The Presence of a Cyclohexyldiamine Moiety Confers Cytotoxicity to Pentacyclic Triterpenoids
Abstract
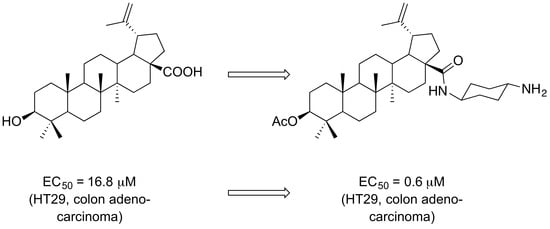
:1. Introduction
2. Results
3. Conclusions
4. Experimental
4.1. Cell Lines and Culture Conditions
4.2. Cytotoxicity Assay (SRB Assay)
4.3. General Procedure for the Synthesis of Acetates 1–4 (GPA)
4.4. General Procedure for the Synthesis of Amides 9–31 (GPB)
- 3β-Acetyloxy-olean-12-en-28-oic acid (1). Following GPA, compound 1 (4.89 g, 89%) was obtained as a colorless solid; Rf = 0.54 (hexanes/ethyl acetate, 3:1); m.p.: 259–263 °C (lit.: [29] 266–268 °C); = +74.1° (c 0.43, CHCl3) [lit.: [29] = +74.0° (c 1, CHCl3)]; MS (ESI, MeOH): m/z 499.1 ([M + H]+, 9), 521.3 (38%, [M + Na]+), 1019.4 (100%, [2M + Na]+).
- 3β-Acetoxy-urs-12-en-28-oic acid (2). Following GPA, compound 2 (4.89 g, 89%) was obtained as a colorless solid; Rf = 0.71 (toluene/ethyl acetate/heptane/formic acid, 80:26:10:5); m.p.: 287–290 °C (lit.: [30] 289–290 °C); = +68.9° (c 0.315, CHCl3) [lit.: [31] = +72.3° (c 0.5, CHCl3)]; MS (ESI, MeOH): m/z 499.0 ([M + H]+, 74), 516.3 (36%, [M + NH4]+), 521.5 (34%, [M + Na]+);
- 3β-Acetoxy-lup-20(29)-en-28-oic acid (3). Following GPA, compound 3 (4.90 g, 89%) was obtained as a colorless solid; Rf = 0.58 (hexanes/ethyl acetate, 4:1); m.p.: 281–283 °C (lit.: [32] 280–282 °C); = +25.6° (c 0.35, CHCl3) [lit.: [33] = +26.4° (c 0.54, CHCl3)]; MS (ESI, MeOH): m/z 487.1 (28%, [M− H]−) 995.3 (100%, [2M − H]−), 1018.2 (28%, [2M − 2H + Na]−).
- 3β-Acetoxy-20-oxo-30-norlupan-28-oic acid (4). Following GPA, compound 4 (13.8 g, 84%) was obtained as a colorless solid; Rf = 0.50 (toluene/ethyl acetate/heptane/formic acid, 80:26:10:5); m.p.: 268–270 °C (decomp.), (lit.: [34] 252–255 °C); = −9.1° (c 0.34, CHCl3) [lit.: [34] = −9.5° (c 0.8, CHCl3)]; MS (ESI, MeOH): m/z 999.3 (100%, [2M − H]−).
- Trans-cyclohexyl-1,4-diamine (5) and cis-cyclohexyl-1,4-diamine (6). These compounds were commercially obtained from Merck and used as received.
- (3β)-28-[(trans-4-Aminocyclohexyl)amino]-28-oxoolean-12-en-3-yl acetate (9). Following GPB, compound 9 (1.01 g, 84%) was obtained as a colorless solid; Rf = 0.66 (CHCl3/MeOH, 8:2); m.p.: 203–205 °C (decomp.); = +23.6° (c 0.35, MeOH); IR (ATR): ν = 3423 w, 2945 m, 1703 m, 1618 m, 1430 s, 1332 s, 1036 s, 817 s. 749 s cm−1; 1H NMR (500 MHz, CD3OD): δ = 6.64 (m, 1H, NH), 5.43 (t, J = 3.6 Hz, 1H, 12-H), 4.48 (dd, J = 11.2, 4.9 Hz, 1H, 3-H), 3.89 (s, 1H, 33-H), 3.35–3.32 (m, 1H, 36-H), 2.85–2.79 (m, 1H, 18-H), 2.20–2.07 (m, 1H, 16-Ha), 2.05 (s, 3H, 32-H), 1.99–1.28 (m, 25H, 34-H, 35-H, 37-H, 38-H, 1-Hb, 22-H, 2-H, 15-Ha, 6-H, 11-H, 7-H, 1-Ha, 21-H, 9-H, 19-Hb), 1.23 (s, 3H, 27-H), 1.22–1.02 (m, 3H, 15-Hb, 16-Hb, 19-Ha), 1.00 (s, 3H, 25-H), 0.98 (s, 3H, 29-H), 0.94 (s, 3H, 30-H), 0.91 (s, 3H, 24-H), 0.91 (s, 3H, 23-H), 0.89 (m, 1H, 5-H), 0.84 (s, 3H, 26-H) ppm; 13C NMR (126 MHz, CD3OD): δ = 179.8 (C-28), 145.5 (C-13), 123.9 (C-12), 82.4 (C-3), 56.6 (C-5), 49.9 (C-9), 49.5 (C-33), 47.7 (C-19), 47.5 (C-17), 46.3 (C-36), 43.8 (C-18), 43.1 (C-14), 40.7 (C-8), 39.3 (C-1), 38.7 (C-4), 38.1 (C-10), 35.1 (C-21), 34.2 (C-34, C-38), 33.8 (C-35, C-37), 33.5 (C-30), 31.6 (C-20), 28.5 (C-15), 28.5 (C-23), 28.1 (C-7), 27.8 (C-22), 26.3 (C-27), 24.5 (C-2), 24.5 (C-11), 24.0 (C-16), 23.9 (C-29), 21.1 (C-32), 19.3 (C-6), 18.1 (C-26), 17.1 (C-24), 15.9 (C-25) ppm; ESI, MeOH): m/z 595.4 (100%, [M + H]+), 1189.3 (5%, [2M + H]+); analysis calcd for C38H62N3O4 (594.91): C 76.72, H 10.50, N 4.71; found: C 76.49, H 10.71, N 4.55. Please see Supplementary Materials.
- (3β)-28-[(cis-4-Aminocyclohexyl)amino]-28-oxoolean-12-en-3-yl acetate (10). Following GPB, compound 10 (1.02 g, 85%) was obtained as a colorless solid; Rf = 0.673 (CHCl3/MeOH, 8:2); m.p.: 197–200 °C (decomposition); = +3.8° (c 0.14, CHCl3); IR (ATR): ν = 3406 w, 2944 m, 1704 m, 1621 m, 1524 s, 1428 s, 1313 s, 1099 m, 1028 s, 821 s, 763 m cm−1; 1H NMR (500 MHz, CD3OD): δ = 6.61–6.55 (m, 1H, NH), 5.37 (t, 1H, 12-H), 4.42 (d, 1H, 3-H), 3.60–3.52 (m, 1H, 33-H), 3.29–3.24 (m, 1H, 36-H), 2.10–1.93 (m, 1H, 18-H), 1.99 (s, 3H, 32-H), 1.93–1.85 (m, 8H, 34-H, 35-H, 37-H, 38-H), 1.85–1.21 (m, 14H, 1-Hb, 22-Ha, 2-H, 16-Ha, 15-Hb, 6-H, 11-Hb, 7-Hb, 1-Ha, 21-Hb, 9-H, 19-Hb), 1.16 (s, 3H, 27-H), 1.16–0.97 (m, 5H, 15-Ha,16-Hb,19-Ha, 21-Ha, 22-Hb), 0.94 (s, 3H, 26-H), 0.91 (s, 3H, 24-H), 0.88 (s, 3H, 25-H), 0.85 (s, 3H, 29-H), 0.84 (s, 3H, 30-H), 0.83–0.81 (m, 1H, 5-H), 0.78 (s, 3H, 23-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 179.8 (C-28), 172.8 (C-31), 145.4 (C-13), 123.9 (C-12), 82.4 (C-3), 56.6 (C-5), 49.8 (C-9), 49.5 (C-33), 47.6 (C-19), 46.2 (C-17), 43.7 (C-18), 43.1 (C-14), 42.8 (C-36), 40.6 (C-21), 39.2 (C-8), 38.6 (C-4), 38.0 (C-1), 37.0 (C-10), 34.2 (C-7), 33.8 (C-34, C-38), 33.5 (C-29, C-30), 31.5 (C-35, C-37), 28.0 (C-20), 27.7 (C-22), 27.3 (C-15), 27.2 (C-16), 26.3 (C-27), 24.5 (C-2), 21.1 (C-32), 19.2 (C-6), 18.3 (C-23), 18.1 (C-26), 17.1 (C-24), 15.9 (C-25) ppm; MS (ESI, MeOH): m/z 595.4 (100%, [M + H]+), 1190.4 (8%, [2M + H]+); analysis calcd for C38H62N3O4 (594.91): C 76.72, H 10.50, N 4.71; found: C 76.59, H 10.75, N 4.46.
- (3β)-28-[(trans-4-Aminocyclohexyl)amino]-28-oxoursan-12-en-3-yl acetate (11). Following GPB, compound 11 (1.02 g, 85%) was obtained as a colorless solid; Rf = 0.66 (CHCl3/MeOH, 8:2); m.p.: 189–193 °C (decomp.); = +34.4° (c 0.31, MeOH); IR (ATR): ν = 3420 w, 2928 m, 1734 m, 1621 m, 1523 m,1314 s, 1244 s, 1096 s, 1027 s, 985 m, 902 m, 822 s cm−1; 1H NMR (500 MHz, CD3OD): δ = 6.90 (m, 1H, NH), 5.28 (t, J = 3.7 Hz, 1H, 12-H), 4.42 (m, 1H, 3-H), 3.64–3.51 (m, 1H, 33-H), 3.10–2.98 (m, 1H, 36-H), 2.16–2.12 (m, 1H, 18-H), 2.07–1.97 (m, 3H, 16-Hb, 35-Ha, 37-Ha), 1.99 (s, 3H, 30), 1.94–1.87 (m, 3H, 11, 21-Ha), 1.84–1.72 (m, 3H, 15-Hb, 34-Hb, 38-Hb), 1.69–1.26 (m, 17H, 1-Hb, 2-H, 6-H, 7-H, 9-H, 16-Ha, 19-H, 22-H, 34-Ha, 35-Hb, 37-Hb, 38-Ha), 1.10 (s, 3H, 27-H), 1.07–0.99 (m, 3H, 1-Ha, 15-Ha, 20-H), 0.95 (s, 3H, 25-H), 0.93 (s, 3H, 32-H), 0.87 (s, 3H, 26-H), 0.85 (s, 3H, 24-H), 0.84 (s, 3H, 23-H), 0.82–0.80 (m, 1H, 5-H), 0.79 (s, 3H, 29-H) ppm; 13C NMR (126 MHz, CD3OD): δ = 179.5 (C-28), 172.8 (C-31), 139.9 (C-13), 126.8 (C-12), 82.4 (C-3), 56.7 (C-5), 53.9 (C-18), 50.6 (C-36), 49.9 (C-9), 48.8 (C-17), 48.7 (C-33), 43.4 (C-14), 40.8 (C-19), 40.2 (C-20), 39.4 (C-1), 38.7 (C-8), 38.0 (C-4), 34.2 (C-10), 31.9 (C-7), 31.1 (C-21), 30.7 (C-38, C-34), 30.5 (C-37, C-35), 28.9 (C-15), 28.6 (C-23), 24.9 (C-16), 24.5 (C-2), 24.4 (C-11), 24.1 (C-27), 21.6 (C-32), 21.1 (C-30), 19.3 (C-6), 18.2 (C-29), 17.2 (C-24), 16.0 (C-25) ppm; MS (ESI, MeOH): m/z 595.4 (100%, [M + H]+), 1211.6 (4%, [2M + Na]+); analysis calcd for C38H62N3O4 (594.91): C 76.72, H 10.50, N 4.71; found: C 76.60, H 10.83, N 4.52.
- (3β)-28-[(Cis-4-Aminocyclohexyl)Amino]-28-Oxoursan-12-En-3-Yl Acetate (12). Following GPB, compound 12 (0.96 g, 80%) was obtained as a colorless solid; Rf = 0.67 (CHCl3/MeOH, 8:2); m.p.: 186–190 °C (decomp.); = +26.7° (c 0.10, CHCl3); IR (ATR): ν = 3416 m, 2929 m, 1625 m, 1520 m, 1326 s, 1245 s, 1028 s, 823 m cm−1; 1H NMR (500 MHz, CD3OD): δ = 6.52 (m, 1H, NH), 5.36 (t, J = 3.6 Hz, 1H, 12-H), 4.42 (dd, J = 11.0, 5.0 Hz, 1H, 3-H), 3.61–3.51 (m, 1H, 33-H), 3.28–3.26 (m, 1H, 36-H), 2.12–2.05 (m, 1H, 18-H), 1.99 (s, 3H, 30-H), 2.06–1.96 (m, 3H, 16-Hb, 35-Ha, 37-Ha), 1.96–1.89 (m, 3H, 11-H, 21-Ha), 1.89–1.75 (m, 3H, 15-Hb, 34-Hb, 38-Hb), 1.74–1.27 (m, 16H, 34-Ha, 35-Hb, 37-Hb,38-Ha, 1-Hb, 22-H, 2-H, 16-H, 6-H, 9-H, 7-H), 1.12 (s, 3H, 27-H), 1.08–1.00 (m, 3H, 1-Ha, 15-Ha, 20-H), 0.95 (s, 3H, 25-H), 0.94 (s, 3H, 32-H), 0.98 (m, 3H, 26-H), 0.85 (s, 3H, 24-H), 0.84 (s, 3H, 23-H), 0.83–0.81 (m, 1H, 5-H), 0.79 (s, 3H, 29-H) ppm; 13C NMR (126 MHz, CD3OD): δ = 179.7 (C-28), 172.8 (C-31), 140.4 (C-13), 127.0 (C-12), 82.3 (C-3), 56.6 (C-5), 54.6 (C-18), 49.8 (C-9), 49.6 (C-36), 49.0 (C-17), 48.8 (C-33), 43.5 (C-14), 40.9 (C-19), 40.0 (C-20), 39.4 (C-1), 38.7 (C-8), 38.0 (C-4), 34.1 (C-22), 31.9 (C-7), 29.0 (C-15), 28.6 (C-23), 28.0 (C-21), 27.1 (C-34, C-38), 27.0 (C-35, C-37), 25.2 (C-16), 24.5 (C-2), 24.4 (C-11), 23.9 (C-27), 21.5 (C-32), 21.1 (C-30), 19.2 (C-6), 18.1 (C-29), 17.6 (C-26), 17.2 (C-24), 16.1 (C-25) ppm; MS (ESI, MeOH): m/z 595.4 (100%, [M + H]+), 1189.4 (10%, [2M + H]+); analysis calcd for C38H62N3O4 (594.91): C 76.72, H 10.50, N 4.71; found: C 76.54, H 10.69, N 4.48.
- (3β)-28-[(trans-4-Aminocyclohexyl)amino]-28-oxolup-20(29)-en-3-yl acetate (13). Following GPB, compound 13 (0.42 g, 70%) was obtained as a colorless solid; Rf = 0.595 (CHCl3/MeOH, 8:2); m.p.: 205–212 °C (decomp.); = +0.1° (c 0.17, MeOH); IR (ATR): ν = 2940 m, 1731 m, 1637 m, 1513 m, 1369 m, 1244 s, 1026 m, 978 m, 882 m, 751 s cm−1; 1H NMR (500 MHz, CDCl3): δ = 4.71 (s, 1H, 29-Ha), 4.57 (s, 1H, 29-Hb), 4.49–4.41 (m, 1H, 3-H), 3.96–3.80 (m, 1H, 36-H), 3.76–3.63 (m, 1H, 33-H), 3.22–3.03 (m, 1H, 19-H), 2.40 (td, J = 12.3, 3.6 Hz, 1H, 13), 2.02 (s, 3H, 32-H), 1.98–1.02 (m, 28H, 37-H, 35-H, 38-H, 34-H, 1-Ha, 22-Ha, 12-Ha, 2-H, 18-H, 16-H, 15-Ha, 6-H, 11-H, 7-H, 1-Hb, 21-Ha, 9-H, 15-Hb), 1.66 (s, 3H, 30-H), 1.01–0.96 (m, 2H, 1-Hb, 12-Hb), 0.94 (s, 3H, 27-H), 0.92 (s, 3H, 26-H), 0.82 (d, J = 1.5 Hz, 6H, 23-H, 24-H), 0.81 (s, 3H, 25-H), 0.78–0.73 (m, 1H, 5-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 175.6 (C-28), 171.1 (C-31), 151.1 (C-20), 109.5 (C-29), 81.1 (C-3), 55.6 (C-17), 55.6 (C-5), 50.7 (C-9), 50.3 (C-18), 50.1 (C-33), 47.4 (C-19), 47.0 (C-36), 42.6 (C-14), 40.9 (C-8), 39.2 (C-13), 38.5 (C-22), 38.5 (C-1), 37.9 (C-10), 37.2 (C-4), 34.4 (C-7), 33.9 (C-16), 33.8 (C-34, C-38), 31.5 (C-35, C-37), 31.0 (C-21), 29.5 (C-15), 28.1 (C-23), 25.7 (C-12), 23.8 (C-2), 21.4 (C-32), 21.1 (C-11), 19.6 (C-30), 18.3 (C-6), 16.6 (C-24), 16.4 (C-25), 16.3 (C-26), 14.7 (C-27) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z 593.3 (100%, [M − H]−), 629.3 (80%, [M + Cl]−); analysis calcd for C38H62N3O4 (594.91): C 76.72, H 10.50, N 4.71; found: C 76.47, H 10.89, N 4.43.
- (3β)-28-[(Cis-4-Aminocyclohexyl)Amino]-28-Oxolup-20(29)-En-3-Yl Acetate (14). Following GPB, compound 14 (0.39 g, 65%) was obtained as a colorless solid; Rf = 0.634 (CHCl3/MeOH, 8:2); m.p.: 230–235 °C (decomp.); = +9.7° (c 0.19, MeOH); IR (ATR): ν = 2940 s, 1731 m, 1620 m, 1505 m, 1368 m, 1244 s, 1027 m, 978 m, 751 s cm−1; 1H NMR (500 MHz, CDCl3): δ = 4.73 (s, 1H, 29-Ha), 4.59 (s, 1H, 29-Hb), 4.46 (dd, J = 10.3, 5.7 Hz, 1H, 3-H), 4.00 (s, 1H, 36-H), 3.47 (s, 1H, 33-H), 3.10 (td, J = 11.0, 3.9 Hz, 1H, 19-H), 2.46 (dd, J = 11.7, 2.0 Hz, 1H, 13-H), 2.03 (s, 3H, 32-H), 2.02–1.05 (m, 28H, 37-H, 35-H, 38-H, 34-H, 1-Ha, 22-Ha, 12-Ha, 2-H, 18-H, 16-H, 15-Ha, 6-H, 11-H, 7-H, 1-Hb, 21-Ha, 9-H, 15-Hb), 1.67 (s, 3H, 30-H), 1.03–0.96 (m, 2H, 1-Ha, 12-Hb), 0.95 (s, 3H, 27-H), 0.92 (s, 3H, 26-H), 0.84 (s, 6H, 23-H, 24-H), 0.83 (s, 3H, 25-H), 0.80–0.75 (m, 1H, 5-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 175.6 (C-28), 171.0 (C-31), 150.8 (C-20),109.5 (C-29), 80.9 (C-3), 55.6 (C-17), 55.4 (C-5), 50.5 (C-9), 50.0 (C-18), 48.0 (C-33), 46.7 (C-19), 44.3 (C-36), 42.4 (C-14), 40.8 (C-8), 38.5 (C-22), 38.4 (C-1), 37.8 (C-10), 37.7 (C-13), 37.1 (C-4), 34.4 (C-7), 33.8 (C-16), 30.9 (C-21), 29.7 (C-34, C-38), 29.4 (C-15), 27.9 (C-23), 26.9 (C-35, C-37), 25.6 (C-12), 23.7 (C-2), 21.3 (C-32), 21.0 (C-11), 19.5 (C-30), 18.0 (C-6), 16.5 (C-24), 16.3 (C-26), 16.2 (C-25), 14.6 (C-27) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z 595.5 (100%, [M + H]+); analysis calcd for C38H62N3O4 (594.91): C 76.72, H 10.50, N 4.71; found: C 76.55, H 10.83, N 4.61.
- (3β)-28-[(trans-4-Aminocyclohexyl)amino]-20,28-dioxo-30-norlupan-3-yl acetate (15). Following GPB, compound 15 (0.385 g, 65%) was obtained as a colorless solid; Rf = 0.595 (CHCl3/MeOH, 8:2); m.p.: 251–255 °C (decomp.); = −17.0° (c 0.15, MeOH); IR (ATR): ν = 3384 w, 2941 s, 1710 m, 1633 m, 1516 m, 1368 m, 1025 m, 751 s cm−1; 1H NMR (500 MHz, CDCl3): δ = 4.49–4.41 (m, 1H, 3-H), 3.84–3.71 (m, 1H, 35-H), 3.46–3.27 (m, 1H, 19-H), 3.25–2.97 (m, 1H, 32-H), 2.33–2.18 (m, 1H, 13-H), 2.15 (s, 3H, 29-H), 2.07 (d, J = 15.8 Hz, 2H, 18-H, 21-Ha), 2.03 (s, 3H, 31-H), 1.94–1.81 (m, 1H, 16-Ha), 1.77–1.04 (m, 27-H, 36-H, 34-H, 37-H, 33-H, 22-H, 12-H, 2-H, 1-Ha, 16-Hb, 21-Hb, 15-Ha, 6-H, 11-H, 7-H, 9-H, 15-Hb), 0.98 (s, 3H, 27-H), 0.96–0.92 (m, 1H, 1-Hb), 0.90 (s, 3H, 26-H), 0.83–0.82 (m, 6H, 24-H, 25-H), 0.81 (s, 3H, 23-H), 0.80–0.76 (m, 1H, 5-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 212.8 (C-20), 175.8 (C-28), 171.1 (C-30), 80.9 (C-3), 55.5 (C-5), 55.4 (C-17) 51.3 (C-19), 50.5 (C-9), 50.3 (C-33), 50.2 (C-18), 47.0 (C-36), 42.4 (C-14), 40.9 (C-8), 38.5 (C-1), 38.2 (C-22), 37.9 (C-10), 37.3 (C-4), 37.0 (C-13), 34.4 (C-7), 33.1 (C-16), 30.9 (C-33, C-37), 30.3 (C-29), 29.7 (C-36, C-34), 29.6 (C-15), 28.7 (C-21), 28.1 (C-23), 27.3 (C-12), 23.8 (C-2), 21.4 (C-31), 21.1 (C-11), 18.3 (C-6), 16.6 (C-24), 16.3 (C-25), 16.3 (C-26), 14.8 (C-27) ppm; MS (ESI, MeOH/CHCl3 4:1): m/z 597.4 (100%, [M + 2H]+); analysis calcd for C37H60N2O4 (596.88): C 74.45, H 10.13, N 4.69; found: C 74.19, H 10.32, N 4.42.
- (3β)-28-[(cis-4-Aminocyclohexyl)amino]-20,28-dioxo-30-norlupan-3-yl acetate (16). Following GPB, compound 16 (465 mg, 78%) was obtained as a colorless solid; Rf = 0.65 (CHCl3/MeOH, 8:2); m.p.: 257–260 °C (decomp.); = −9.1° (c 0.14, CHCl3); IR (ATR): ν = 2936 m, 1729 m, 1600 s, 1517 m, 1369 m, 1245 s, 988 s, 804 m, 7451 m cm−1; 1H NMR (500 MHz, CDCl3): δ = 4.58–4.26 (m, 1H, 3-H), 4.00–3.83 (m, 1H, 35-H), 3.43 (dt, J = 11.4, 6.0 Hz, 1H, 19-H), 3.11–2.85 (m, 1H, 32-H), 2.21 (dt, J = 11.8, 4.5 Hz, 1H, 13-H), 2.14 (s, 3H, 29-H), 2.09–2.03 (m, 2H, 18-H, 21-Ha), 2.01 (s, 3H, 31-H), 1.95–1.85 (m, 1H, 16-Hb), 1.77–1.01 (m, 27-H, 36-H, 34-H, 37-H, 33-H, 22-H, 12-H, 2-H, 1-Hb, 16-Hb, 15-H, 21-Hb, 6-H, 11-H, 7-H, 9-H), 0.97 (s, 3H, 27-H), 0.88 (s, 3H, 26-H), 0.87 (s, 1H, 1-Ha), 0.82 (s, 3H, 25-H), 0.81 (s, 3H, 24-H), 0.80 (s, 3H, 23-H), 0.79–0.73 (m, 1H, 5-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 213.0 (C-20), 175.3 (C-28), 171.0 (C-30), 81.0 (C-3), 55.5 (C-17), 55.4 (C-5), 51.3 (C-19), 50.5 (C-9), 50.2 (C-18), 47.6 (C-32), 45.1 (C-35), 42.3 (C-14), 40.8 (C-8), 38.5 (C-1), 38.2 (C-22), 37.9 (C-10), 37.2 (C-4), 36.9 (C-13), 34.3 (C-7), 33.2 (C-16), 30.8 (C-37, C-33), 30.4 (C-29), 29.6 (C-15), 28.7 (C-21), 28.0 (C-23), 27.9 (C-36, C-34), 27.6 (C-12), 23.8 (C-2), 21.4 (C-31), 21.1 (C-11), 18.3 (C-6), 16.6 (C-24), 16.3 (C-25), 14.8 (C-27) ppm; MS (ESI, MeOH/CHCl3, 4:1): m/z 597.2 (95%, [M − H]−), 631.3 (100%, [M + Cl]−); analysis calcd for C37H60N2O4 (596.88): C 74.45, H 10.13, N 4.69; found: C 74.23, H 10.39, N 4.37.
- (3β)-28-[(2-Aminoethyl)amino]-28-oxoolean-12-en-3-yl acetate (17). This compound (0.69 g, 83%) was obtained from 1 following GPB as a colorless solid; [35,36] m.p. 211–214 °C (lit.: [36] 212–215 °C); = +38.3° (c 0.4, CHCl3) [lit.: [36] = +37.8° (c 0.35, CHCl3); MS (ESI, MeOH): m/z 541.2 (100%, [M + H]+).
- (3β)-28-[(2-Aminoethyl)amino]-28-oxours-12-en-3-yl acetate (18). This compound (0.81 g, 87%) was obtained from 2 following GPB as a colorless solid; [37,38,39,40] m.p. 202–205 °C (lit.: [37] 140–142 °C); = +39.0° (c 0.2, CHCl3) [lit.: [18] = +39.4° (c 0.555, CHCl3); MS (ESI, MeOH): m/z 541.3 (100%, [M + H]+).
- (3β)-28-[(2-Aminoethyl)amino]-28-oxolup-20(29)-en-3-yl acetate (19). This compound (0.86 g, 93%) was obtained from 3 following GPB as a colorless solid; [41] m.p. 150–153 °C (lit.: [18] 152–154 °C); = +8.1° (c 0.25, CHCl3) [lit.: [18] = +8.4° (c 0.33, CHCl3); MS (ESI, MeOH): m/z 541.2 (100%, [M + H]+).
- (3β)-28-[(2-Aminoethyl)amino]-20,28-dioxo-30-norlupan-3-yl acetate (20). This compound (0.80 g, 88%) was obtained from 4 following GPB as a colorless solid; [42] m.p. 231–234 °C (lit.: [19] 230–234 °C); = −8.5° (c 0.20, CHCl3) [lit.: [19] = −8.5° (c 0.16, CHCl3); MS (ESI, MeOH): m/z 543.1 (100%, [M + H]+).
- (3β)28-(1,4-Diazabicyclo[3.2.2]nonyl-4-yl)-28-oxoolean-12-en-3-yl acetate (25). Following GPB from 1 (626 mg, 1.26 mmol) and 7 (500 mg, 2.51 mmol), 25 (462 mg, 73%) was obtained as colorless solid; m.p. 271–274 °C; Rf = 0.7 (CHCl3/MeOH, 9:1); [α]D = +20.5° (c 0.15, CHCl3); IR (ATR): ν = 2943 br, 1732 m, 1621 m, 1463 w, 1393 m, 1363 m, 1243 s, 1174 m, 1140 m, 1115 w, 1026 m, 1005 m, 749 m cm−1; 1H NMR (400 MHz, CDCl3): δ = 6.15–5.99 (m, 2H, 34-H), 5.27–5.24 (m, 1H, 12-H), 4.76 (m, 1H, 39-H), 4.51–4.44 (m, 1H, 3-H), 4.38–3.99 (m, 5H, 35-Ha + 37-H2 + 40-H2), 3.75 (t, J = 12.0 Hz, 1H, 35-Hb), 3.04 (d, J = 13.6 Hz, 1H, 18-H), 2.38–2.25 (m, 2H, 38-H2), 2.24–2.11 (m, 2H, 41-H2), 2.03 (s, 3H, 32-H3), 2.00–1.81 (m, 3H, 11-H2 + 16-Ha), 1.61 (m, 7H, 1-Ha + 6-Ha + 9-H + 15-Ha + 19-Ha + 22-H2), 1.48–1.16 (m, 10H, 1-Hb + 2-H2 + 6-Hb + 7-H2 + 16-Hb + 19-Hb + 21-H2), 1.13 (s, 3H, 26-H3), 0.92 (s, 3H, 23-H3), 0.91 (s, 3H, 25-H3), 0.90 (s, 3H, 30-H3), 0.86 (s, 3H, 29-H3), 0.84 (s, 3H, 24H3), 0.81 (s, 1H, 5-H), 0.67 (s, 3H, 27-H3) ppm; 13C NMR (101 MHz, CDCl3): δ = 174.9 (C-28), 171.0 (C-31), 144.1 (C-13), 122.0 (C-12), 80.9 (C-3), 72.4 (C-34), 55.4 (C-35), 55.3 (C-5), 47.9 (C-37), 47.6 (C-10), 46.1 (C-17), 45.2 (C-39), 43.7 (C-18), 41.9 (C-14), 40.9 (C-40), 39.1 (C8), 38.1 (C-22), 37.7 (C-20), 37.0 (C-4), 33.8 (C-1), 33.5 (C-21), 32.9 (C-23), 32.8 (C-16), 32.5 (C-7), 28.0 (C-29), 27.9 (C-15), 25.8 (C-26), 24.0 (C-38), 23.5 (C-41), 23.3 (C-19), 22.6 (C-2), 22.5 (C-11), 21.3 (C-32), 18.2 (C-6), 17.0 (C-25), 16.6 (C-24), 15.4 (C-27), 14.1 (C-30), 8.6 (C-9) ppm; MS (ESI, MeOH): m/z = 607.5 (100%, [M + H]+), 608.5 (40%, [M + 2H]+); analysis calcd for C39H62N2O3 (606.94): C 77.18, H 10.30, N 4.62; found: C 76.84, H 10.58, N 4.45.
- (3β)28-(1,4-Diazabicyclo[3.2.2]non-4-yl)-28-oxolup-20(29)en-3-yl acetate (26). Following GPB from 3 (250 mg, 0.50 mmol) and 7 (249 mg, 1.25 mmol), 26 (228 mg, 74%) was obtained as a colorless solid; m.p. 242–246 °C; Rf = 0.4 (DCM/MeOH, 9:1); [α]D = −0.9° (c 0.17, CHCl3); IR (ATR): ν = 2941 m, 1731 m, 1624 m, 1475 m, 1398 m, 1385 m, 1242 s, 1117 m, 1028 m, 978 m, 749 m cm−1; 1H NMR (500 MHz, CDCl3): δ = 4.72 (d, J = 2.3 Hz, 1H, 29-Ha), 4.62 (dq, J = 4.6, 2.5 Hz, 1H, 39-H), 4.58 (dt, J = 2.4, 1.4 Hz, 1H, 29-Hb), 4.49–4.45 (m, 1H, 3-H), 3.79–3.64 (m, 2H, 34-H2), 3.16–2.88 (m, 7H, 13-H + 19-H2 + 35-H + 37-H2 + 40-H2), 2.12 (dt, J = 13.5, 3.5 Hz, 1H, 41-Ha), 2.04 (s, 3H. 32-H3), 2.02–1.89 (m, 5H, 1-Ha + 16-Ha + 21-Ha + 22-Ha + 41-Hb), 1.89–1.45 (m, 14H, 2-H2 + 7-H2 + 12-H2 + 15-Ha + 18-H2 + 30-H3 + 38-H2), 1.44–1.06 (m, 7H, 6-H2 + 9-H + 11-H2 + 16-Hb + 22-Hb), 0.96 (s, 3H, 27-H3), 0.94 (s, 3H, 25-H3), 0.92–0.89 (m, 3H, 1-Hb + 15-Hb + 21-Hb), 0.86 (s, 3H, 23-H3), 0.85 (s, 3H, 26-H3), 0.84 (s, 3H, 24-H3), 0.79 (dd, J = 8.7, 3.3 Hz, 1H, 5-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 173.3 (C-28), 171.0 (C-31), 151.4 (C-20), 109.1 (C-29), 81.0 (C-3), 55.6 (C-5), 55.2 (C-17), 53.0 (C-18), 50.8 (C-9), 47.4 (C-39), 46.8 (C-37), 46.3 (C-35), 45.7 (C-19), 45.2 (C-34), 41.9 (C-14), 40.7 (C-8), 38.4 (C-1), 37.8 (C-4), 37.2 (C-10), 36.9 (C-13), 36.2 (C-22), 34.3 (C-7), 32.5 (C-40), 31.5 (C-16), 29.9 (C-21), 27.9 (C-23), 26.6 (C-15), 26.5 (C41 + C38), 25.6 (C-12), 23.7 (C-2), 21.3 (C-32), 21.2 (C-11), 19.7 (C-30), 18.2 (C-6), 16.5 (C-24), 16.3 (C-25), 16.1 (C-26), 14.7 (C-27) ppm; MS (ESI, MeOH): m/z = 607.6 (100%, [M + H]+), 608.6 (45%, [M + 2H]+), 1214.2 (5%, [2M + 2H]+); analysis calcd for C39H62N2O3 (606.94): C 77.18, H 10.30, N 4.62; found: C 76.97, H 10.51, N 4.44.
- (3β)28-(1,4-Diazabicyclo[3.2.2]non-4-yl)-20,28-dioxo-30-norlupan-3-yl acetate (27). Following GPB from 4 (250 mg, 0.49 mmol) and 7 (238 mg, 1.19 mmol), 27 (270 mg, 99%) was obtained as a colorless solid; m.p. 253 °C (decomp.); Rf = 0.3 (DCM/MeOH/, 9:1); [α]D = −8.7° (c 0.21, CHCl3); IR (ATR): ν = 2940 br, 1731 m, 1622 m, 1367 m, 1243 s, 1026 m, 978 m, 772 s cm−1; 1H NMR (400 MHz, CDCl3): δ = 4.60 (s, 1H, 39-H), 4.46 (dd, J = 10.5, 5.5 Hz, 1H, 3-H), 3.71 (m, 2H, 34-H2), 3.25 (td, J = 11.3, 3.5 Hz, 1H, 19-H), 3.18–2.88 (m, 6H, 35-H2 + 37-H2 + 40-H2), 2.79 (td, J = 12.0, 3.8 Hz, 1H, 13-H), 2.16 (s, 3H, 29-H3), 2.15–2.05 (m, 2H, 18-H + 21-Ha), 2.03 (s, 3H, 32-H3), 2.01–1.80 (m, 2H, 16-Ha + 21-Hb), 1.79–1.55 (m, 6H, 15-Ha + 16-Hb + 38-H2 + 41-H2), 1.54–1.11 (m, 14H, 1-Ha + 2-Ha + 6-H2 + 7-H2 + 9-H + 11-H2 + 12-H2 + 15-Hb + 22-H2), 1.05 (dd, J = 13.4, 3.5 Hz, 1H, 2-Hb), 0.98 (s, 3H, 27-H3), 0.96 (s, 1H, 1-Hb), 0.93 (s, 3H, 26-H3), 0.83 (m, 6H, 23-H3 + 25-H3), 0.82 (s, 3H, 24-H3), 0.81–0.76 (m, 1H, 5-H) ppm; 13C NMR (126 MHz CDCl3): δ = 213.1 (C-20), 173.3 (C-28), 171.0 (C-31), 80.9 (C-3), 55.5 (C-5), 55.1 (C-17), 52.9 (C-18), 50.7 (C-9), 50.1 (C-19), 47.4 (C-39), 46.8 (C-35), 46.2 (C-37), 46.2 (C-40), 44.9 (C-34), 41.8 (C-14), 40.6 (C-8), 38.4 (C-1), 37.8 (C-4), 37.1 (C-10), 37.1 (C-22), 35.9 (C-13), 35.7 (C-16), 34.2 (C-7), 32.0 (C-38), 30.3 (C-29), 29.9 (C-15), 28.9 (C-21), 27.9 (C-24), 27.5 (C-41), 27.3 (C-12), 23.7 (C-2), 21.3 (C-32), 21.2 (C-11), 18.1 (C-6), 16.5 (C-23), 16.2 (C-25), 16.0 (C-26), 14.7 (C-27) ppm; MS (ESI, MeOH): m/z = 609.5 (25%, [M + H]+); analysis calcd for C38H60N2O4 (608.91): C 74.96, H 9.93, N 4.60; found: C 74.72, H 10.13, N 4.48.
- (3β)28-(1,3-Diazabicyclo[3.2.2]nonyl-3-yl)-28-oxoolean-12-en-3-yl acetate (28). Following GPB from 1 (375 mg, 0.75 mmol) and 8 (300 mg, 1.52 mmol), 28 (462 mg, 73%) was obtained as an off-white solid; m.p. 130 °C (decomp.); Rf = 0.3 (CHCl3/MeOH, 98:2); [α]D = +10.5° (c 0.16, CHCl3); IR (ATR): ν = 3221 brw, 2942 brm, 1731 m, 1610 m, 1530 m, 1446 s, 1366 m, 1244 s, 1026 s, 655 s cm−1; 1H NMR (400 MHz, MeOH-d4): δ = 5.83 (ddt, J = 9.9, 3.7, 1.7 Hz, 1H, 34-Ha), 5.72–5.66 (m, 1H, 34-Hb), 5.36 (t, J = 3.7 Hz, 1H, 12-H), 4.45 (dd, J = 10.9, 5.0 Hz, 1H, 3-H), 3.46 (d, J = 6.5 Hz, 1H, 39-Ha), 3.27 (d, J = 6.7 Hz, 1H, 39-Hb), 3.21 (dt, J = 8.9, 2.8 Hz, 1H, 18-H), 2.84 (dq, J = 15.0, 5.9 Hz, 2H, 36-H2), 2.73 (m, 3H, 18-H + 40-H2), 2.28 (q, J = 2.5 Hz, 2H, 37-H2), 2.14–2.05 (m, 2H, 9-H + 11-Ha), 2.02 (s, 3H, 32-H3), 1.97–1.87 (m, 4H, 2-H2 + 11-Hb + 15-Ha) 1.84–1.73 (m, 2H, 6-Ha + 19-Ha), 1.69–1.51 (m, 7H, 16-Ha + 21-H2 + 38-H2 + 41-H2), 1.51–1.20 (m, 7H, 1-H2 + 6-Hb + 7-H2 + 22-H2), 1.18 (s, 3H, 26-H3), 1.16–0.99 (m, 3H, 15-Hb + 16-Hb + 19-Hb), 0.97 (s, 3H, 25-H3), 0.94 (s, 3H, 30-H3), 0.91 (s, 3H, 23-H3), 0.88 (s, 3H, 29-H3), 0.87 (s, 3H, 24-H3), 0.85 (s, 1H, 5-H), 0.79 (s, 3H, 27-H3) ppm; 13C NMR (101 MHz, MeOH-d4): δ = 179.3 (C-28), 171.5 (C-31), 143.7 (C-13), 124.9 (C-34), 123.2 (C-34),122.7 (C-12), 81.1 (C-3), 56.1 (C-40), 55.3 (C-5), 49.6 (C36), 47.1 (C-38), 46.3 (C-17), 46.2 (C-19), 41.5 (C-14), 41.3 (C-18), 39.3 (C-8), 37.9 (C-1), 37.3 (C-20), 36.7 (C-4), 35.7 (C-39), 34.1 (C-21), 33.7 (C-10), 32.8 (C-16), 32.5 (C-22), 32.1(C-23), 30.2(C-7), 27.2 (C-29), 27.1 (C-15), 25.0 (C-26), 24.6 (C-41),23.4 (C-38) 23.2 (C-37), 23.1 (C-11), 22.6 (C-30), 22.2 (C-2), 19.7 (C-32), 17.9 (C-6), 16.5 (C-27), 15.7 (C-24), 14.5 (C-25) ppm; MS (ESI, MeOH): m/z = 607.4 (100%, [M + H]+), 608.4 (60%, [M + 2H]+); analysis calcd for C39H62N2O3 (606.94): C 77.18, H 10.30, N 4.62; found: C 76.93, H 10.56, N 4.49.
- (3β)28-(1,3-Diazabicyclo[3.2.2]nonyl)-3-yl)-28-oxours-12-en-3-yl acetate (29). Following GPB from 2 (375 mg, 0.75 mmol) and 8 (404 mg, 2.03 mmol), 29 (332 mg, 73%) was obtained as a colorless solid; m.p. 135–139 °C (decomp.); Rf = 0.35 (DCM/MeOH, 96:4); [α]D = +28.6° (c 0.14, CHCl3); IR (ATR): ν = 2924 br, 1733 s, 1651 m, 1455 m, 1369 m, 1243 s, 1141 w, 1026 m, 653 m cm−1; 1H NMR (500 MHz, CDCl3): δ = 5.77 (m, 1H, 34-Ha), 5.67 (m, 1H, 34-Hb), 5.27 (t, J = 3.7 Hz, 1H, 12-H), 4.60–4.44 (dd, J = 10.0, 6.0 Hz, 1H, 3-H), 3.41–3.19 (m, 2H, 39-H2), 3.07 –2.82 (m, 2H, 36-H2), 2.64 (dt, J = 10.8, 5.2 Hz, 1H, 40-Ha), 2.53 (dt, J = 9.0, 5.5 Hz, 2H, 18-H, 40-Hb), 2.29–2.10 (m, 3H, 11-H2 + 16-Ha), 2.06 (s, 3H, 32-H3), 2.02–1.83 (m, 6H, 19-H + 1-Ha + 2-H2 + 7-H2), 1.80–1.70 (m, 3H, 15-Ha + 37-H2), 1.64 (m, 4H, 6-Ha + 21-Ha + 22-H2), 1.59–1.25 (m, 10H, 6-Hb + 9-H + 16-Hb + 20-H2 + 21-Hb + 38-H2 + 41-H2), 1.09 (s, 3H, 27-H3), 1.08–0.95 (m, 2H, 1-Hb + 15-Hb), 0.96 (s, 3H, 25-H3), 0.94 (s, 3H, 30-H3) 0.89 (s, 3H, 29-H3), 0.88 (s, 3H, 24-H3), 0.87 (s, 3H, 23-H3), 0.85–0.81 (m, 1H, 5-H), 0.79 (s, 3H, 26-H3) ppm; 13C NMR (126 MHz, CDCl3): δ = 178.1 (C-28), 171.0 (C-31), 139.2 (C-13), 125.8(C-12), 125.2 (C-34), 125.1 (C-34), 80.9 (C-3), 56.7 (C-18), 55.2(C-5), 53.9 (C-19), 52.3 (C-36), 50.3 (C-40), 47.8 (C-17), 47.5 (C-9), 42.4 (C-14), 39.8 (C-38), 39.6 (C-8), 39.1 (C-20), 38.3 (C-1), 37.7 (C-4), 37.3 (C-37), 36.9 (C-10), 36.2 (C-7), 36.2 (C-39), 32.7(C-22), 31.0 (C-21), 28.1 (C-24), 27.9 (C-16), 26.3 (C-11), 24.9 (C-41), 23.5 (C-2), 23.5 (C-15), 23.2 (C-27), 21.3 (C-32), 21.2 (C-25), 18.2 (C-6), 17.3 (C-29), 17.0 (C-26), 16.7 (C-23), 15.6 (C-30) ppm; MS (ESI, MeOH): m/z = 607.3 (100%, [M + H]+), 608.3 (65%; [M + 2H]+; m/z = 605.3 (100%, [M − H]−); analysis calcd for C39H62N2O3 (606.94): C 77.18, H 10.30, N 4.62; found: C 76.87, H 10.57, N 4.43.
- (3β)28-(1,3-Diazabicyclo[3.2.2]non-3-yl)-28-oxolup-20(29)-en-3-yl acetate (30). Following GPB from 3 (200 mg, 0.40 mmol) and 8 (238 mg, 1.19 mmol), 30 (125 mg, 95%) was obtained as an amorphous colorless solid; Rf = 0.30 (DCM/MeOH, 98:2); [α]D = +5.5° (c 0.17, CHCl3); IR (ATR): ν = 2942 m, 1732 m, 1638 m, 1450 m, 1368 m, 1243 s, 1027 m, 978 m, 881 m, 772 m, 653 m cm−1; 1H NMR (400 MHz, CDCl3): δ = 5.75 (m, 1H, 34-Ha), 5.66 (m, 1H, 34-Hb), 4.72 (d, J = 2.4 Hz, 1H, 29-Ha), 4.59 (dt, J = 2.5, 1.4 Hz, 1H, 29-Hb), 4.51–4.42 (m, 1H, 3-H), 3.37 (qt, J = 13.9, 6.8 Hz, 2H, 39-H2), 3.11–2.92 (m, 3H, 19-H + 36-Ha + 40-Ha), 2.65–2.52 (m, 4H, 36-Hb + 40-Hb), 2.37 (td, J = 12.4, 3.6 Hz, 1H, 9-H), 2.17 (tp, J = 5.7, 2.9, 2.3 Hz, 2H, 13-H + 18-H), 2.03 (s, 3H, 32-H3), 2.00–1.72 (m, 4H, 1-Ha + 12-Ha + 21-Ha + 22-Ha), 1.68 (m, 4H, 21-Hb + 30-H3), 1.66–1.56 (m, 6H, 2-H2 + 6-Ha + 11-Ha + 15-Ha + 22-Hb), 1.55–0.97 (m, 14H, 1-Hb + 6-Hb + 7-H2 + 11-Hb + 12-Hb + 15-Hb + 16-H2 + 37-H2 + 38-H + 41-H2), 0.95 (s, 3H, 27-H3), 0.93 (s, 3H, 26-H3), 0.84 (s, 3H, 23-H3), 0.83 (s, 3H, 25-H3), 0.82 (s, 3H, 24-H3), 0.80–0.74 (m, 1H, 5-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 177.7 (C-28), 171.0 (C-31), 151.0 (C-20), 118.9 (C-34), 109.4 (C-29), 80.9 (C-3), 55.9 (C36), 55.4 (C-5), 50.5 (C-9), 50.3(C-40), 50.1 (C-18), 49.2 (C-17), 46.8 (C-19), 42.4 (C-14), 40.8 (C-8), 38.4 (C-13), 38.1 (C-1), 37.8 (C-4), 37.7 (C-38), 37.1 (C-10), 34.3 (C-22), 34.2 (C-7), 33.0 (C-16), 30.9 (C-21), 29.5 (C-15), 27.9 (C-23), 25.6 (C-12), 25.4 (C-41), 23.7 (C-2), 21.8 (C-37), 21.3 (C-32), 21.0 (C-11), 19.4 (C-30), 18.2 (C-6), 16.5 (C-24), 16.2 (C-25), 16.2 (C-26), 14.6 (C-27) ppm; MS (ESI, MeOH): m/z = 607.3 (100%, [M + H]+, 1241.9 (5%, [2M + 3H2O + 2H]2+); analysis calcd for C39H62N2O3 (606.94): C 77.18, H 10.30, N 4.62; found: C 76.81, H 10.53, N 4.41.
- (3β)28-(1,4-Diazabicyclo[3.2.2]non-4-yl)-20,28-dioxo-30-norlupan-3-yl-acetate (31). Following GPB from 4 (250 mg, 0.39 mmol) and 8 (238 mg, 1.19 mmol), 31 (178 mg, 73%) was obtained as a colorless solid; m.p. 130–132 °C; Rf = 0.40 (DCM/MeOH, 95:5); [α]D = −7.0° (c 0.15, CHCl3); IR (ATR): ν = 2942 brm, 1732 m, 1656 m, 1517 m, 1449 m, 1363 m, 1244 m, 1195 m, 1026 m, 979 m, 772 m, 654 m cm−1; 1H NMR (500 MHz, CDCl3): δ = 5.89–5.64 (m, 2H, 34-H2), 4.45 (dd, J = 11.0, 5.2 Hz, 1H, 3-H), 3.52 (m, 2H, 39-H2), 3.37 (m, 2H, 36-H2), 3.33–3.18 (m, 1H, 19-H), 2.87 (dt, J = 26.8, 6.1 Hz, 2H, 40-H2), 2.42–2.30 (m, 2H, 37-H2), 2.29–2.17 (m, 1H, 13-H), 2.14 (s, 3H, 29-H3), 2.12–2.04 (m, 2H, 18-H + 38-H), 2.02 (s, 3H, 32-H3), 2.00–1.81 (m, 1H, 21-Ha), 1.67–1.11 (m, 18H, 1-Ha + 2-Ha + 6-H2 + 7-H2 + 9-H + 11-H2 + 12-H2 + 15-H2 + 16-Ha + 22-H2 + 41-H2), 1.05 (m, 3H, 2-Hb + 16-Hb + 21-Hb), 0.97 (s, 3H, 27-H3), 0.94–0.91 (m, 1H, 1-Hb), 0.89 (s, 3H, 26-H3), 0.84–0.80 (m, 9H, 23-H3 + 24-H3 + 25-H3), 0.79–0.73 (m, 1H, 5-H) ppm; 13C NMR (126 MHz, CDCl3): δ = 212.7 (C-20), 176.8 (C-28), 170.9 (C-31), 125.6 (C-34), 80.8 (C-3), 56.2 (C36), 55.7 (C-17), 55.4 (C-5), 51.3 (C-4), 51.2 (C-19), 50.4 (C-9), 50.0 (C-18), 49.4 (C-40), 42.2 (C-14), 40.7 (C-8), 38.3 (C-1), 37.8 (C-38), 37.8 (C-22), 37.1 (C-10), 36.8 (C-13), 35.0 (C-39), 34.2 (C-7), 32.6 (C-16), 30.1 (C-29), 29.5 (C-15), 28.6 (C-21), 27.9 (C-24), 27.9 (C-25), 27.7 (C-12), 27.2 (C-41), 23.8 (C-2), 23.6 (C-37), 21.3 (C-32), 20.9 (C-11), 18.2 (C-6), 16.5 (C-23), 16.2 (C-26), 14.6 (C-27) ppm; MS (ESI, MeOH): m/z = 609.2 (100%, [M + H]+), 610.2 (50%; [M + 2H]+); analysis calcd for C38H60N2O4 (608.91): C 74.96, H 9.93, N 4.60; found: C 74.76, H 10.14, N 4.41.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Al-Harrasi, A.; Khan, A.L.; Rehman, N.U.; Csuk, R. Biosynthetic diversity in triterpene cyclization within the Boswellia genus. Phytochemistry 2021, 184, 112660. [Google Scholar] [CrossRef]
- Almahli, H. Pentacyclic triterpene acids: Use, mode of action, biological activity and synthesis. J. Chem. Biol. Phys. Sci. 2020, 10, 294–315. [Google Scholar]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef] [Green Version]
- Ghiulai, R.; Rosca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macasoi, I.; Olariu, T.; Dehelean, C.; Cretu, O.M.; et al. Tetracyclic and pentacyclic triterpenes with high therapeutic efficiency in wound healing approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef] [Green Version]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic acid-based derivatives as potential anti-cancer agents: An update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Manez, S. New Pharmacological Opportunities for Betulinic Acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvador, J.A.R.; Leal, A.S.; Valdeira, A.S.; Goncalves, B.M.F.; Alho, D.P.S.; Figueiredo, S.A.C.; Silvestre, S.M.; Mendes, V.I.S. Oleanane-, ursane-, and quinone methide friedelane-type triterpenoid derivatives: Recent advances in cancer treatment. Eur. J. Med. Chem. 2017, 142, 95–130. [Google Scholar] [CrossRef]
- Sharma, N.; Palia, P.; Chaudhary, A.; Shalini; Verma, K.; Kumar, I. A review on pharmacological activities of lupeol and its triterpene derivatives. J. Drug Deliv. Ther. 2020, 10, 325–332. [Google Scholar] [CrossRef]
- Sun, Q.; He, M.; Zhang, M.; Zeng, S.; Chen, L.; Zhou, L.; Xu, H. Ursolic acid: A systematic review of its pharmacology, toxicity and rethink on its pharmacokinetics based on PK-PD model. Fitoterapia 2020, 147, 104735. [Google Scholar] [CrossRef]
- Lowitz, J.T. Über eine neue fast benzoesäureartige Substanz der Birken. Crell’s Ann. Chem. 1788, 2, 312–317. [Google Scholar]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of Betulinic Acid as a Selective Inhibitor of Human-Melanoma That Functions by Induction of Apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Feng, J.-H.; Chen, W.; Zhao, Y.; Ju, X.-L. Anti-tumor activity of oleanolic, ursolic and glycyrrhetinic acid. Open Nat. Prod. J. 2009, 2, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Paduch, R.; Kandefer-Szerszen, M. Antitumor and Antiviral Activity of Pentacyclic Triterpenes. Mini-Rev. Org. Chem. 2014, 11, 262–268. [Google Scholar] [CrossRef]
- Paszel-Jaworska, A.; Romaniuk, A.; Rybczynska, M. Molecular Mechanisms of Biological Activity of Oleanolic Acid—A Source of Inspiration for A New Drugs Design. Mini-Rev. Org. Chem. 2014, 11, 330–342. [Google Scholar] [CrossRef]
- Csuk, R. Betulinic acid and its derivatives: A patent review (2008–2013). Expert Opin. Ther. Pat. 2014, 24, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Hoenke, S.; Heise, N.V.; Kahnt, M.; Deigner, H.-P.; Csuk, R. Betulinic acid derived amides are highly cytotoxic, apoptotic and selective. Eur. J. Med. Chem. 2020, 207, 112815. [Google Scholar] [CrossRef]
- Kahnt, M.; Fischer, L.; Al-Harrasi, A.; Csuk, R. Ethylenediamine derived carboxamides of betulinic and ursolic acid as potential cytotoxic agents. Molecules 2018, 23, 2558. [Google Scholar] [CrossRef] [Green Version]
- Kahnt, M.; Heller, L.; Al-Harrasi, A.; Schaefer, R.; Kluge, R.; Wagner, C.; Otgonbayar, C.; Csuk, R. Platanic acid-derived methyl 20-amino-30-norlupan-28-oates are potent cytotoxic agents acting by apoptosis. Med. Chem. Res. 2018, 27, 1757–1769. [Google Scholar] [CrossRef]
- Kahnt, M.; Heller, L.; Grabandt, P.; Al-Harrasi, A.; Csuk, R. Platanic acid: A new scaffold for the synthesis of cytotoxic agents. Eur. J. Med. Chem. 2018, 143, 259–265. [Google Scholar] [CrossRef]
- Siewert, B.; Pianowski, E.; Csuk, R. Esters and amides of maslinic acid trigger apoptosis in human tumor cells and alter their mode of action with respect to the substitution pattern at C-28. Eur. J. Med. Chem. 2013, 70, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Siewert, B.; Pianowski, E.; Obernauer, A.; Csuk, R. Towards cytotoxic and selective derivatives of maslinic acid. Bioorg. Med. Chem. 2014, 22, 594–615. [Google Scholar] [CrossRef]
- Wolfram, R.K.; Fischer, L.; Kluge, R.; Stroehl, D.; Al-Harrasi, A.; Csuk, R. Homopiperazine-rhodamine B adducts of triterpenoic acids are strong mitocans. Eur. J. Med. Chem. 2018, 155, 869–879. [Google Scholar] [CrossRef]
- Sommerwerk, S.; Heller, L.; Kerzig, C.; Kramell, A.E.; Csuk, R. Rhodamine B conjugates of triterpenoic acids are cytotoxic mitocans even at nanomolar concentrations. Eur. J. Med. Chem. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Hoenke, S.; Serbian, I.; Deigner, H.-P.; Csuk, R. Mitocanic Di- and triterpenoid rhodamine B conjugates. Molecules 2020, 25, 5443. [Google Scholar] [CrossRef]
- Kahnt, M.; Loesche, A.; Serbian, I.; Hoenke, S.; Fischer, L.; Al-Harrasi, A.; Csuk, R. The cytotoxicity of oleanane derived aminocarboxamides depends on their aminoalkyl substituents. Steroids 2019, 149, 108422. [Google Scholar] [CrossRef]
- De Costa, B.R.; He, X.; Linders, J.T.M.; Dominguez, C.; Gu, Z.Q.; Williams, W.; Bowen, W. Synthesis and evaluation of conformationally restricted N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamines at sigma receptors. 2. Piperazines, bicyclic amines, bridged bicyclic amines, and miscellaneous compounds. J. Med. Chem. 1993, 36, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Mikhlina, E.E.; Rubtsov, M.V. Reaction of 3-quinuclidone with hydrazoic acid. Zh. Obshch. Khim. 1963, 33, 2167–2172. [Google Scholar]
- Takemoto, T.; Kometani, K. Triterpene glycosides (mubenins) from the seeds of Stauntonia hexaphylla. Justus Liebigs Ann. Chem. 1965, 685, 237–246. [Google Scholar] [CrossRef]
- Ewen, E.S.; Spring, F.S. Triterpene resinols and related acids. XIV. The oxidation of acetylursolic acid. J. Chem. Soc. 1943, 523–525. [Google Scholar] [CrossRef]
- Taketa, A.T.C.; Breitmaier, E.; Schenkel, E.P. Triterpenes and triterpenoidal glycosides from the fruits of Ilex paraguariensis (Mate). J. Braz. Chem. Soc. 2004, 15, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Petrenko, N.I.; Elantseva, N.V.; Petukhova, V.Z.; Shakirov, M.M.; Shul’ts, E.E.; Tolstikov, G.A. Synthesis of Betulonic Acid Derivatives Containing Amino-Acid Fragments. Chem. Nat. Compd. 2002, 38, 331–339. [Google Scholar] [CrossRef]
- Thibeault, D.; Gauthier, C.; Legault, J.; Bouchard, J.; Dufour, P.; Pichette, A. Synthesis and structure-activity relationship study of cytotoxic germanicane- and lupane-type 3β-O-monodesmosidic saponins starting from betulin. Bioorg. Med. Chem. 2007, 15, 6144–6157. [Google Scholar] [CrossRef]
- Vystrcil, A.; Budesinsky, M. Triterpenes. XVI. Unusual epimerization of the C-19 acetyl group in 20-oxo-30-norlupane derivatives. Collect. Czech. Chem. Commun. 1970, 35, 295–311. [Google Scholar] [CrossRef]
- Brandes, B.; Hoenke, S.; Fischer, L.; Csuk, R. Design, synthesis and cytotoxicity of BODIPY FL labelled triterpenoids. Eur. J. Med. Chem. 2020, 185, 111858. [Google Scholar] [CrossRef]
- Loesche, A.; Kahnt, M.; Serbian, I.; Brandt, W.; Csuk, R. Triterpene-based carboxamides act as good inhibitors of butyrylcholinesterase. Molecules 2019, 24, 948. [Google Scholar] [CrossRef] [Green Version]
- Bai, K.-K.; Yu, Z.; Chen, F.-L.; Li, F.; Li, W.-Y.; Guo, Y.-H. Synthesis and evaluation of ursolic acid derivatives as potent cytotoxic agents. Bioorg. Med. Chem. Lett. 2012, 22, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.S.; Chukanov, N.V.; Grigor’ev, I.A. New Amino-Bisphosphonate Building Blocks in the Synthesis of Bisphosphonic Derivatives Based on Lead Compounds. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 1201–1212. [Google Scholar] [CrossRef]
- Kahnt, M.; Hoenke, S.; Fischer, L.; Al-Harrasi, A.; Csuk, R. Synthesis and cytotoxicity evaluation of DOTA-conjugates of ursolic acid. Molecules 2019, 24, 2254. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jiang, Z.; Xiang, L.; Li, Y.; Ou, M.; Yang, X.; Shao, J.; Lu, Y.; Lin, L.; Chen, J.; et al. Synergism of ursolic acid derivative US597 with 2-deoxy-D-glucose to preferentially induce tumor cell death by dual-targeting of apoptosis and glycolysis. Sci. Rep. 2014, 4, 5006. [Google Scholar] [CrossRef] [Green Version]
- Pettit, G.R.; Melody, N. Betulastatin Compounds for Cancer Cell Growth Inhibition. International Patent Application No WO2019094709A1, 16 May 2019. [Google Scholar]
- Heller, L.; Kahnt, M.; Loesche, A.; Grabandt, P.; Schwarz, S.; Brandt, W.; Csuk, R. Amino derivatives of platanic acid act as selective and potent inhibitors of butyrylcholinesterase. Eur. J. Med. Chem. 2017, 126, 652–668. [Google Scholar] [CrossRef] [PubMed]
- Brandes, B.; Koch, L.; Hoenke, S.; Deigner, H.-P.; Csuk, R. The presence of a cationic center is not alone decisive for the cytotoxicity of triterpene carboxylic acid amides. Steroids 2020, 163, 108713. [Google Scholar] [CrossRef]
- Friedrich, S.; Serbian, I.; Hoenke, S.; Wolfram, R.K.; Csuk, R. Synthesis and cytotoxic evaluation of malachite green derived oleanolic and ursolic acid piperazineamides. Med. Chem. Res. 2020, 29, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Kahnt, M.; Wiemann, J.; Fischer, L.; Sommerwerk, S.; Csuk, R. Transformation of asiatic acid into a mitocanic, bimodal-acting rhodamine B conjugate of nanomolar cytotoxicity. Eur. J. Med. Chem. 2018, 159, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Luo, J.-G.; Wang, X.-B.; Yin, H.; Sun, H.-B.; Yao, H.-Q.; Kong, L.-Y. Synthesis of new α-glucosidase inhibitors based on oleanolic acid incorporating cinnamic amides. Chem. Pharm. Bull. 2011, 59, 1051–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, S.-X.; Huang, R.-Z.; Ye, M.-Y.; Pan, Y.-M.; Yao, G.-Y.; Zhang, Y.; Wang, H.-S. Design, synthesis and in vitro evaluation of novel ursolic acid derivatives as potential anticancer agents. Eur. J. Med. Chem. 2015, 95, 435–452. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Jiang, W.; Ou, M.; Chen, Y.; Xu, Y.; Wu, Q.; Zheng, Q.; Wu, F.; Wang, L.; et al. Synthesis and Biological Evaluation of Novel Ursolic acid Derivatives as Potential Anticancer Prodrugs. Chem. Biol. Drug Des. 2015, 86, 1397–1404. [Google Scholar] [CrossRef]



| Compound | A375 | HT29 | MCF-7 | A2780 | FaDu | NIH 3T3 |
|---|---|---|---|---|---|---|
| OA | >30 | >30 | >30 | >30 | >30 | >30 |
| UA | 15.4 ± 1.0 | 12.4 ± 1.1 | 14.7 ± 0.4 | 17.3 ± 0.9 | 18.2 ± 1.7 | 16.3 ± 1.4 |
| BA | 17.7 ± 0.4 | 16.8 ± 2.0 | 12.3 ± 1.1 | 9.4 ± 1.1 | 13.7 ± 0.9 | 19.3 ± 1.1 |
| PA | >30 | >30 | >30 | >30 | >30 | >30 |
| 1 | 13.1 ± 1.1 | 20.5 ± 1.7 | 12.9 ± 1.9 | 9.4 ± 0.5 | 11.8 ± 0.9 | 17.5 ± 1.5 |
| 2 | 11.4 ± 1.4 | 17.3 ± 1.5 | 12.1 ± 1.2 | 8.3 ± 0.9 | 10.7 ± 0.8 | 16.4 ± 1.7 |
| 3 | 19.2 ± 1.7 | 21.3 ± 2.0 | 11.0 ± 0.5 | 18.3 ± 0.5 | 7.2 ± 1.2 | >30 |
| 4–8 | >30 | >30 | >30 | >30 | >30 | >30 |
| 9 | 1.8 ± 0.3 | 1.7 ± 0.2 | 2.0 ± 0.3 | 2.1 ± 0.2 | 2.0 ± 0.1 | 1.8 ± 0.4 |
| 10 | 2.4 ± 0.2 | 2.9 ± 0.1 | 2.8 ± 0.6 | 2.7 ± 0.2 | 2.9 ± 0.2 | 2.2 ± 0.2 |
| 11 | 1.9 ± 0.2 | 2.6 ± 0.1 | 2.5 ± 0.4 | 2.6 ± 0.3 | 2.6 ± 0.1 | 1.9 ± 0.3 |
| 12 | 1.9 ± 0.3 | 2.4 ± 0.1 | 2.4 ± 0.4 | 2.3 ± 0.2 | 2.5 ± 0.1 | 1.9 ± 0.2 |
| 13 | 0.9 ± 0.1 | 0.6 ± 0.1 | 1.3 ± 0.4 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| 14 | 1.3 ± 0.2 | 0.8 ± 0.1 | 1.2 ± 0.4 | 1.2 ± 0.35 | 1.1 ± 0.2 | 1.1 ± 0.1 |
| 15 | 0.9 ± 0.1 | 2.1 ± 0.1 | 2.8 ± 0.4 | 1.8 ± 0.2 | 1.5 ± 0.1 | 0.5 ± 0.1 |
| 16 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| DX | n.d. | 0.9 ± 0.2 | 1.1 ± 0.3 | 0.02 ± 0.01 | 1.7 ± 0.3 | 0.06 ± 0.03 |
| Compound | HT29 | MCF-7 | A2780 | NIH 3T3 |
|---|---|---|---|---|
| 17 | 2.0 ± 0.2 | 1.7 ± 0.2 | 3.1 ± 0.1 | 2.1 ± 0.1 |
| 18 | 1.8 ± 0.1 | 2.0 ± 0.1 | 2.3 ± 0.1 | 2.6 ± 0.3 |
| 19 | 1.0 ± 0.3 | 1.3 ± 0.1 | 1.4 ± 0.2 | 1.4 ± 0.1 |
| 20 | 3.3 ± 1.2 | 3.1 ± 0.1 | 3.2 ± 0.2 | 2.1 ± 0.1 |
| 21 | 1.3 ± 0.1 | 1.7 ± 0.2 | 1.7 ± 0.1 | 1.7 ± 0.1 |
| 22 | 1.9 ± 0.3 | 2.0 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 |
| 23 | 1.0 ± 0.1 | 1.4 ± 0.1 | 1.9 ± 0.1 | 0.9 ± 0.1 |
| 24 | 2.4 ± 0.3 | 2.8 ± 0.1 | 3.1 ± 0.1 | 0.7 ± 0.1 |
| Compound | A375 | HT29 | MCF-7 | A2780 | FaDu | NIH 3T3 |
|---|---|---|---|---|---|---|
| 25, 26 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| 27 | 2.3 ± 0.2 | 5.2 ± 0.2 | 4.2 ± 0.8 | 3.9 ± 0.4 | 2.7 ± 0.4 | 2.2 ± 0.2 |
| 28, 29 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| 30 | 4.7 ± 0.2 | 4.8 ± 0.3 | 6.0 ± 0.9 | 5.5 ± 0.4 | 6.3 ± 0.5 | 9.3 ± 0.7 |
| 31 | 6.0 ± 0.5 | 8.2 ± 0.3 | 6.3 ± 0.5 | 6.4 ± 0.3 | 6.2 ± 0.6 | 5.0 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoenke, S.; Christoph, M.A.; Friedrich, S.; Heise, N.; Brandes, B.; Deigner, H.-P.; Al-Harrasi, A.; Csuk, R. The Presence of a Cyclohexyldiamine Moiety Confers Cytotoxicity to Pentacyclic Triterpenoids. Molecules 2021, 26, 2102. https://doi.org/10.3390/molecules26072102
Hoenke S, Christoph MA, Friedrich S, Heise N, Brandes B, Deigner H-P, Al-Harrasi A, Csuk R. The Presence of a Cyclohexyldiamine Moiety Confers Cytotoxicity to Pentacyclic Triterpenoids. Molecules. 2021; 26(7):2102. https://doi.org/10.3390/molecules26072102
Chicago/Turabian StyleHoenke, Sophie, Martin A. Christoph, Sander Friedrich, Niels Heise, Benjamin Brandes, Hans-Peter Deigner, Ahmed Al-Harrasi, and René Csuk. 2021. "The Presence of a Cyclohexyldiamine Moiety Confers Cytotoxicity to Pentacyclic Triterpenoids" Molecules 26, no. 7: 2102. https://doi.org/10.3390/molecules26072102