Theoretical Studies on the Mechanism of deNOx Process in Cu–Zn Bimetallic System—Comparison of FAU and MFI Zeolites
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
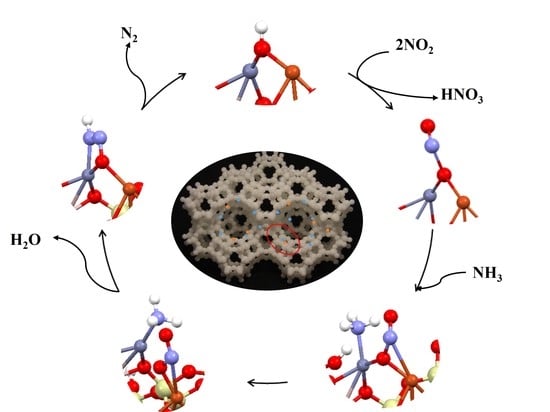
- The bimetallic dimer selected in this study, Cu–Zn, showed high stability in the FAU zeolite, while their adsorption on the MFI zeolite proceeded with an energy barrier. The deNOx reaction mechanism was the same regardless of zeolite type.
- All NO and NH3 coadsorption variants occurred spontaneously with energy release.
- To propose the mechanism of the deNOx process, the most stable structures (emitting the highest amount of energy) were chosen because of the most probable formation of these systems inside the zeolite frame.
- Two types of mechanism were proposed depending on the type of dimer hydration. It was shown that energy barriers occurred between different stages depending on the type of hydration.
- The most efficient reaction mechanism was represented by the FAU zeolite with bimetallic Cu–Zn dimer and hydrated zinc center because during the process the energy barrier appeared only in one stage and was relatively low.
- The real advantage of the Cu–Zn system over FAU and MFI in hydrothermal conditions was demonstrated in comparison to a conventional Cu–Cu catalyst.
- This study provided a good basis for comparison with experimental results to confirm the theoretically obtained adsorption mechanisms.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mera, Z.; Matzer, C.; Hausberger, S.; Fonseca, N. Performance of selective catalytic reduction (SCR) system in a diesel passenger car under real-world conditions. Appl. Therm. Eng. 2020, 181, 115983. [Google Scholar] [CrossRef]
- Carslaw, D.C.; Farren, N.J.; Vaughan, A.R.; Drysdale, W.S.; Young, S.; Lee, J.D. The diminishing importance of nitrogen dioxide emissions from road vehicle exhaust. Atmos. Environ. X 2019, 1, 100002. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Y.; Chen, Z.; Yu, Q.; Wu, S. High-efficiency removal of NOx over natural mordenite using an enhanced plasma-catalytic process at ambient temperature. Fuel 2018, 224, 323–330. [Google Scholar] [CrossRef]
- Herreros, J.M.; George, P.; Umar, M.; Tsolakis, A. Enhancing selective catalytic reduction of NOx with alternative reactants/promoters. Chem. Eng. J. 2014, 252, 47–54. [Google Scholar] [CrossRef]
- Yao, X.; Ma, K.; Zou, W.; He, S.; An, J.; Yang, F.; Dong, L. Influence of preparation methods on the physicochemical properties and catalytic performance of MnOx-CeO2 catalysts for NH3-SCR at low temperature. Chin. J. Catal. 2017, 38, 146–159. [Google Scholar] [CrossRef]
- Meng, D.; Zhan, W.; Guo, Y.; Guo, Y.; Wang, L.; Lu, G. A Highly Effective Catalyst of Sm-MnOx for the NH3-SCR of NOx at Low Temperature: Promotional Role of Sm and Its Catalytic Performance. ACS Catal. 2015, 5, 5973–5983. [Google Scholar] [CrossRef]
- Bosch, H.; Janssen, F. Formation and control of nitrogen oxides. Catal. Today 1988, 2, 369–379. [Google Scholar] [CrossRef]
- Yang, S.; Pan, X.; Han, Z.; Zhao, D.; Liu, B.; Zheng, D.; Yan, Z. Removal of NOx and SO2 from simulated ship emissions using wet scrubbing based on seawater electrolysis technology. Chem. Eng. J. 2018, 331, 8–15. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total Environ. 2020, 714, 136712. [Google Scholar] [CrossRef]
- Baran, R.; Grzybek, T.; Onfroy, T.; Dzwigaj, S. High activity of mononuclear copper present in the framework of CuSiBEA zeolites in the selective catalytic reduction of NO with NH3. Microporous Mesoporous Mater. 2016, 226, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Current Understanding of Cu-Exchanged Chabazite Molecular Sieves for Use as Commercial Diesel Engine DeNOx Catalysts. Top. Catal. 2013, 56, 1441–1459. [Google Scholar] [CrossRef]
- Qian, F.; Ma, D.; Zhu, N.; Li, P.; Xu, X. Research on Optimization Design of SCR Nozzle for National VI Heavy Duty Diesel Engine. Catalyst 2019, 9, 452. [Google Scholar] [CrossRef] [Green Version]
- Szymaszek, A.; Samojeden, B.; Motak, M. The Deactivation of Industrial SCR Catalysts—A Short Review. Energies 2020, 13, 3870. [Google Scholar] [CrossRef]
- Klimczak, M.; Kern, P.; Heinzelmann, T.; Lucas, M.; Claus, P. High-throughput study of the effects of inorganic additives and poisons on NH3-SCR catalysts—Part I: V2O5–WO3/TiO2 catalysts. Appl. Catal. B Environ. 2010, 95, 39–47. [Google Scholar] [CrossRef]
- Andana, T.; Rappe, K.G.; Gao, F.; Szanyi, J.; Pereira-Hernandez, X.; Wang, Y. Recent advances in hybrid metal oxide–zeolite catalysts for low-temperature selective catalytic reduction of NOx by ammonia. Appl. Catal. B Environ. 2021, 291, 120054. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Q.; Zhang, Z. Zeolitic Materials for DeNOx Selective Catalytic Reduction. ChemCatChem 2018, 10, 29–41. [Google Scholar] [CrossRef]
- Liu, Q.; Bian, C.; Ming, S.; Guo, L.; Zhang, S.; Pang, L.; Liu, P.; Chen, Z.; Li, T. The opportunities and challenges of iron-zeolite as NH3-SCR catalyst in purification of vehicle exhaust. Appl. Catal. A Gen. 2020, 607, 117865. [Google Scholar] [CrossRef]
- Ghasemian, N.; Falamaki, C. Zn2+, Fe2+, Cu2+, Mn2+, H+ Ion-exchanged and Raw Clinoptilolite Zeolite Catalytic Performance in the Propane-SCR-NOx Process: A Comparative Study. Int. J. Chem. React. Eng. 2017, 16, 20160192. [Google Scholar] [CrossRef]
- Pereda-Ayo, B.; De La Torre, U.; Romero-Saez, M.; Aranzabal, A.; Gonzalez-Marcos, J.A.; Gonzalez-Velasco, J.R. Influence of the washcoat characteristics on NH3-SCR behavior of Cu-zeolite monoliths. Catal. Today 2013, 216, 82–89. [Google Scholar] [CrossRef]
- Grzybek, J.; Gil, B.; Roth, W.J.; Skoczek, M.; Kowalczyk, A.; Chmielarz, L. Characterization of Co and Fe-MCM-56 catalysts for NH3-SCR and N2O decomposition: An in situ FTIR study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 196, 281–288. [Google Scholar] [CrossRef]
- Man, H.; Wen, C.; Luo, W.; Bian, J.; Wang, W.; Li, C. Simultaneous deSOx and deNOx of marine vessels flue gas on ZnO-CuO/rGO: Photocatalytic oxidation kinetics. J. Ind. Eng. Chem. 2020, 92, 77–87. [Google Scholar] [CrossRef]
- Mytareva, A.I.; Bokarev, D.A.; Stakheev, A.Y. Seven Modern Trends in the DeNOx Catalyst Development. Kinet. Catal. 2021, 62, 1–32. [Google Scholar] [CrossRef]
- Moliner, M.; Corma, A. From metal-supported oxides to well-defined metal site zeolites: The next generation of passive NO x adsorbers for low-temperature control of emissions from diesel engines. React. Chem. Eng. 2019, 4, 223–234. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Y.; Yang, R.T. Selective Catalytic Reduction of Nitric Oxide with Ammonia over ZSM-5 Based Catalysts for Diesel Engine Applications. Catal. Lett. 2008, 121, 111–117. [Google Scholar] [CrossRef]
- Begum, S.H.; Hung, C.-T.; Chen, Y.-T.; Huang, S.-J.; Wu, P.-H.; Han, X.; Liu, S.-B. Acidity-activity correlation over bimetallic iron-based ZSM-5 catalysts during selective catalytic reduction of NO by NH3. J. Mol. Catal. A Chem. 2016, 423, 423–432. [Google Scholar] [CrossRef]
- Yuan, E.; Han, W.; Zhang, G.; Zhao, K.; Mo, Z.; Lu, G.; Tang, Z. Structural and Textural Characteristics of Zn-Containing ZSM-5 Zeolites and Application for the Selective Catalytic Reduction of NOx with NH3 at High Temperatures. Catal. Surv. Asia 2016, 20, 41–52. [Google Scholar] [CrossRef]
- Tarach, K.A.; Jablonska, M.; Pyra, K.; Liebau, M.; Reiprich, B.; Glaser, R.; Gora-Marek, K. Effect of zeolite topology on NH3-SCR activity and stability of Cu-exchanged zeolites. Appl. Catal. B Environ. 2021, 284, 119752. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Li, J.; Ren, X.; Liu, S.; Gao, J.; Schwank, J.W.; Zhang, T.; Su, W.; Chang, H. Synthesis and evaluation of mesopore structured ZSM-5 and a CuZSM-5 catalyst for NH3-SCR reaction: Studies of simulated exhaust and engine bench testing. RSC Adv. 2016, 6, 102570–102581. [Google Scholar] [CrossRef]
- Mohan, S.; Dinesha, P.; Kumar, S. NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: A review. Chem. Eng. J. 2020, 384, 123253. [Google Scholar] [CrossRef]
- Xu, R.; Wang, Z.; Liu, N.; Dai, C.; Zhang, J.; Chen, B. Understanding Zn Functions on Hydrothermal Stability in a One-Pot-Synthesized Cu&Zn-SSZ-13 Catalyst for NH3 Selective Catalytic Reduction. ACS Catal. 2020, 10, 6197–6212. [Google Scholar] [CrossRef]
- Saeidi, M.; Hamidzadeh, M. Co-doping a metal (Cr, Mn, Fe, Co, Ni, Cu, and Zn) on Mn/ZSM-5 catalyst and its effect on the catalytic reduction of nitrogen oxides with ammonia. Res. Chem. Intermed. 2017, 43, 2143–2157. [Google Scholar] [CrossRef]
- Shelyapina, M.G.; Gurgul, J.; Łątka, K.; Sánchez-López, P.; Bogdanov, D.; Kotolevich, Y.; Petranovskii, V.; Fuentes, S. Mechanism of formation of framework Fe3+ in bimetallic Ag-Fe mordenites - effective catalytic centers for deNOx reaction. Microporous Mesoporous Mater. 2020, 299, 109841. [Google Scholar] [CrossRef]
- Chen, M.; Sun, O.; Yang, X.; Yu, J. A dual-template method for the synthesis of bimetallic CuNi/SSZ-13 zeolite catalysts for NH3-SCR reaction. Inorg. Chem. Commun. 2019, 105, 203–207. [Google Scholar] [CrossRef]
- Jouini, H.; Mejri, I.; Petitto, C.; Martinez-Ortigosa, J.; Vidal-Moya, A.; Mhamdi, M.; Blascod, T.; Delahay, G. Characterization and NH3-SCR reactivity of Cu-Fe-ZSM-5 catalysts prepared by solid state ion exchange: The metal exchange order effect. Microporous Mesoporous Mater. 2018, 260, 217–226. [Google Scholar] [CrossRef]
- Jouini, H.; Martinez-Ortigosa, J.; Mejri, I.; Mhamdi, M.; Blasco, T.; Delahay, G. On the performance of Fe-Cu-ZSM-5 catalyst for the selective catalytic reduction of NO with NH3: The influence of preparation method. Res. Chem. Intermed. 2019, 45, 1057–1072. [Google Scholar] [CrossRef]
- Janssens, T.V.W.; Falsig, H.; Lundegaard, L.F.; Vennestrøm, P.N.R.; Rasmussen, S.B.; Moses, P.G.; Giordanino, F.; Borfecchia, E.; Lomachenko, K.A.; Lamberti, C.; et al. A Consistent Reaction Scheme for the Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. ACS Catal. 2015, 5, 2832–2845. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Wang, H.-F.; Hu, P. Theoretical Investigation of NH3-SCR Processes over Zeolites: A Review. Int. J. Quantum Chem. 2015, 115, 618–630. [Google Scholar] [CrossRef]
- Kurzydym, I.; Czekaj, I. Theoretical Studies of deNOx SCR over Cu-, Fe- and Mn-FAU Catalysts. Chem. Chem. Technol. 2021, 15, 16–25. [Google Scholar] [CrossRef]
- Battiston, A.A.; Bitter, J.H.; Koningsberger, O.C. Reactivity of binuclear Fe complexes in over-exchanged Fe/ZSM5, studied by in situ XAFS spectroscopy 2. Selective catalytic reduction of NO with isobutane. J. Catal. 2003, 218, 163–177. [Google Scholar] [CrossRef]
- Abdullah, A.Z.; Abdullah, H.; Bhatia, S. Selective Catalytic Reduction of Nitric Oxide in Diesel Engine Exhaust over Monolithic Catalysts Washcoated with Bimetallic Cu-Zn/ZSM-5. Environ. 2009, 1, 10–16. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: https://asia.iza-structure.org/IZA-SC/ftc_table.php (accessed on 11 June 2021).
- Czekaj, I.; Brandenberger, S.; Kröcher, O. Theoretical studies of HNCO adsorption at stabilized iron complexes in the ZSM-5 framework. Microporous Mesoporous Mater. 2013, 169, 97–102. [Google Scholar] [CrossRef]
- Jodłowski, P.J.; Czekaj, I.; Stachurska, P.; Kuterasiński, Ł.; Chmielarz, L.; Jędrzejczyk, R.J.; Jeleń, P.; Sitarz, M.; Górecka, S.; Mazur, M.; et al. Experimental and Theoretical Studies of Sonically Prepared Cu–Y, Cu–USY and Cu–ZSM-5 Catalysts for SCR deNOx. Catalysts 2021, 11, 824. [Google Scholar] [CrossRef]
- Bendrich, M.; Scheuer, A.; Hayes, R.E.; Votsmeier, M. Unified mechanistic model for Standard SCR, Fast SCR, and NO2 SCR over a copper chabazite catalyst. Appl. Catal. B Environ. 2018, 222, 76–87. [Google Scholar] [CrossRef]
- Liu, C.; Kubota, H.; Toyao, T.; Maeno, Z.; Shimizu, K. Mechanistic insights into the oxidation of copper(I) species during NH3-SCR over Cu-CHA zeolites: A DFT study. Catal. Sci. Technol. 2020, 10, 3586–3593. [Google Scholar] [CrossRef]
- Hermann, K.; Pettersson, L.G.M.; Casida, M.E.; Daul, C.; Goursot, A.; Koester, A.; Proynov, E.; St-Amant, A.; Salahub, D.R.; Carravetta, V.; et al. StoBe-deMon; deMon Software: Stockholm, Sweden; Berlin, Germany, 2005; Available online: http://www.fhi-berlin.mpg.de/KHsoftware/StoBe/ (accessed on 11 June 2021).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B 1999, 59, 7413. [Google Scholar] [CrossRef] [Green Version]
- Broclawik, E.; Salahub, D.R. Density functional theory and quantum chemistry: Metals and metal oxides. J. Mol. Catal. 1993, 82, 117. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. II. Overlap Populations, Bond Orders, and Covalent Bond Energies. J. Chem. Phys. 1955, 23, 1833. [Google Scholar] [CrossRef] [Green Version]
- Mayer, I. Charge, bond order and valence in the AB initio SCF theory. Chem. Phys. Lett. 1983, 97, 270. [Google Scholar] [CrossRef]
- Mayer, I.J. Bond orders and valences: Role of d-orbitals for hypervalent Sulphur. Mol. Struct. THEOCHEM 1987, 149, 81. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzydym, I.; Czekaj, I. Theoretical Studies on the Mechanism of deNOx Process in Cu–Zn Bimetallic System—Comparison of FAU and MFI Zeolites. Molecules 2022, 27, 300. https://doi.org/10.3390/molecules27010300
Kurzydym I, Czekaj I. Theoretical Studies on the Mechanism of deNOx Process in Cu–Zn Bimetallic System—Comparison of FAU and MFI Zeolites. Molecules. 2022; 27(1):300. https://doi.org/10.3390/molecules27010300
Chicago/Turabian StyleKurzydym, Izabela, and Izabela Czekaj. 2022. "Theoretical Studies on the Mechanism of deNOx Process in Cu–Zn Bimetallic System—Comparison of FAU and MFI Zeolites" Molecules 27, no. 1: 300. https://doi.org/10.3390/molecules27010300