CrossRef]
- Zhou, Z.; Wakamiya, A.; Kushida, T.; Yamaguchi, S. Planarized Triarylboranes: Stabilization by Structural Constraint and Their Plane-to-Bowl Conversion. J. Am. Chem. Soc. 2012, 134, 4529–4532. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Schellhammer, K.S.; Machata, P.; Ortmann, F.; Cuniberti, G.; Fu, Y.; Hunger, J.; Tang, R.; Popov, A.A.; et al. Synthesis of NBN-Type Zigzag-Edged Polycyclic Aromatic Hydrocarbons: 1,9-Diaza-9a-boraphenalene as a Structural Motif. J. Am. Chem. Soc. 2016, 138, 11606–11615. [Google Scholar] [CrossRef]
- Yang, D.-T.; Nakamura, T.; He, Z.; Wang, X.; Wakamiya, A.; Peng, T.; Wang, S. Doping Polycyclic Arenes with Nitrogen–Boron–Nitrogen (NBN) Units. Org. Lett. 2018, 20, 6741–6745. [Google Scholar] [CrossRef]
- Lorenz, T.; Lik, A.; Plamper, F.A.; Helten, H. Dehydrocoupling and Silazane Cleavage Routes to Organic-Inorganic Hybrid Polymers with NBN Units in the Main Chain. Angew. Chem. Int. Edit. 2016, 55, 7236–7241. [Google Scholar] [CrossRef]
- Wan, W.-M.; Tian, D.; Jing, Y.-N.; Zhang, X.-Y.; Wu, W.; Ren, H.; Bao, H.-L. NBN-Doped Conjugated Polycyclic Aromatic Hydrocarbons as an AIEgen Class for Extremely Sensitive Detection of Explosives. Angew. Chem. Int. Edit. 2018, 57, 15510–15516. [Google Scholar] [CrossRef]
- Peng, H.Q.; Niu, L.Y.; Chen, Y.Z.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. Biological Applications of Supramolecular Assemblies Designed for Excitation Energy Transfer. Chem. Rev. 2015, 115, 7502–7542. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Bonder, E.M.; Jakle, F. Electron-Deficient Triaryl borane Block Copolymers: Synthesis by Con-trolled Free Radical Polymerization and Application in the Detection of Fluoride Ions. J. Am. Chem. Soc. 2013, 135, 17286–17289. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.H.M.; Molander, G.A. Synthesis of Functionalized 1,3,2-Benzodiazaborole Cores Using Bench-Stable Components. J. Org. Chem. 2016, 81, 3771–3779. [Google Scholar] [CrossRef]
- Hudson, Z.; Wang, S. Impact of Donor−Acceptor Geometry and Metal Chelation on Photophysical Properties and Applications of Triarylboranes. Acc. Chem. Res. 2009, 42, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Makedonas, C.; Mitsopoulou, C.A. The Relationship Between Aromaticity and Charge-Transfer Transitions: A Combined Study of Square-Planar Metal Complexes Based on DFT, NMR and Nucleus-Independent Chemical Shifts. Eur. J. Inorg. Chem. 2006, 2006, 2460–2468. [Google Scholar] [CrossRef]
- Chan, T.L.; Xie, Z. Synthesis, structure and aromaticity of carborane-fused carbo- and heterocycles. Chem. Sci. 2018, 9, 2284–2289. [Google Scholar] [CrossRef] [Green Version]
- Long, S.; Miao, L.; Li, R.; Deng, F.; Qiao, Q.; Liu, X.; Yan, A.; Xu, Z. Rapid Identification of Bacteria by Mem-brane-Responsive Aggregation of a Pyrene Derivative. ACS Sens. 2019, 4, 281–285. [Google Scholar] [CrossRef]
- Aratani, N.; Yamada, H.; Mei, P.; Kurosaki, R.; Matsumoto, A. One-Pot Synthesis of a Cyclic Pyrene Octamer from Two Bifunctionalized Pyrene Monomers. Synthesis 2020, 53, 344–347. [Google Scholar]
- Orita, A.; Watanabe, H.; Nakajima, K.; Ekuni, K.; Edagawa, R.; Akagi, Y.; Okuda, Y.; Wakamatsu, K. Custom-Made Pyrene Photocatalyst-Promoted Desulfonylation of Arylethenyl Sulfones Using Green-Light-Emitting Diodes. Synthesis 2021, 53, 2984–2994. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, H.; Peng, Y.; Zheng, J.; Wu, J. Visualization of the self-healing process by directly observing the evolution of fluorescence intensity. Polym. Chem. 2021, 12, 494–500. [Google Scholar] [CrossRef]
- Wu, B.Y.; Le, X.X.; Jian, Y.K.; Lu, W.; Yang, Z.Y.; Zheng, Z.K.; Theato, P.; Zhang, J.W.; Zhang, A.; Chen, T. pH and Thermo Dual-Responsive Fluorescent Hydrogel Actuator. Macromol. Rapid Commun. 2019, 40, e1800648. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Jing, Y.N.; Bao, H.L.; Wan, W.M. Triarylmethanolation as A Aersatile Strategy for the Conversion of PAHs into Amorphization-Induced Emission Luminogens for Extremely Sensitive Explosive Detection and Fabrication of Artificial Light-Harvesting Systems. Mater. Chem. Front. 2020, 4, 2435–2442. [Google Scholar] [CrossRef]
- Sun, X.-L.; Liu, N.-M.; Tian, D.; Zhang, X.-Y.; Wu, W.; Wan, W.-M. The introduction of the Barbier reaction into polymer chemistry. Nat. Commun. 2017, 8, 1210. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.N.; Li, S.S.; Su, M.; Bao, H.; Wan, W.M. Barbier Hyperbranching Polymerization-Induced Emission toward Facile Fabrication of White LED and Light Harvesting Film. J. Am. Chem. Soc. 2019, 141, 16839–16848. [Google Scholar] [CrossRef]
- Li, S.-S.; Jing, Y.-N.; Bao, H.; Wan, W.-M. Exploitation of Monofunctional Carbonyl Resources by Barbier Polymerization for Materials with Polymerization-Induced Emission. Cell Rep. Phys. Sci. 2020, 1, 100116. [Google Scholar] [CrossRef]
- Li, S.S.; Zhu, N.B.; Jing, Y.N.; Li, Y.J.; Bao, H.L.; Wan, W.M. Barbier Self-Condensing Ketyl Polymerization-Induced Emission: A Polarity Reversal Approach to Reversed Polymerizability. iScience 2020, 23, 58. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Li, T.; Sun, X.-L.; Wan, W.-M.; Bao, H.; Qian, Q.; Chen, Q. Unpredicted Concentration-Dependent Sensory Properties of Pyrene-Containing NBN-Doped Polycyclic Aromatic Hydrocarbons. Molecules 2022, 27, 327. https://doi.org/10.3390/molecules27010327
Xiao H, Li T, Sun X-L, Wan W-M, Bao H, Qian Q, Chen Q. Unpredicted Concentration-Dependent Sensory Properties of Pyrene-Containing NBN-Doped Polycyclic Aromatic Hydrocarbons. Molecules. 2022; 27(1):327. https://doi.org/10.3390/molecules27010327
Chicago/Turabian StyleXiao, Hang, Tao Li, Xiao-Li Sun, Wen-Ming Wan, Hongli Bao, Qingrong Qian, and Qinghua Chen. 2022. "Unpredicted Concentration-Dependent Sensory Properties of Pyrene-Containing NBN-Doped Polycyclic Aromatic Hydrocarbons" Molecules 27, no. 1: 327. https://doi.org/10.3390/molecules27010327






