Nitro Fatty Acids (NO2-FAs): An Emerging Class of Bioactive Fatty Acids
Abstract
:1. Introduction
2. Bioactivities of NO2-FAs
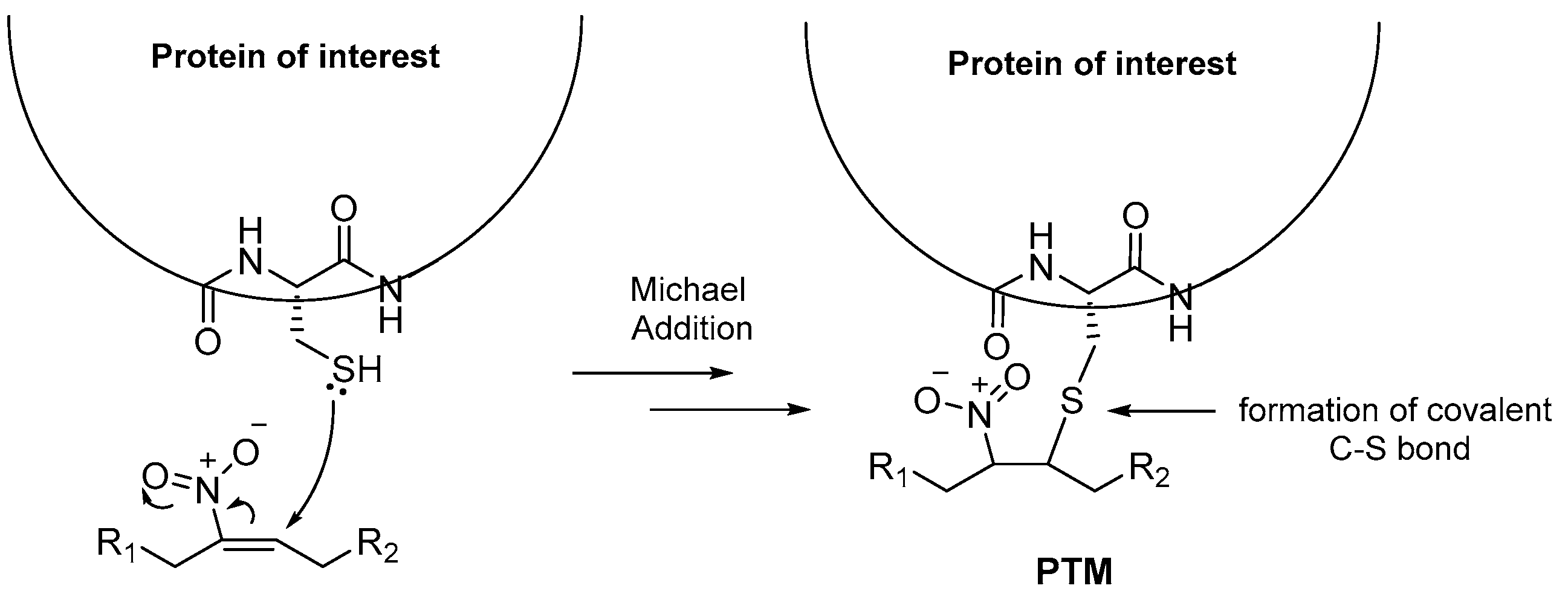
2.1. Interaction of Cysteine Residues with NO2-FAs
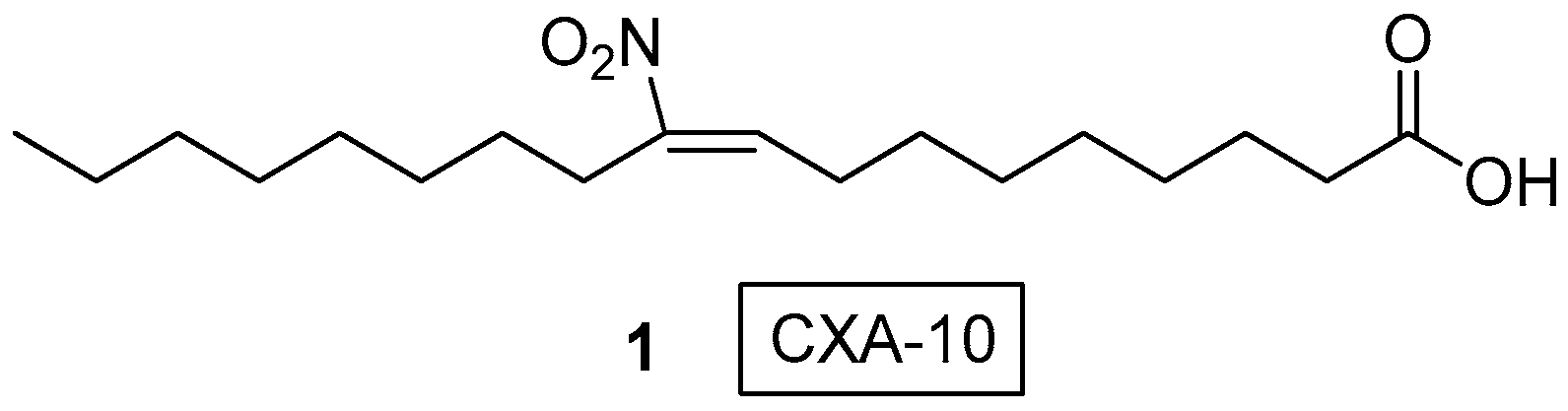
2.2. 10-Nitro Oleic Acid (CXA-10) and Its Derivatives as Anti-Inflammatory and Cytotoxic Agents
2.3. PPARγ-Regulated Diseases Attenuated by NO2-FAs
2.4. NO2-FAs as Regulators of Miscellaneous Targets
3. Syntheses of NO2-FAs
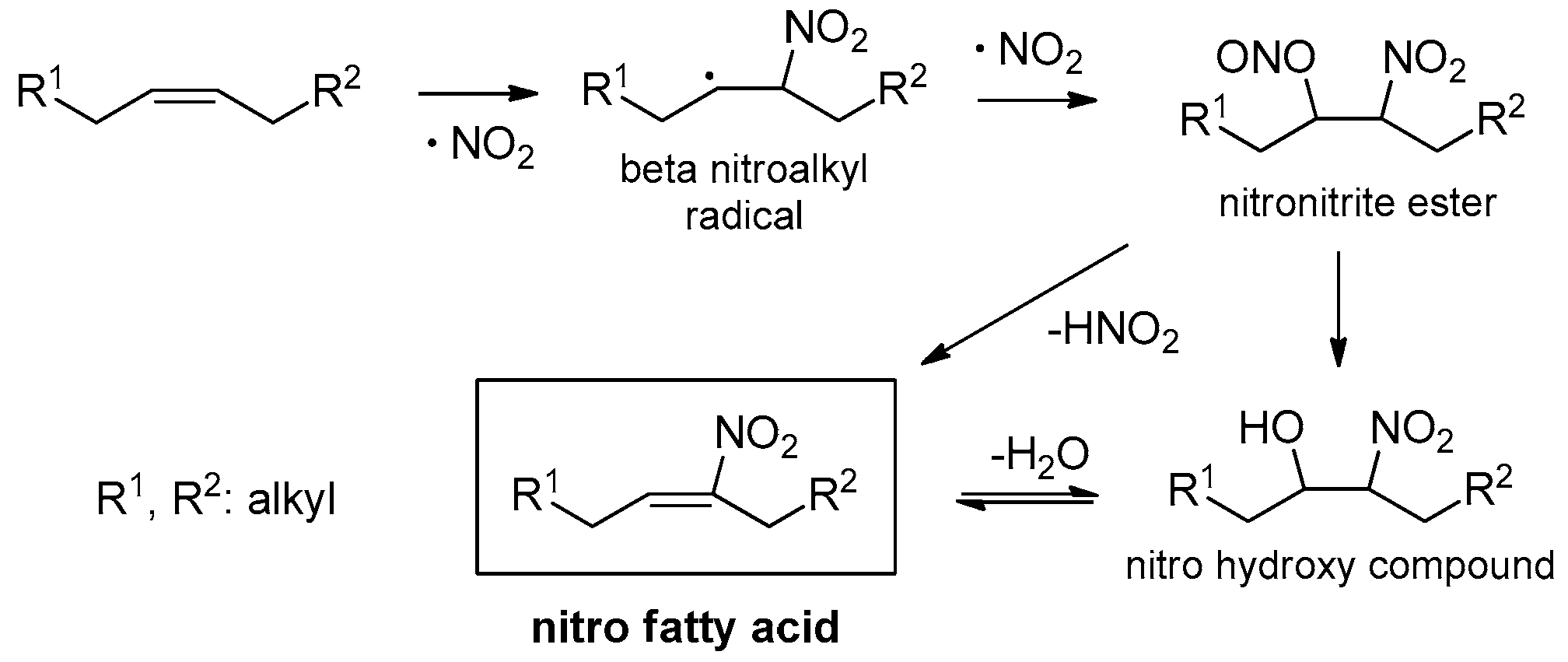
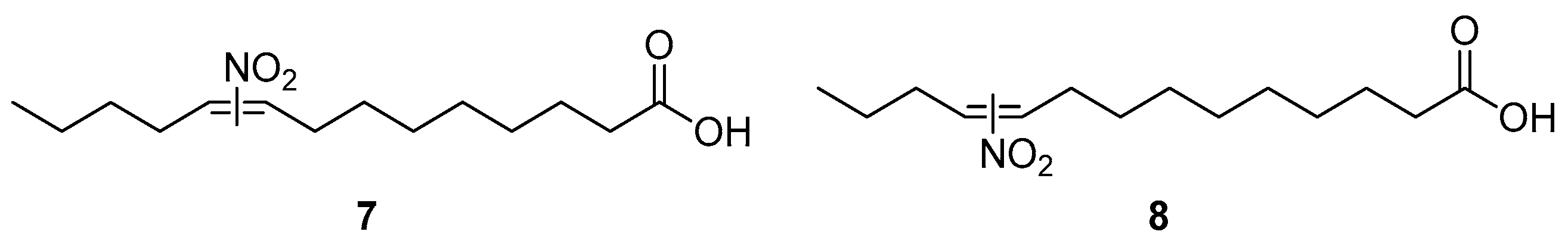
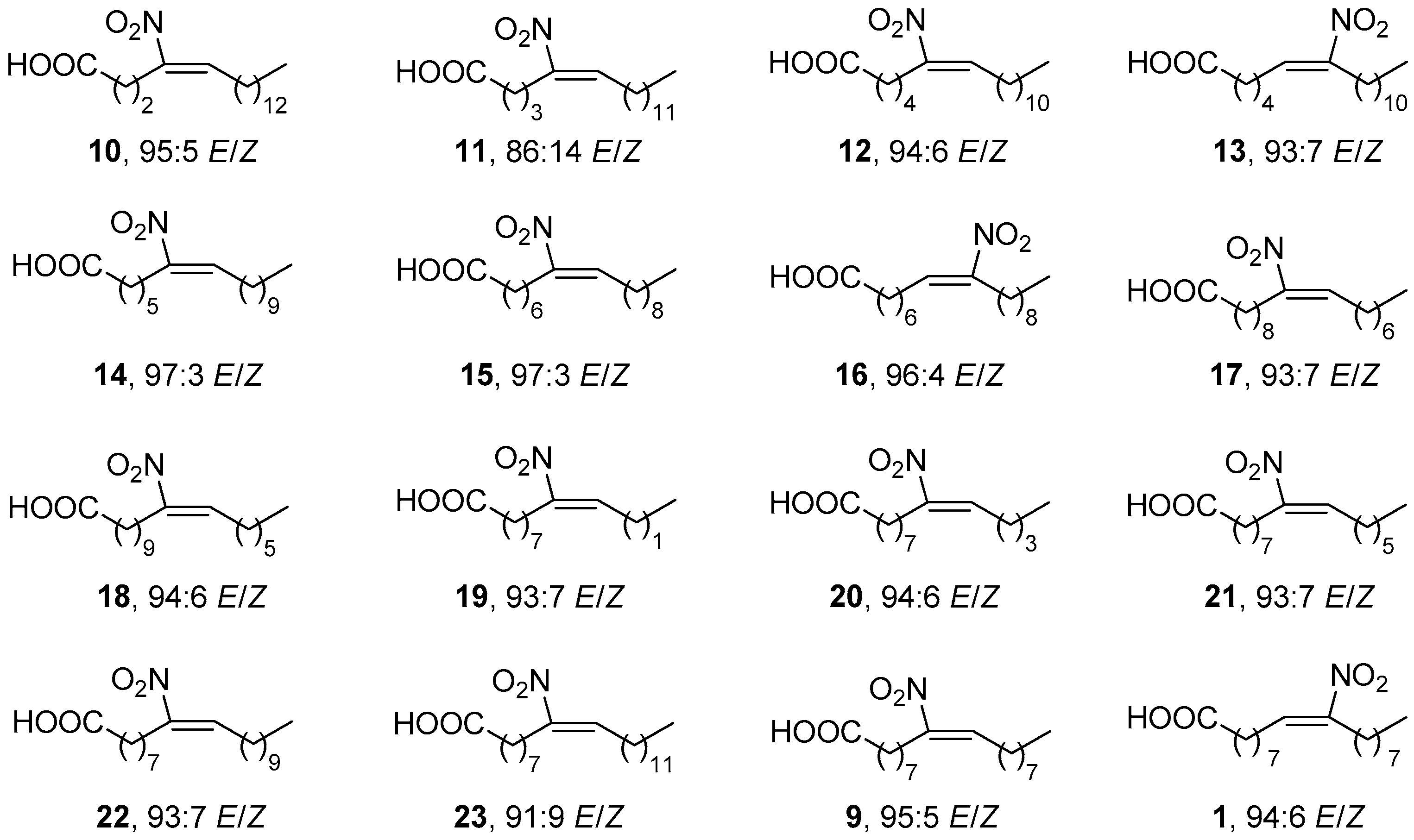
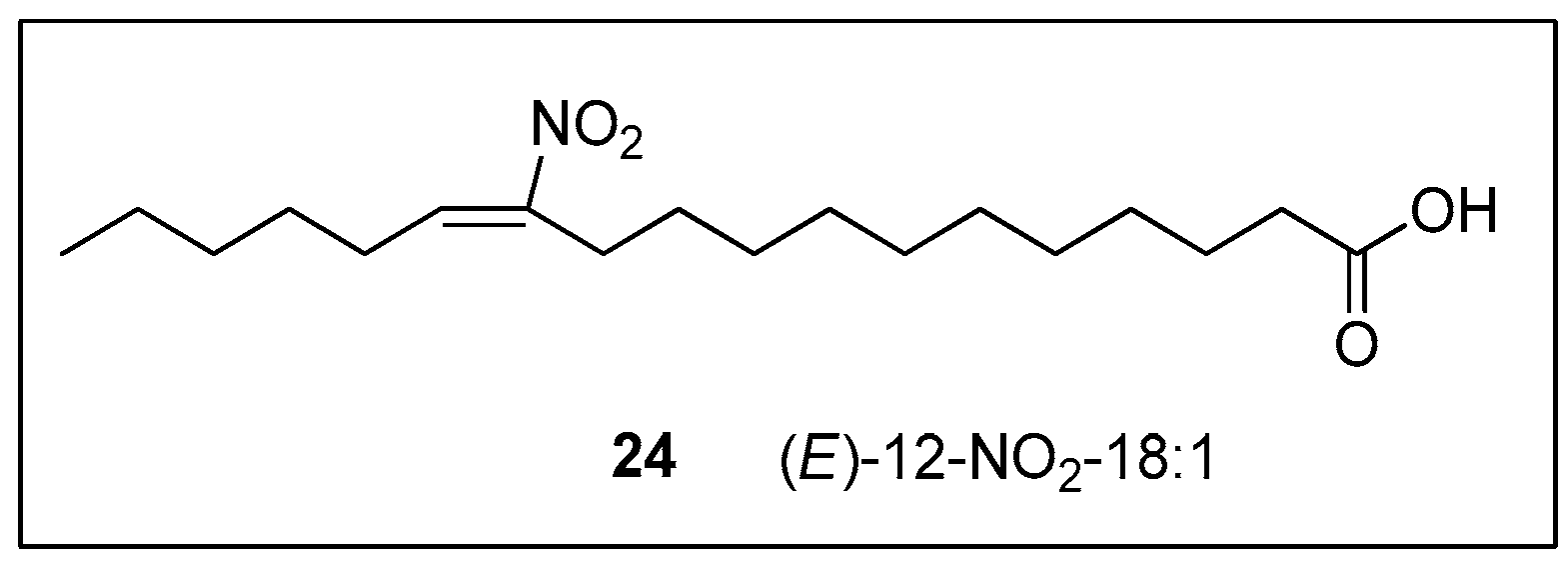
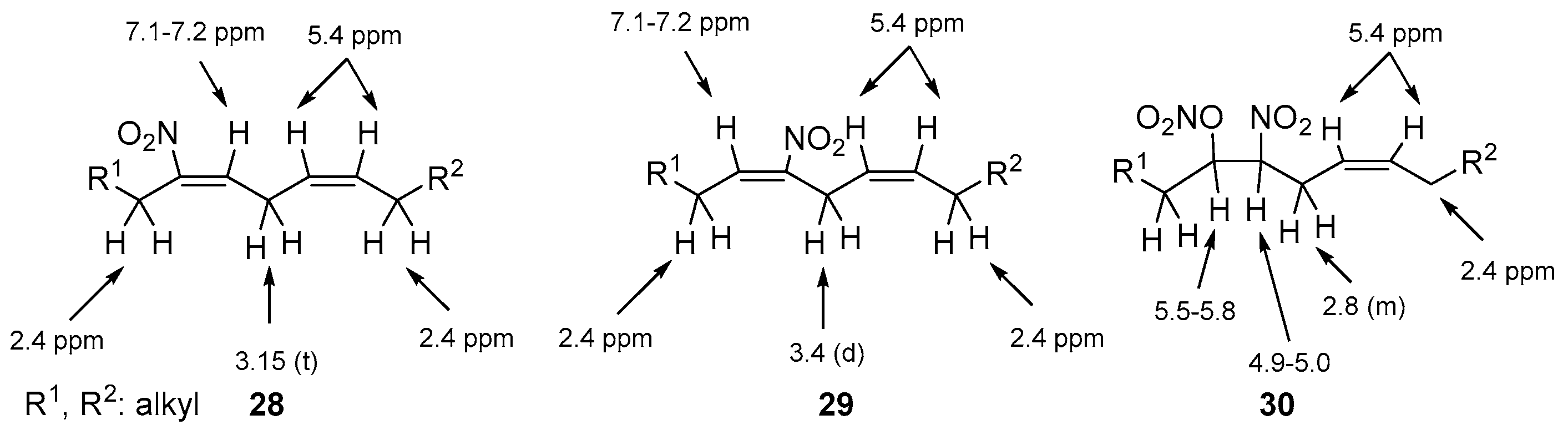
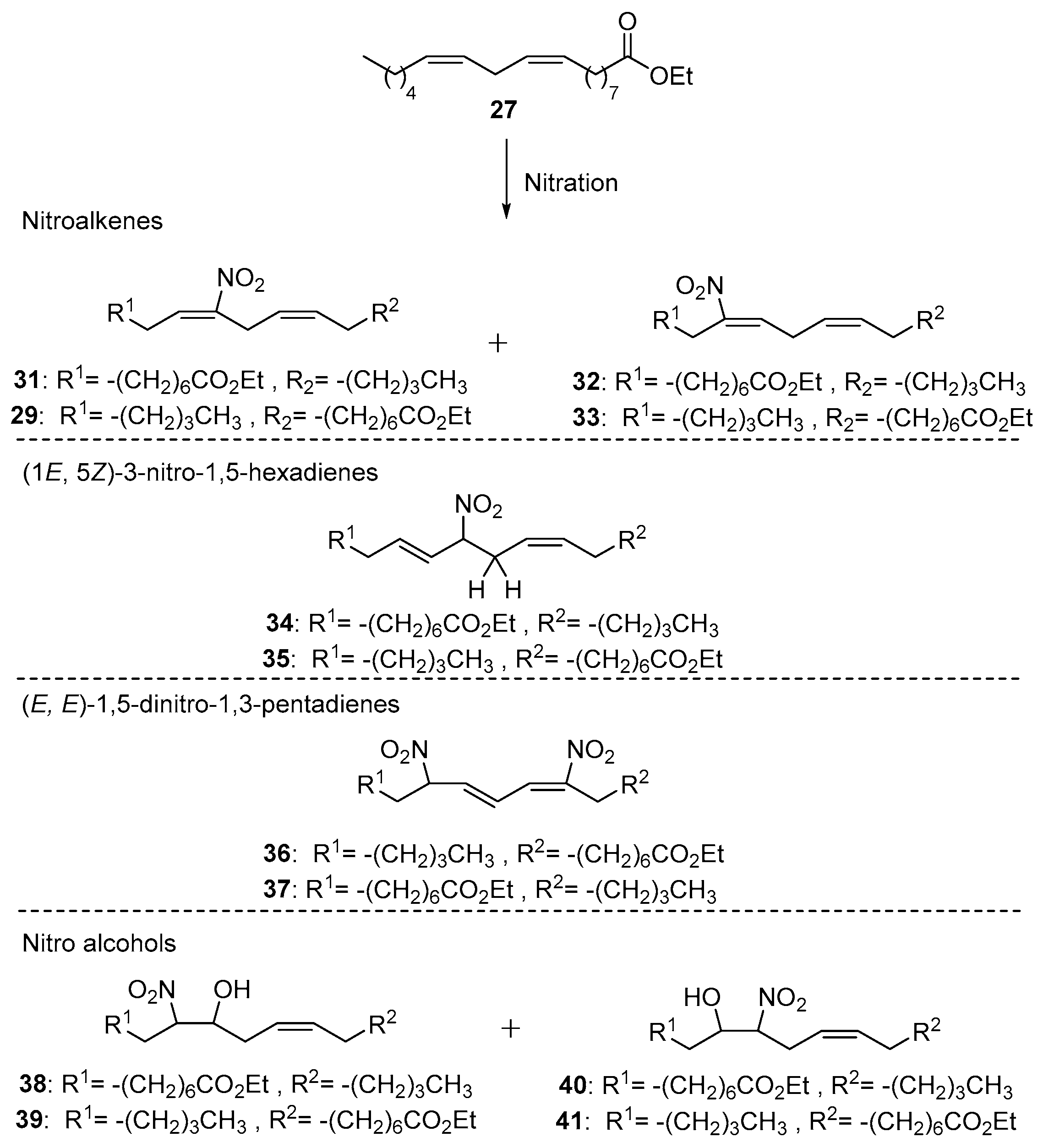
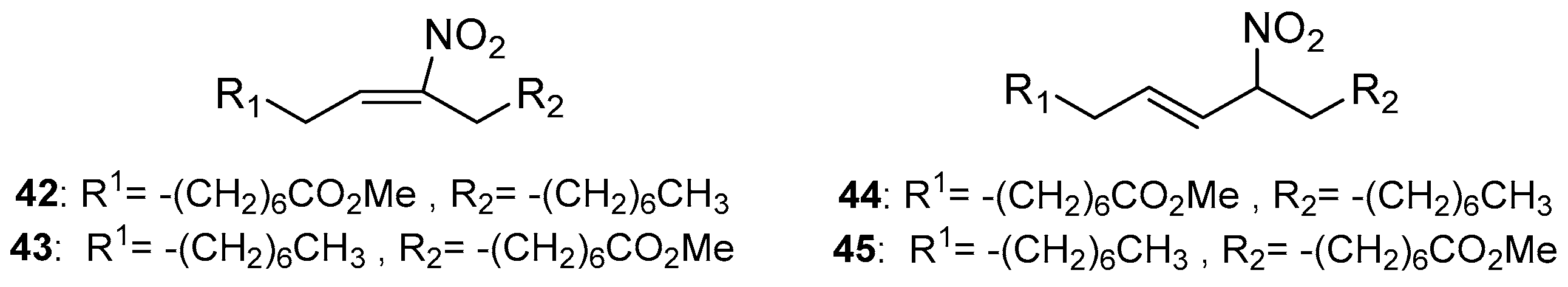
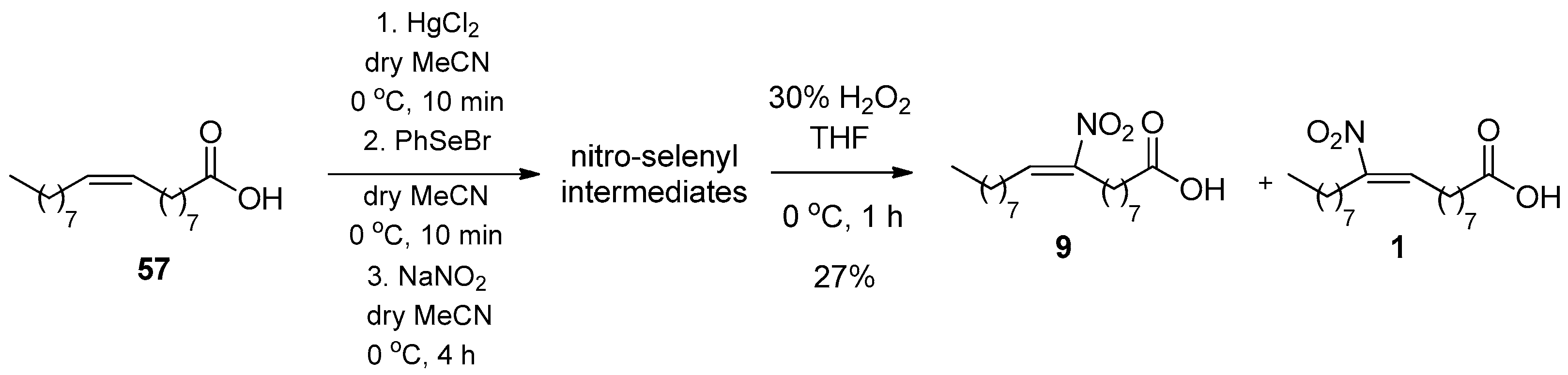
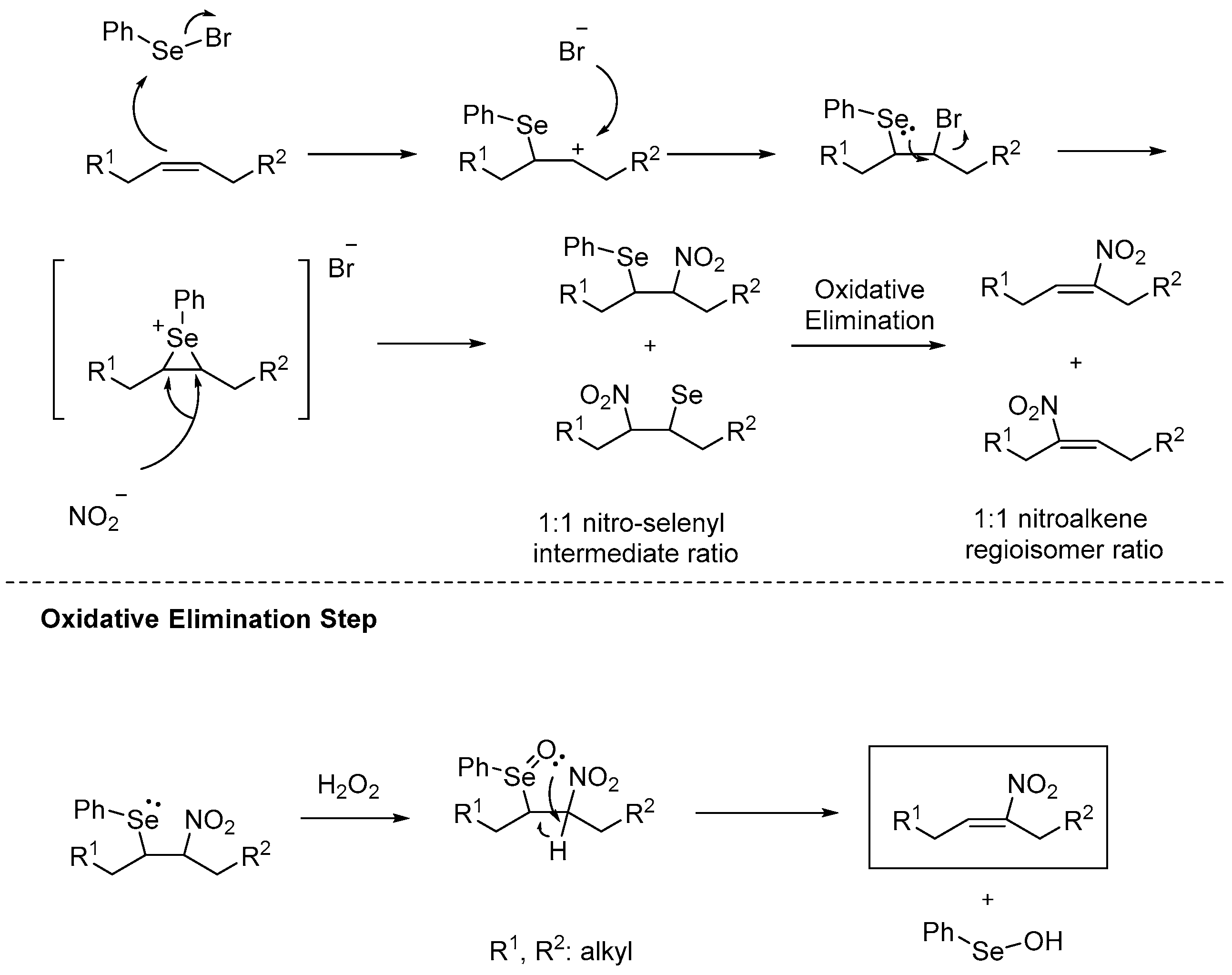
3.1. Direct Nitration of Unsaturated Lipids Yielding Derivatives of NO2-FAs
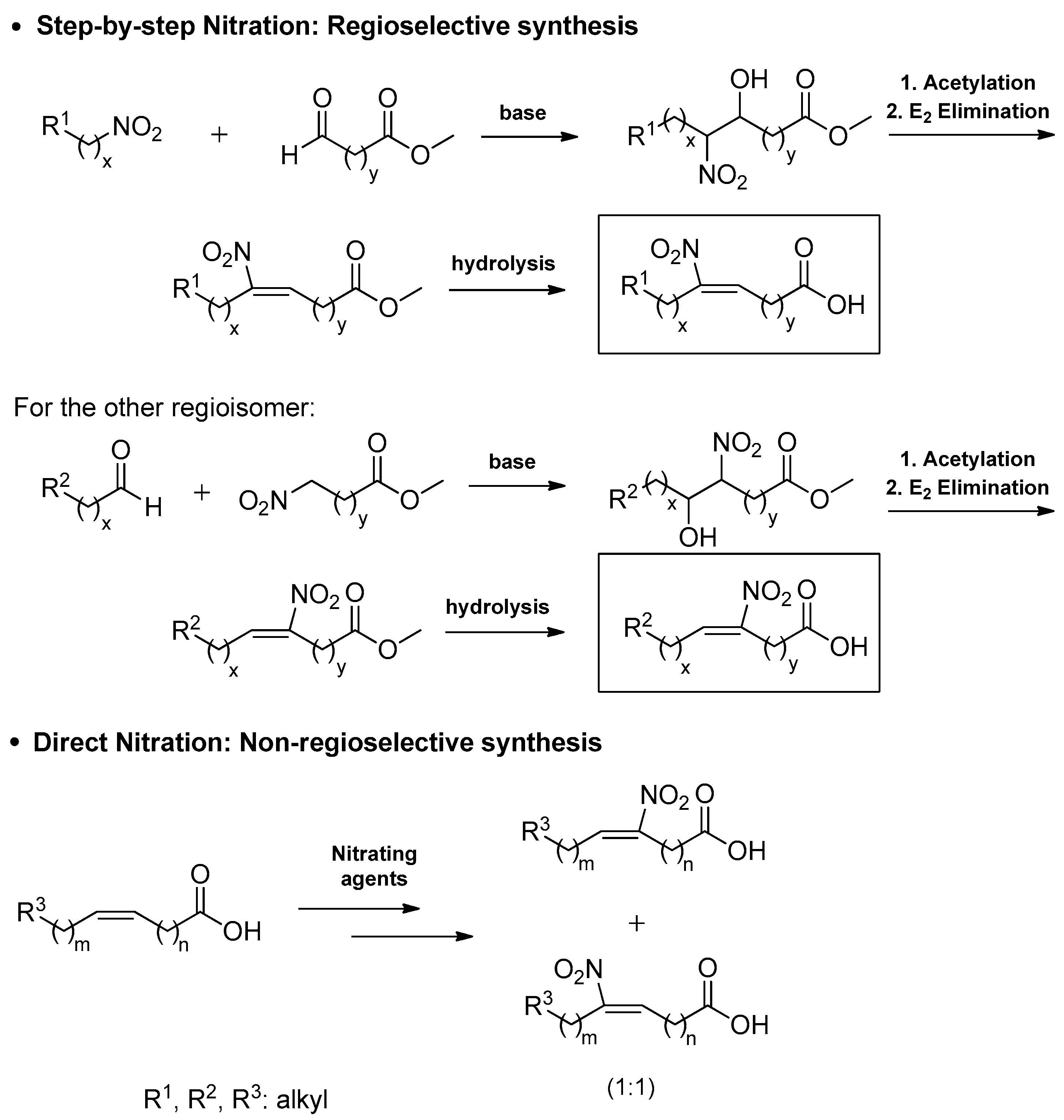
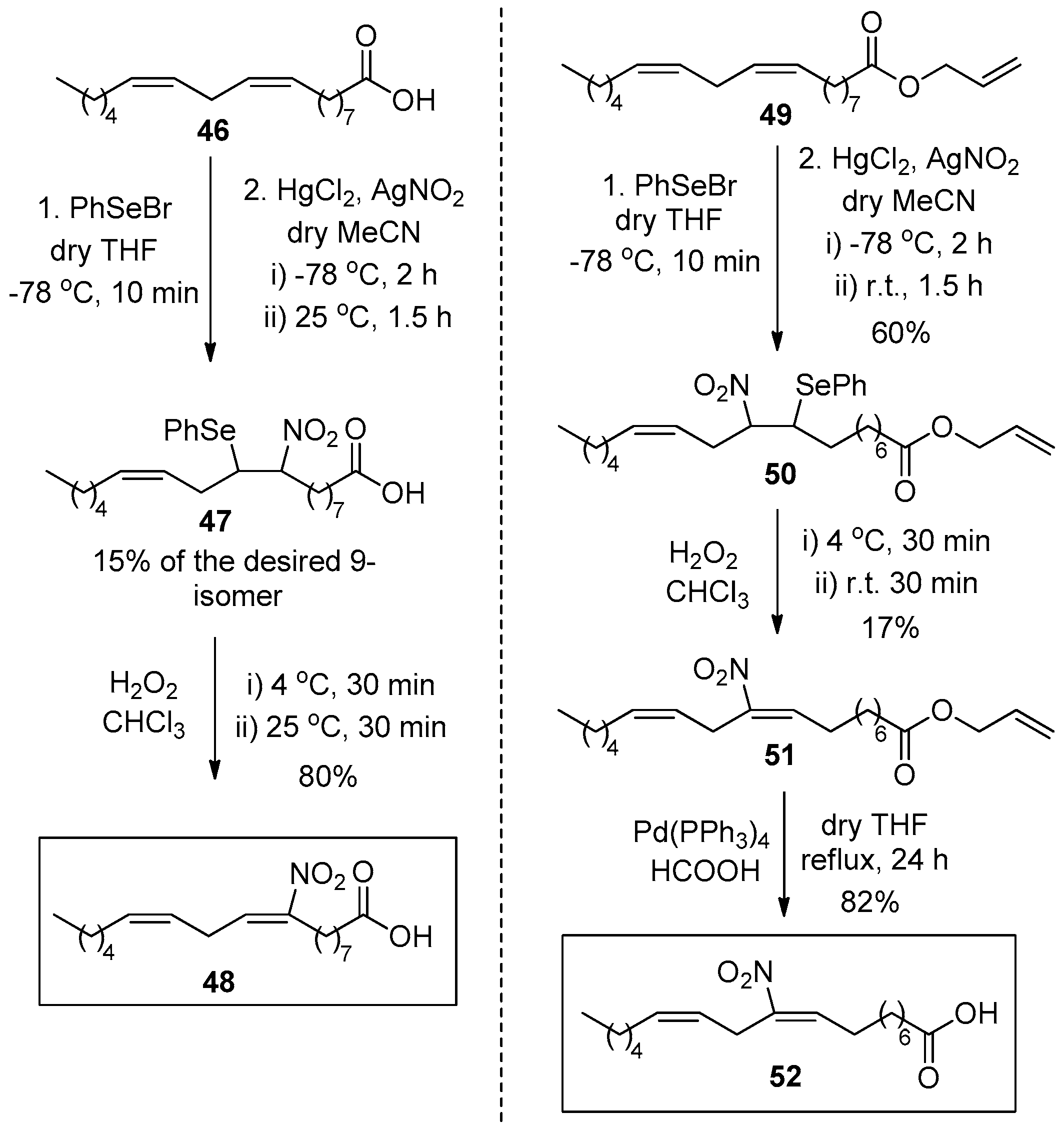
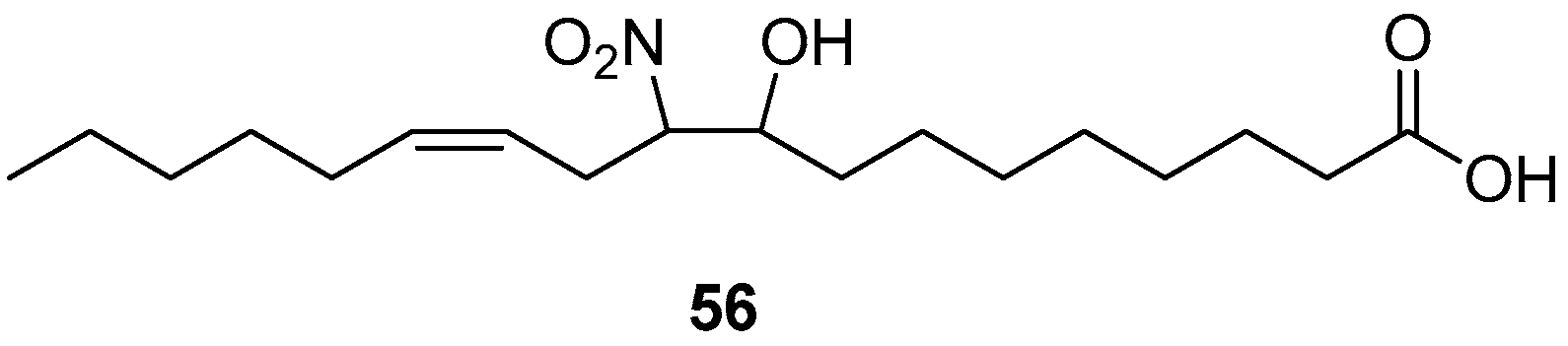
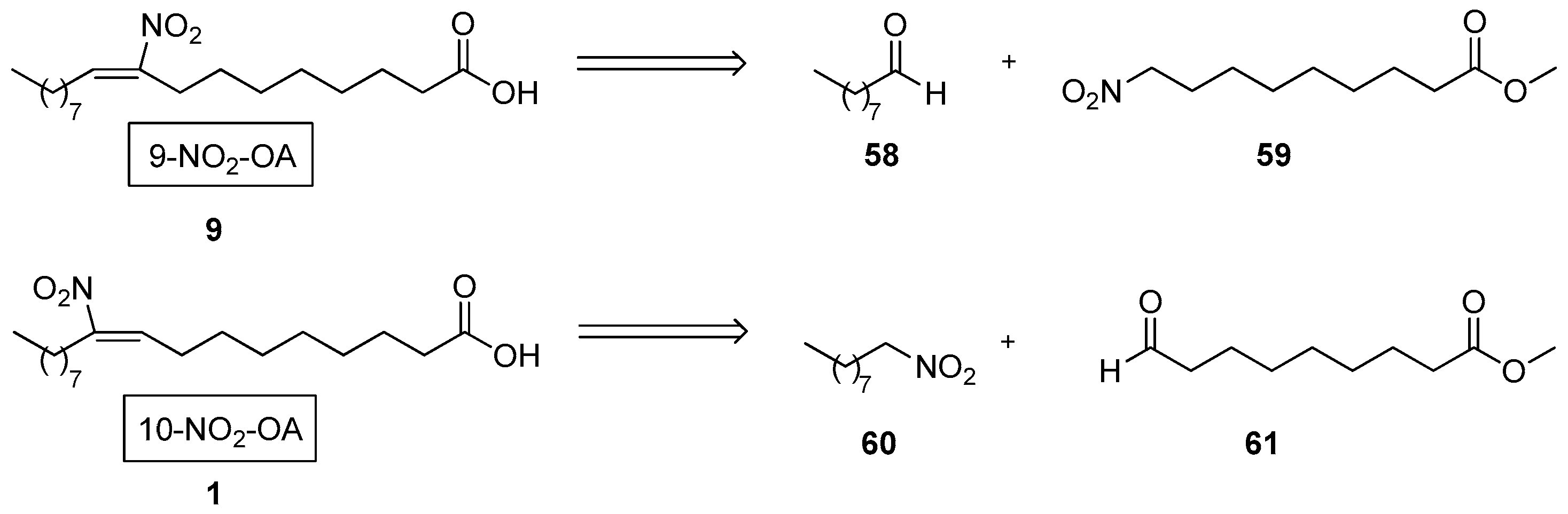
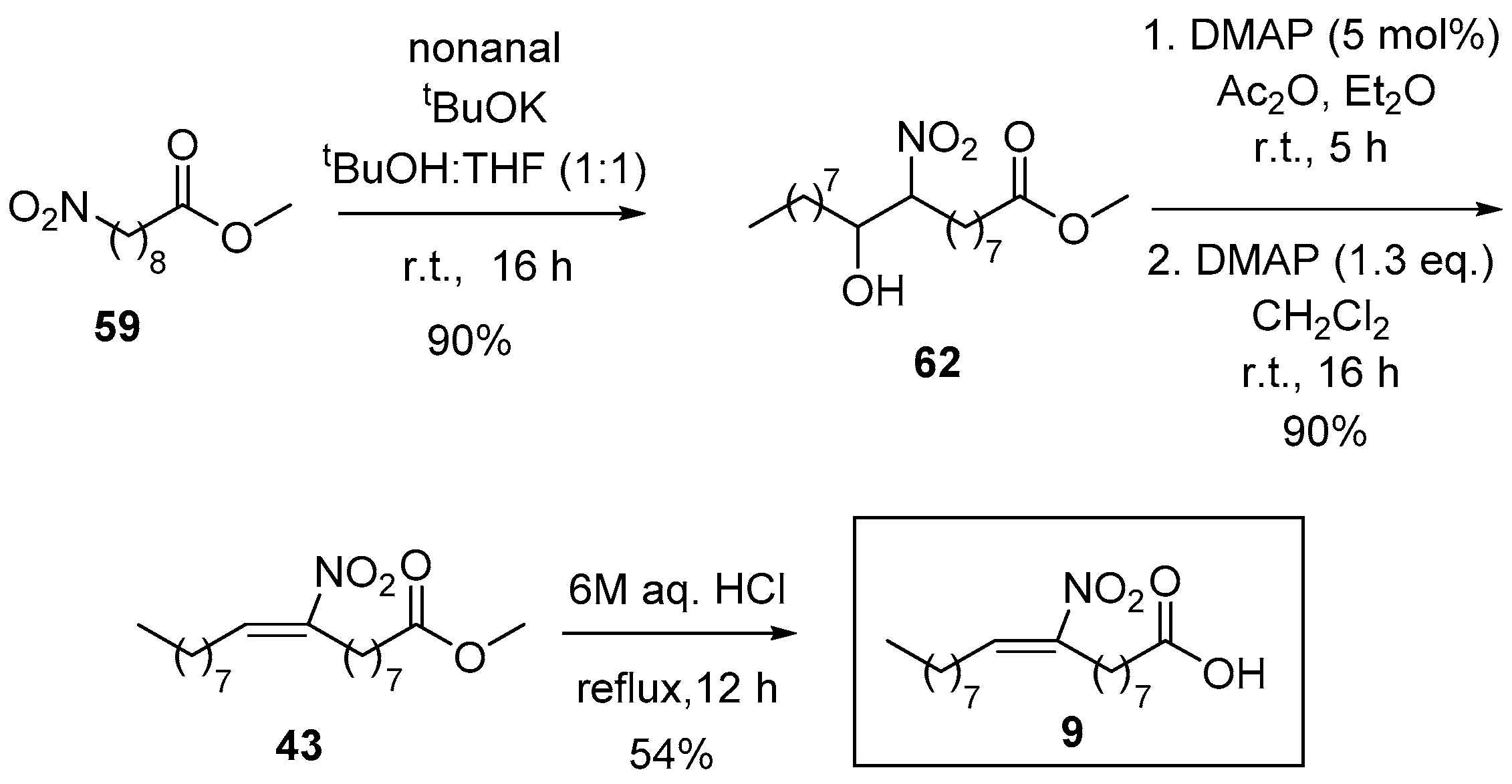
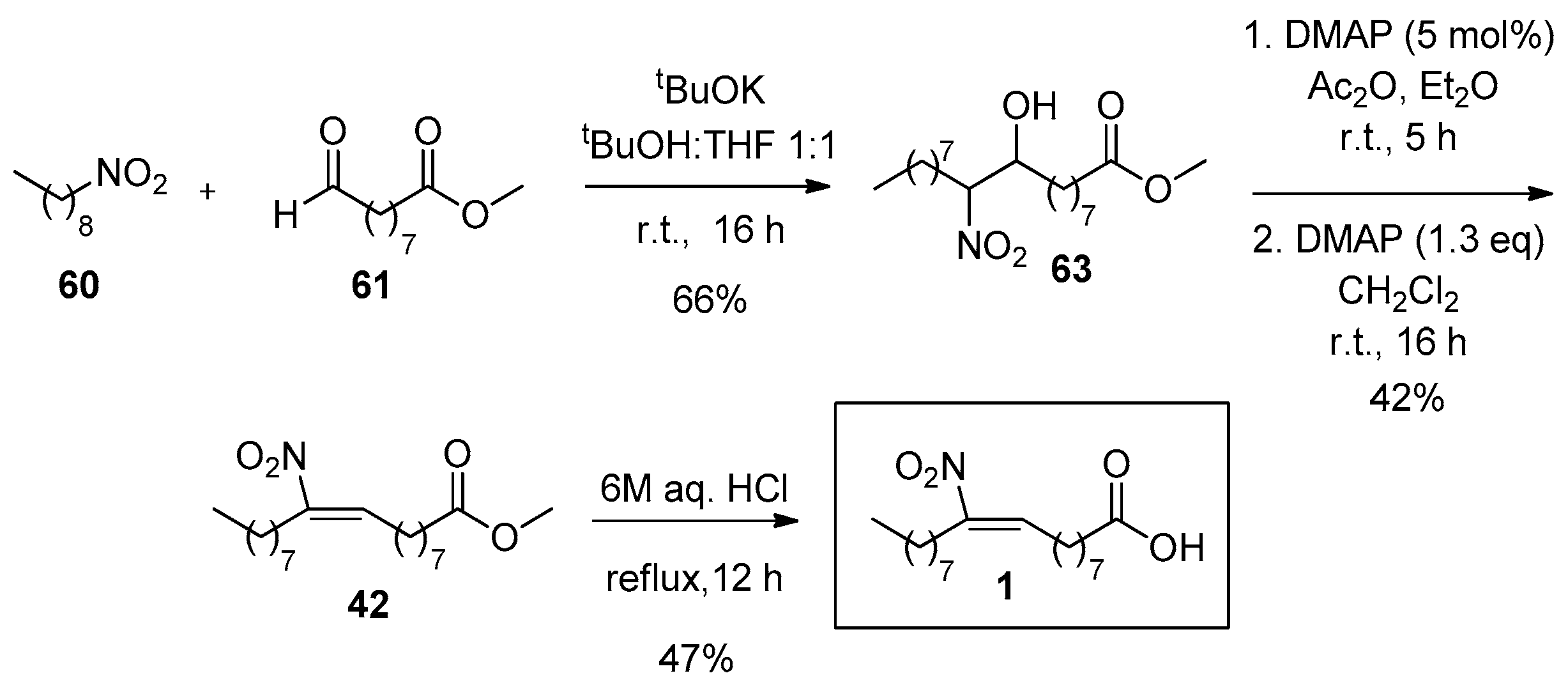
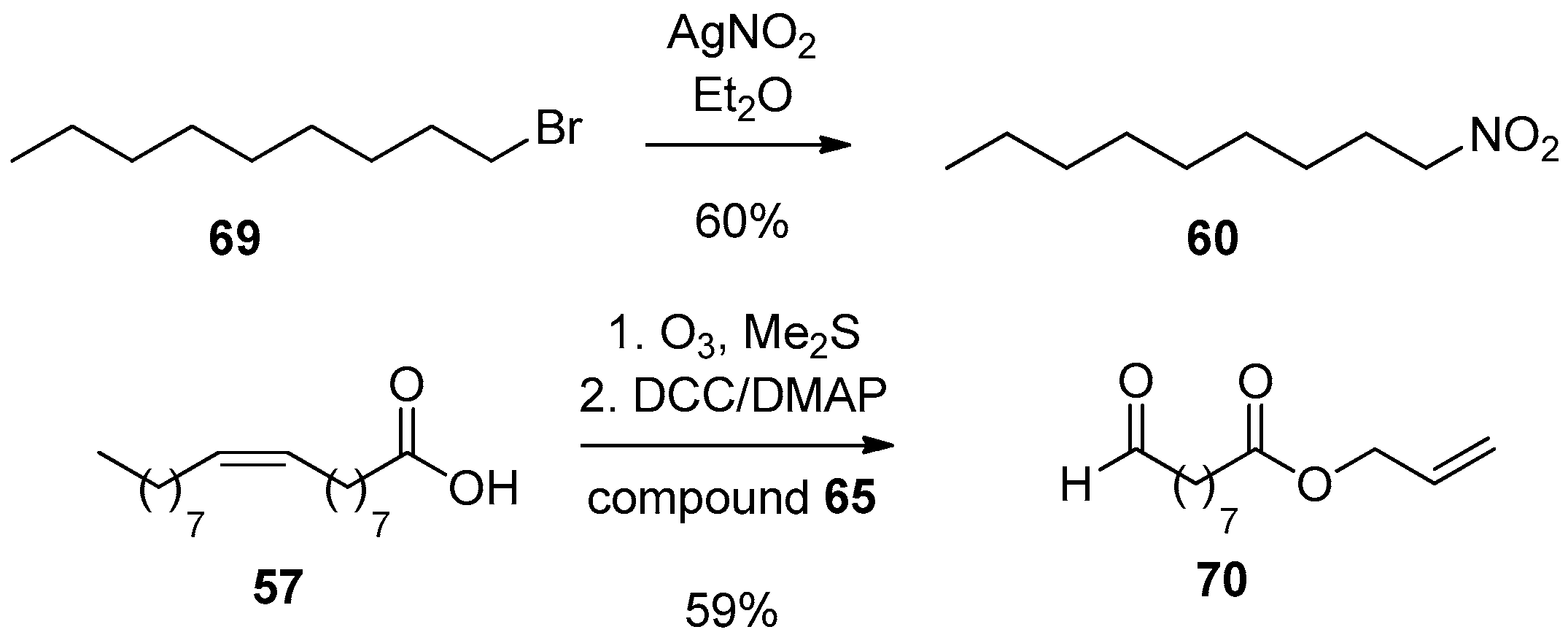
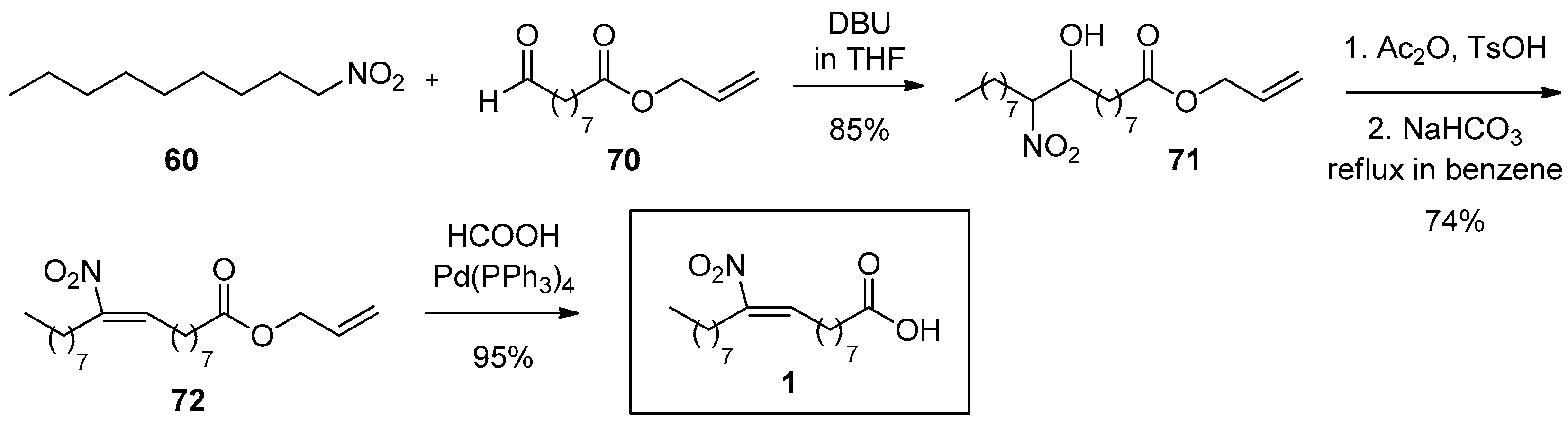
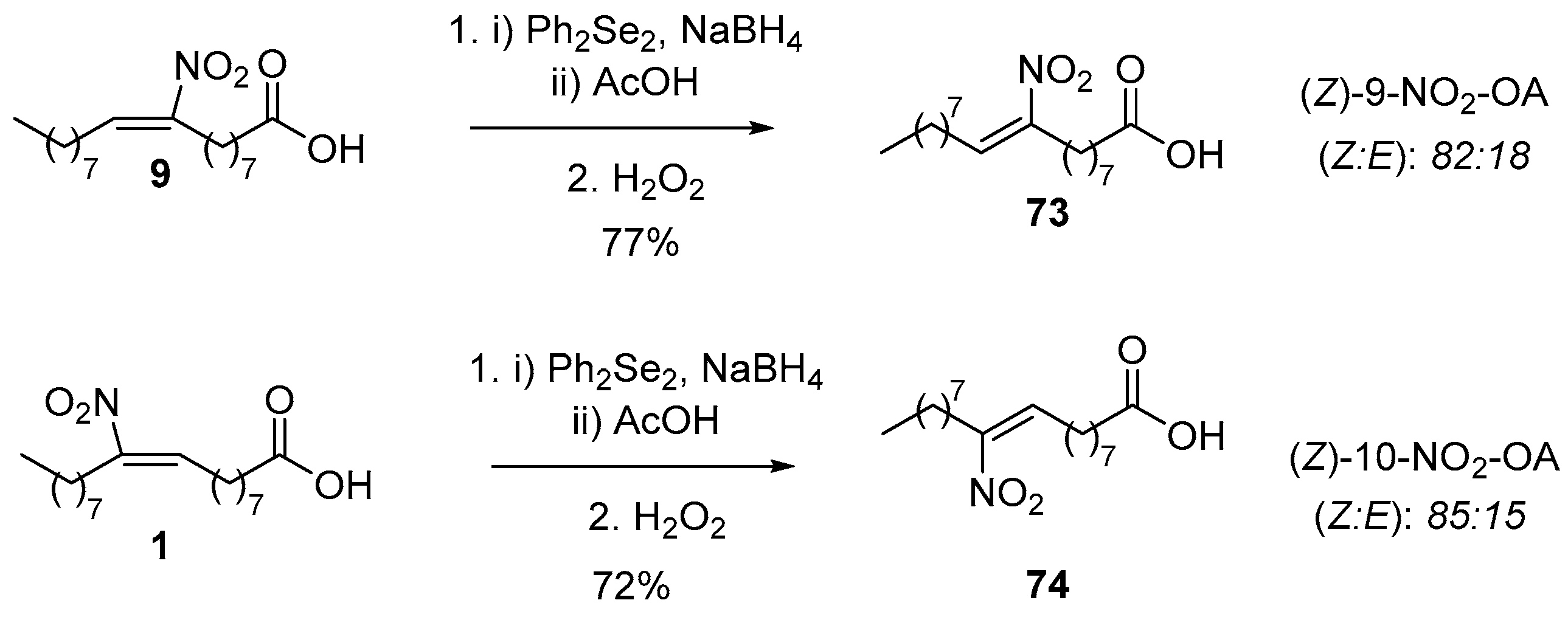
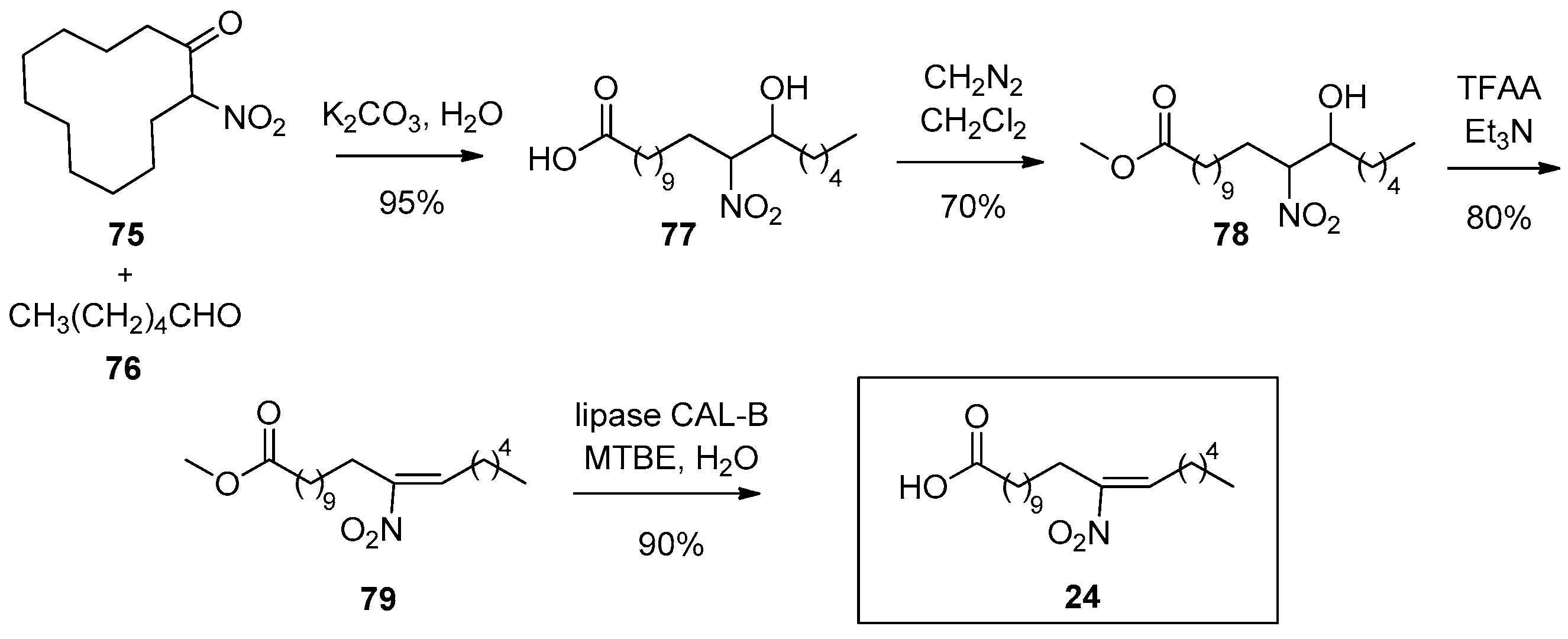
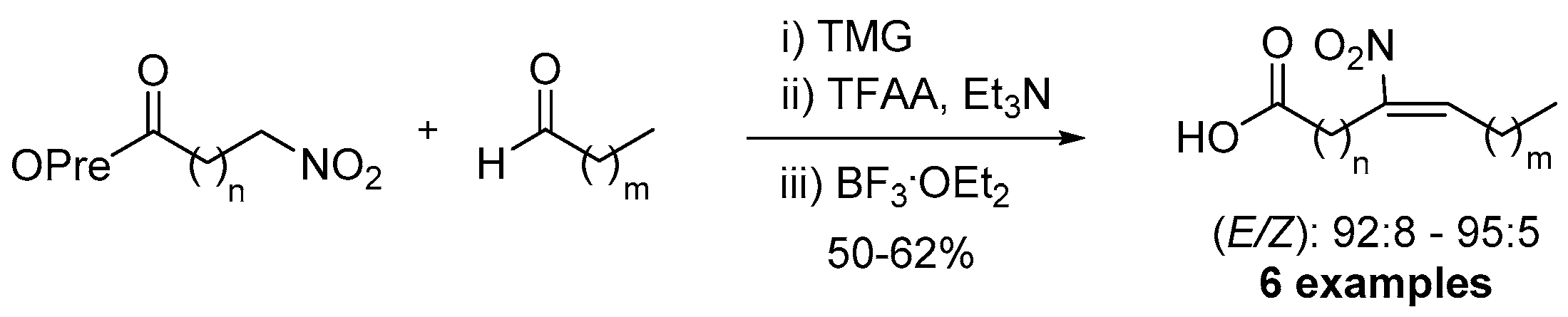
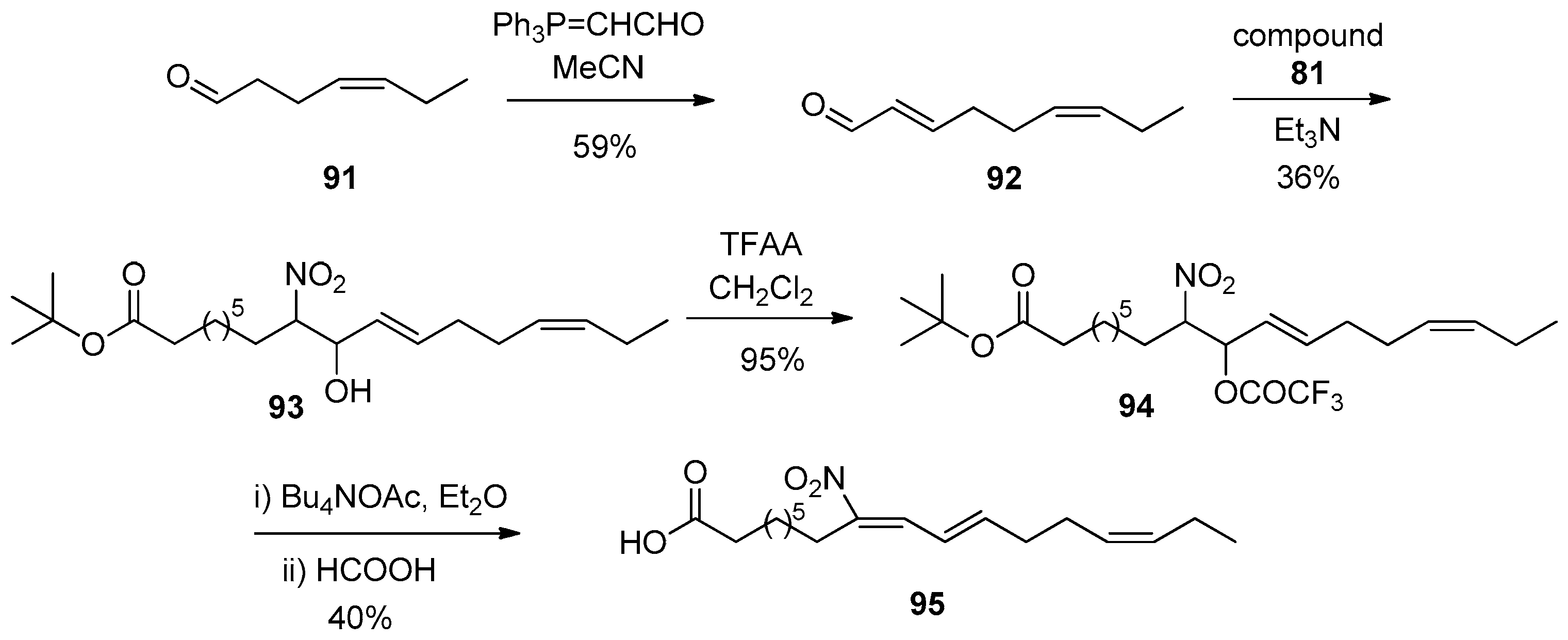
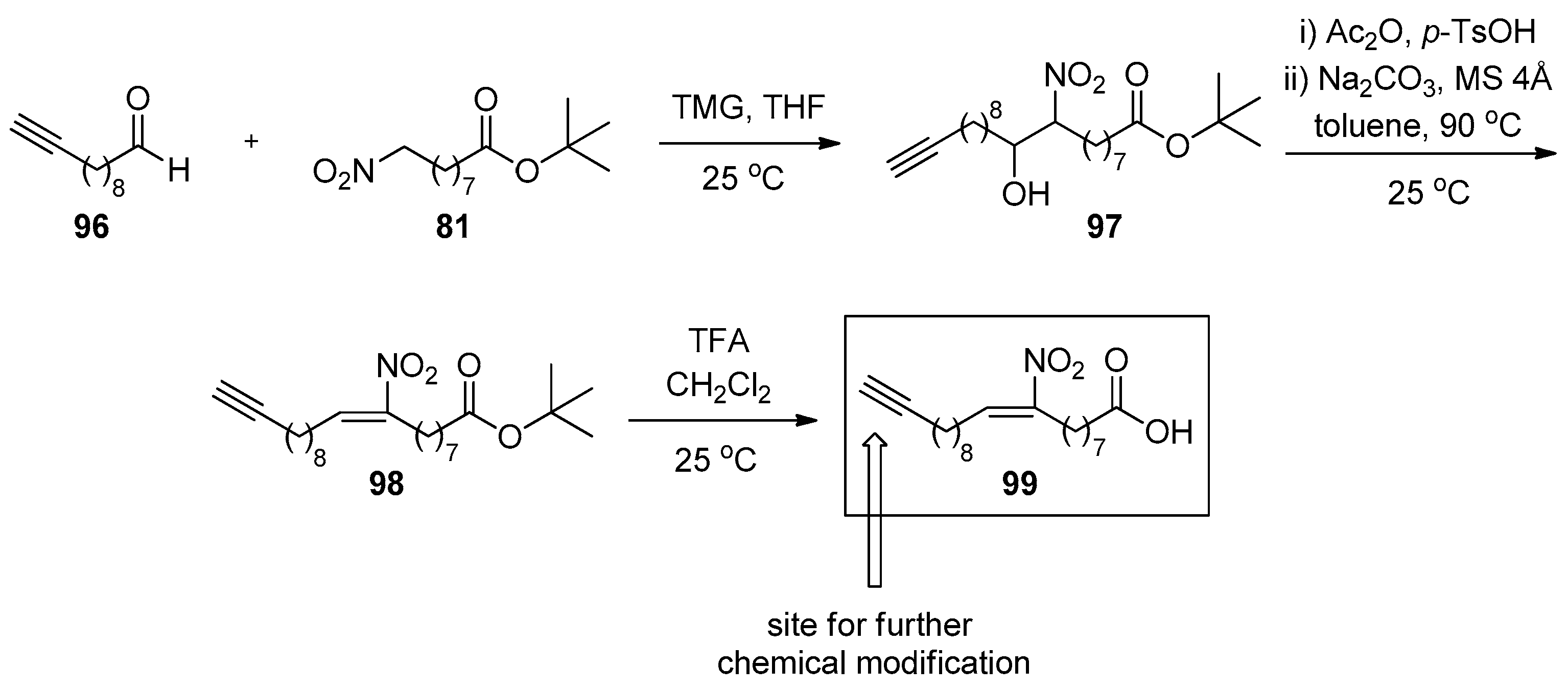
3.2. Step-By-Step Synthesis of NO2-FAs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delmastro-Greenwood, M.; Hughan, K.S.; Vitturi, D.A.; Salvatore, S.R.; Grimes, G.; Potti, G.; Shiva, S.; Schopfer, F.J.; Gladwin, M.T.; Freeman, B.A.; et al. Nitrite and nitrate-dependent generation of anti-inflammatory fatty acid nitroalkenes. Free Radic. Biol. Med. 2015, 89, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Vitturi, D.A.; Minarrieta, L.; Salvatore, S.R.; Postlethwait, E.M.; Fazzari, M.; Ferrer-Sueta, G.; Lancaster, J.R., Jr.; Freeman, B.A.; Schopfer, F.J. Convergence of biological nitration and nitrosation via symmetrical nitrous anhydride. Nat. Chem. Biol. 2015, 11, 504–510. [Google Scholar] [CrossRef]
- Freeman, B.A.; Baker, P.R.S.; Schopfer, F.J.; Woodcock, S.R.; Napolitano, A.; D’Ischia, M. Nitro-fatty acid formation and signaling. J. Biol. Chem. 2008, 283, 15515–15519. [Google Scholar] [CrossRef] [Green Version]
- Lima, E.S.; Di Mascio, P.; Rubbo, H.; Abdalla, D.S.P. Characterization of linoleic acid nitration in human blood plasma by mass spectrometry. Biochemistry 2002, 41, 10717–10722. [Google Scholar] [CrossRef]
- Baker, P.R.; Lin, Y.; Schopfer, F.J.; Woodcock, S.R.; Groeger, A.L.; Batthyany, C.; Sweeney, S.; Long, M.H.; Iles, K.E.; Baker, L.M.S.; et al. Fatty acid transduction of nitric oxide signaling: Multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J. Biol. Chem. 2005, 280, 42464–42475. [Google Scholar] [CrossRef] [Green Version]
- Tsikas, D.; Zoerner, A.A.; Mitschke, A.; Gutzki, F.-M. Nitro-fatty acids occur in human plasma in the picomolar range: A targeted nitro-lipidomics GC-MS/MS study. Lipids 2009, 44, 855–865. [Google Scholar] [CrossRef]
- Schoeman, J.C.; Harms, A.C.; van Weeghel, M.; Berger, R.; Vreeken, R.J.; Hankemeier, T. Development and application of a UHPLC–MS/MS metabolomics based comprehensive systemic and tissue-specific screening method for inflammatory, oxidative and nitrosative stress. Anal. Bioanal. Chem. 2018, 410, 2551–2568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, T.; Montero-Bullόn, J.-F.; Domingues, P.; Domingues, M.R. Discovery of bioactive nitrated lipids and nitro-lipid-protein adducts using mass spectrometry-based approaches. Redox Biol. 2019, 23, 101106. [Google Scholar] [CrossRef]
- Salvatore, S.R.; Rowart, P.; Schopfer, F.J. Mass spectrometry-based study defines the human urine nitrolipidome. Free Radic. Biol. Med. 2021, 162, 327–337. [Google Scholar] [CrossRef]
- Baker, L.M.S.; Baker, P.R.S.; Golin-Bisello, F.; Schopfer, F.J.; Fink, M.; Woodcock, S.R.; Branchaud, B.P.; Radi, R.; Freeman, B.A. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J. Biol. Chem. 2007, 282, 31085–31093. [Google Scholar] [CrossRef] [Green Version]
- Batthyany, C.; Schopfer, F.J.; Baker, P.R.; Duran, R.; Baker, L.M.; Huang, Y.; Cervenansky, C.; Branchaud, B.P.; Freeman, B.A. Reversible post-translational modification of proteins by nitrated fatty acids in vivo. J. Biol. Chem. 2006, 281, 20450–20463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turell, L.; Vitturi, D.A.; Coitiño, E.L.; Lebrato, L.; Möller, M.N.; Sagasti, C.; Salvatore, S.R.; Woodcock, S.R.; Alvarez, B.; Schopfer, F.J. The chemical basis of thiol addition to nitro-conjugated linoleic acid, a protective cell-signaling lipid. J. Biol. Chem. 2017, 292, 1145–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grippo, V.; Mojovic, M.; Pavicevic, A.; Kabelac, M.; Hubatka, F.; Turanek, J.; Zatloukalova, M.; Freeman, B.A.; Vacek, J. Electrophilic characteristics and aqueous behavior of fatty acid nitroalkenes. Redox Biol. 2021, 38, 101756. [Google Scholar] [CrossRef]
- Tsujita, T.; Li, L.; Nakajima, H.; Iwamoto, N.; Nakajima-Takagi, Y.; Ohashi, K.; Kawakami, K.; Kumagai, Y.; Freeman, B.A.; Yamamoto, M.; et al. Nitro-fatty acids and cyclopentenone prostaglandins share strategies to activate the Keap1-Nrf2 system: A study using green fluorescent protein transgenic zebrafish. Genes Cells 2011, 16, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Kansanen, E.; Jyrkkänen, H.K.; Volger, O.L.; Leinonen, H.; Kivelä, A.M.; Häkkinen, S.-K.; Woodcock, S.R.; Schopfer, F.J.; Horrevoets, A.J.; Ylä-Herttuala, S.; et al. Nrf2-dependent and –independent responses to nitro-fatty acids in human endothelial cells: Identification of heat shock response as the major pathway activated by nitro-oleic acid. J. Biol. Chem. 2009, 284, 33233–33241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, M.P.; Rudolph, T.K.; Khoo, N.K.H.; Motanya, U.N.; Golin-Bisello, F.; Wertz, J.W.; Schopfer, F.J.; Ruldoph, V.; Woodcock, S.R.; Bolisetty, S.; et al. Nitro-fatty acid inhibition of Neointima formation after endoluminal vessel injury. Circ. Res. 2009, 105, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Cui, T.; Schopfer, F.J.; Zhang, J.; Chen, K.; Ichikawa, T.; Baker, P.R.S.; Batthyany, C.; Chacko, B.K.; Feng, X.; Patel, R.P.; et al. Nitrated-fatty acids: Endogenous anti-inflammatory signaling mediators. J. Biol. Chem. 2006, 281, 35686–35698. [Google Scholar] [CrossRef] [Green Version]
- Villacorta, L.; Chang, L.; Salvatore, S.R.; Ichikawa, T.; Zhang, J.; Petrovic-Djergovic, D.; Jia, L.; Carlsen, H.; Schopfer, F.J.; Freeman, B.A.; et al. Electrophilic nitro-fatty acids inhibit vascular inflammation by disrupting LPS-dependent TLR4 signalling in lipid rafts. Cardiovasc. Res. 2013, 98, 116–124. [Google Scholar] [CrossRef]
- Ambrozova, G.; Martiskova, H.; Koudelka, A.; Ravekes, T.; Rudolph, T.K.; Klinke, A.; Rudolph, V.; Freeman, B.A.; Woodcock, S.R.; Kubala, L.; et al. Nitro-oleic acid modulates classical and regulatory activation of macrophages and their involvement in profibrotic responses. Free Radic. Biol. Med. 2016, 90, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, T.K.; Freeman, B.A. Transduction of redox signaling by electrophile-protein reactions. Sci. Signal 2009, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Netto, L.E.S.; Antonio de Oliveira, M.; Monteiro, G.; Demasi, A.P.D.; Cussiol, J.R.R.; Discola, K.F.; Demasi, M.; Silva, G.M.; Alves, S.V.; Faria, V.G.; et al. Reactive cysteine in proteins: Protein folding, antioxidant defence, redox signaling and more. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 180–193. [Google Scholar] [CrossRef]
- Derakhshan, B.; Hao, G.; Gross, S.S. Balancing reactivity against selectivity: The evolution of protein S-nitrosylation as an effector of cell signaling by nitric oxide. Cardiovasc. Res. 2007, 75, 210–219. [Google Scholar] [CrossRef] [Green Version]
- Complexa. Complexa Announces Successful Completion of Four Phase 1 Studies of CXA-10 and Planned Phase 2 Initiation in Multiple Orphan Indications. Available online: https://www.complexarx.com/nov12 (accessed on 12 October 2021).
- Chieffo, C.; Botbyl, J.; Perry, K.; Blok, T.M.; Jorkasky, D.K. Use of an obese population in phase 1 to evaluate the pharmacology of oral CXA-10, an endogenous, nitrofatty acid singaling agent. In Proceedings of the Annual Meeting of the American College of Clinical Pharmacology, Bethesda, MD, USA, 24–27 September 2016. [Google Scholar]
- Clinical Trial of CXA-10 against Asthma, Lead Sponsor: University of Colorado, Denver; Anti Inflammatory Lipid Mediators in Asthma: A Double Blind Placebo Control Cross Over, Proof of Concept Study of CXA-10 to Reduce Bronchial Hyperresponsiveness in Obese Asthmatics. Available online: Ichgcp.net/clinical-trials-registry/NCT03762395 (accessed on 12 October 2021).
- Garner, R.M.; Mould, D.R.; Chieffo, C.; Jorkasky, D.K. Pharmacokinetic and pharmacodynamic effects of oral CXA-10, a nitro fatty acid, after single and multiple ascending doses in healthy and obese subjects. Clin. Transl. Sci. 2019, 12, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Klinke, A.; Μöller, A.; Pekarova, M.; Ravekes, T.; Friedrichs, K.; Berlin, M.; Scheu, K.M.; Kubala, L.; Kolarova, H.; Ambrozova, G.; et al. Protective effects of 10-nitro oleic acid in hypoxia-induced murine model of pulmonary hypertension. Am. J. Respir. Cell. Mol. Biol. 2014, 51, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, E.E.; Baust, J.; Bonacci, G.; Golin-Bisello, F.; Devlin, J.E.; St Croix, C.M.; Watkins, S.C.; Gor, S.; Cantu-Medellin, N.; Weidert, E.R.; et al. Fatty acid nitroalkenes ameliorate glucose intolerance and pulmonary hypertension in high-fat diet-induced obesity. Cardiovasc. Res. 2014, 101, 352–363. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, M.L.; Abramova, E.; Guo, C.; Gow, J.G.; Murray, A.; Koudelka, A.; Cechova, V.; Freeman, B.A.; Gow, A.J. Fatty acid nitroalkenes inhibit the inflammatory response to bleomycin-mediated lung injury. Toxicol. Appl. Pharmacol. 2020, 407, 115236. [Google Scholar] [CrossRef] [PubMed]
- Alparslan, A.; Skoko, J.J.; Woodcock, C.-S.C.; Wingert, B.M.; Woodcock, S.R.; Normolle, D.; Huang, Y.; Stark, J.M.; Camacho, C.J.; Freeman, B.A.; et al. Electrophilic fatty acids impair RAD51 function and potentiate the effects of DNA-damaging agents on growth of triple-negative breast cells. J. Biol. Chem. 2018, 294, 397–404. [Google Scholar]
- Woodcock, C.-S.C.; Huang, Y.; Woodcock, S.R.; Salvatore, S.R.; Singh, B.; Golin-Bisello, F.; Davidson, N.E.; Neumann, C.A.; Freeman, B.A.; Wendell, S.G. Nitro-fatty acid inhibition of triple-negative breast cancer cell viability, migration, invasion and tumor growth. J. Biol. Chem. 2018, 293, 1120–1137. [Google Scholar] [CrossRef] [Green Version]
- Khoo, N.K.H.; Li, L.; Salvatore, S.R.; Schopfer, F.J.; Freeman, B.A. Electrophilic fatty acid nitroalkenes regulate Nrf2 and NF-κB signaling: A medicinal chemistry investigation of structure-function relationships. Sci. Rep. 2018, 8, 2295–2311. [Google Scholar] [CrossRef]
- Kühn, B.; Brat, C.; Fettel, J.; Hellmuth, N.; Maucher, I.V.; Bulut, U.; Hock, K.J.; Grimmer, J.; Manolikakes, G.; Rühl, M.; et al. Anti-inflammatory nitro-fatty acids suppress tumor growth by triggering mitochondrial dysfunction and activation of the intrinsic apoptotic pathway in colorectal cancer cell lines. Biochem. Pharmacol. 2018, 155, 48–60. [Google Scholar] [CrossRef]
- Hellmuth, N.; Brat, C.; Awad, O.; George, S.; Kahnt, A.; Bauer, T.; Phuoc, H.P.H.; Steinhilber, D.; Angioni, C.; Hassan, M.; et al. Structural modifications yield novel insights into the intriguing pharmacodynamic potential of anti-inflammatory nitro-fatty acids. Front. Pharmacol. 2021, 12, 715076. [Google Scholar] [CrossRef]
- Rudolph, T.K.; Rudolph, V.; Edreira, M.M.; Cole, M.P.; Bonacci, G.; Schopfer, F.J.; Woodcock, R.S.; Franek, A.; Pekarova, M.; Khoo, N.K.H.; et al. Nitro-fatty acids reduce atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 938–945. [Google Scholar] [CrossRef]
- Marchioli, R.; Barzi, F.; Bomba, E.; Chieffo, C.; Di Gregorio, D.; Di Mascio, R.; Franzosi, M.G.; Geraci, E.; Levantesi, G.; Maggioni, A.P.; et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: Time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002, 105, 1897–1903. [Google Scholar] [CrossRef] [Green Version]
- Braumann, S.; Schumacher, W.; Im, N.G.; Nettersheim, F.S.; Mehrkens, D.; Bokredenghel, S.; Hof, A.; Nies, R.J.; Adler, C.; Winkels, H.; et al. Nitro-oleic (NO2-OA) improves systolic function in dilated cardiomyopathy by attenuating myocardial fibrosis. Int. J. Mol. Sci. 2021, 22, 9052. [Google Scholar] [CrossRef]
- Wang, G.; Ji, Y.; Li, Z.; Han, X.; Guo, N.; Song, Q.; Quan, L.; Wang, T.; Han, W.; Pang, D.; et al. Nitro-oleic acid downregulates lipoprotein-associated phospholipase A2 expression via the p42/p44 MAPK and NFκB pathways. Sci. Rep. 2014, 4, 4905–4912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coles, B.; Bloodsworth, A.; Clark, S.R.; Lewis, M.J.; Cross, A.R.; Freeman, B.A.; O’Donnell, V.B. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: Novel anti-inflammatory properties of nitric-oxide-derived reactives species in vascular cells. Circ. Res. 2002, 91, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, V.; Rudolph, T.K.; Schopfer, F.J.; Bonacci, G.; Woodcock, S.R.; Cole, M.P.; Baker, P.R.S.; Ramani, R.; Freeman, B.A. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc. Res. 2010, 85, 155–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabatine, M.S.; Morrow, D.A.; O’Donoghue, M.; Jablonski, K.A.; Rice, M.M.; Solomon, S.; Rosenberg, Y.; Domanski, M.J.; Hsia, J. Prognostic utility of lipoprotein-associated phospholipase A2 for cardiovascular outcomes in patients with stable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2463–2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsimikas, S.; Willeit, J.; Knoflach, M.; Mayr, M.; Egger, G.; Notdurfter, M.; Witztum, J.L.; Wiedermann, C.J.; Xu, Q.; Kiechl, S. Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: Results from the Bruneck study. Eur. Heart J. 2009, 30, 107–115. [Google Scholar] [CrossRef]
- Mollenhauer, M.; Mehrkens, D.; Klinke, A.; Lange, M.; Remane, L.; Friedrichs, K.; Braumann, S.; Geißen, S.; Simsekyilmaz, S.; Nettersheim, F.S.; et al. Nitro-fatty acids suppress ischemic ventricular arrhythmias by preserving calcium homeostasis. Sci. Rep. 2020, 10, 15319. [Google Scholar] [CrossRef]
- Koenitzer, J.R.; Bonacci, G.; Woodcock, S.R.; Chen, C.-S.; Cantu-Medellin, N.; Kelley, E.E.; Schopfer, F.J. Fatty acid nitroalkenes induce resistance to ischemic cardiac injury by modulating mitochondrial respiration at complex II. Redox Biol. 2016, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Montuschi, P. Leukotrienes, antileukotrienes and asthma. Mini Rev. Med. Chem. 2008, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [Green Version]
- Montuschi, P.; Peters-Golden, M.L. Leukotriene modifiers for asthma treatment. Clin. Exp. Allergy 2010, 40, 1732–1741. [Google Scholar] [CrossRef]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Dennis, E.A.; Cao, J.; Hsu, Y.-H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, R.W.; Gale, P.H. Inhibition of mammalian 5-lipoxygenase by aromatic disulfides. J. Biol. Chem. 1985, 260, 11554–11559. [Google Scholar] [CrossRef]
- Awwad, K.; Steinbrink, S.D.; Frömel, T.; Lill, N.; Isaak, J.; Häfner, A.-K.; Roos, J.; Hofmann, B.; Heide, H.; Geisslinger, G.; et al. Electrophilic fatty acid species inhibit 5-lipoxygenase and attenuate sepsis-induced pulmonary inflammation. Antioxid. Redox Signal 2014, 20, 2667–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häfner, A.K.; Cernescu, M.; Hofmann, B.; Ermisch, M.; Hörnig, M.; Metzner, J.; Schneider, G.; Brutschy, B.; Steinhilber, D. Dimerization of human 5-lipoxygenase. Biol. Chem. 2011, 392, 1011–1097. [Google Scholar] [CrossRef] [Green Version]
- Maucher, I.V.; Rühl, M.; Kretschmer, S.B.M.; Hoffman, B.; Kühn, B.; Fettel, J.; Vogel, A.; Flügel, K.T.; Manolikakes, G.; Hellmuth, N.; et al. Michael acceptor containing drugs are a novel class of 5-lipoxygenase inhibitor targeting the surface cysteines C416 and C418. Biochem. Pharm. 2016, 25, 55–74. [Google Scholar] [CrossRef]
- Michalik, L.; Auwerx, J.; Berger, J.P.; Chatterjee, V.K.; Glass, C.K.; Gonzalez, F.J.; Grimaldi, P.A.; Kadowaki, T.; Lazar, M.A.; O’Rahilly, S.; et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 2006, 58, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Anastasi, M.R.; Zhang, V.J.; Russell, D.L.; Robker, R.L. Regulation of fatty acid oxidation in mouse cumulus-oocyte complexes during maturation and modulation by PPAR agonists. PLoS ONE 2014, 9, e87327. [Google Scholar]
- Belfiore, A.; Genua, M.; Malaguarnera, R. PPAR-gamma agonists and their effects on IGF-I receptor signaling: Implications for cancer. PPAR Res. 2009, 2009, 830501. [Google Scholar] [CrossRef] [Green Version]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef] [Green Version]
- Feige, J.N.; Gelman, L.; Michalik, L.; Desvergne, B.; Wahli, W. From molecular action to physiological outputs: Peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog. Lipid Res. 2006, 45, 120–159. [Google Scholar] [CrossRef]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARγ signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, E.-K.; Lee, Y.-R.; Kim, Y.-R.; Yeom, J.-Y.; Yoo, C.-H.; Kim, H.-K.; Park, H.-M.; Kang, H.-S.; Kim, J.-S.; Park, H.-M.; et al. NAADP mediates insulin-stimulated glucose uptake and insulin sensitization by PPARγ in adipocytes. Cell Rep. 2012, 2, 1607–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peluso, I.; Morabito, G.; Urban, L.; Ioannone, F.; Serafini, M. Oxidative stress in atherosclerosis development: The central role of LDL and oxidative burst. Endocr. Metab. Immune 2012, 12, 351–360. [Google Scholar] [CrossRef]
- Schopfer, F.J.; Cole, M.P.; Groeger, A.L.; Chen, C.S.; Khoo, N.K.; Woodcock, S.R.; Golin-Bisello, F.; Motanya, U.N.; Li, Y.; Zhang, J.; et al. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: Selective ligand activity and anti-diabetic signaling actions. J. Biol. Chem. 2010, 285, 12321–12333. [Google Scholar] [CrossRef] [Green Version]
- Villacorta, L.; Schopfer, F.J.; Zhang, J.; Freeman, B.A.; Chen, Y.E. PPAR-gamma and its ligands: Therapeutic implications in cardiovascular disease. Clin. Sci. 2009, 116, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Schopfer, F.J.; Lin, Y.; Baker, P.R.; Cui, T.; Garcia-Barrio, M.; Zhang, J.; Chen, K.; Chen, Y.E.; Freeman, B.A. Nitrolinoleic acid: An endogenous peroxisome proliferator-activated receptor gamma ligand. Proc. Natl. Acad. Sci. USA 2005, 102, 2340–2345. [Google Scholar] [CrossRef] [Green Version]
- Baker, P.R.; Schopfer, F.J.; Sweeney, S.; Freeman, B.A. Red cell membrane and plasma linoleic acid nitration products: Synthesis, clinical identification and quantitation. Proc. Natl. Acad. Sci. USA 2004, 101, 11577–11582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, H.; Jia, Z.; Guan, G.; Yang, T. Effects of endogenous PPAR agonist nitro-oleic acid on metabolic syndrome in obese zucker rats. PPAR Res. 2010, 2010, 601562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef]
- Ratziu, V. Pharmacological agents for NASH. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 676–685. [Google Scholar] [CrossRef]
- Khoo, K.H.; Fazzari, M.; Chartoumpekis, D.; Li, L.; Guimaraes, D.A.; Arteel, G.E.; Shiva, S.; Freeman, B.A. Electrophilic nitro-oleic acid reverses obesity-induced hepatic steatosis. Redox Biol. 2019, 22, 101132. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Xu, G.; Guo, Y.; Zhu, Y.; Wang, H.; Zhang, J.; Fan, Y.; Liang, W.; Lu, H.; Liu, Y.; et al. Nitro-fatty acids protect against steatosis and fibrosis during development of nonalcoholic fatty liver disease in mice. EBioMedicine 2019, 14, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Arbeeny, C.M.; Ling, H.; Smith, M.M.; O’Brien, S.; Wawersik, S.; Ledbetter, S.R.; McAlexander, A.; Schopfer, F.J.; Wilette, R.N.; Jorkasky, D.K. CXA-10, a nitrated fatty acid, is renoprotective in deoxycorticosterone acetate-salt nephropathy. J. Pharmacol. Exp. Ther. 2019, 369, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Nie, H.; Xue, X.; Li, J.; Liu, X.; Shasha, L.; Guan, G.; Liu, H.; Liu, S.; Chen, Z. Nitro-oleic acid attenuates OGD/R-triggered apoptosis in renal tubular cells via inhibition of Bax mitochrondrial translocation in a PPAR-γ-dependent manner. Cell Physiol. Biochem. 2015, 35, 1201–1218. [Google Scholar] [CrossRef]
- Schopfer, F.J.; Vitturi, D.A.; Jorkasky, D.K.; Freeman, B.A. Nitro-fatty acids: New drug candidates for chronic inflammatory and fibrotic diseases. Nitric Oxide 2018, 79, 31–37. [Google Scholar] [CrossRef]
- Gorczynski, M.J.; Smitherman, P.K.; Akiyama, T.E.; Wood, H.B.; Berger, J.P.; King, B.S.; Morrow, C.S. Activation of Peroxisome Proliferator-Activated Receptor γ (PPARγ) by nitroalkene fatty acids: Importance of nitration position and degree of unsaturation. J. Med. Chem. 2009, 52, 4631–4639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, R.L.; Wright, M.W.; Gorcjynski, M.J.; Smitherman, P.K.; Akiyama, T.E.; Wood, H.B.; Berger, J.P.; King, B.S.; Morrow, C.S. Differential potencies of naturally occurring regio-isomers of nitrolinoleic acid in PPARγ activation. Biochemistry 2009, 48, 492–498. [Google Scholar] [CrossRef]
- Remels, A.H.V.; Langen, R.C.J.; Gosker, H.R.; Russell, A.P.; Spaapen, F.; Voncken, J.W.; Schrauwen, P.; Schols, W.J. PPARγ inhibits NF-κB dependent transcriptional activity in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 174–183. [Google Scholar] [CrossRef]
- Friedman, J.E.; Kirwan, J.P.; Jing, M.; Presley, L.; Catalano, P.M. Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes 2008, 57, 606–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lappas, M.; Permezel, M.; Rice, G.E. Release of proinflammatory cytokines and 8-isoprostane from placenta, adipose tissue, and skeletal muscle from normal pregnant women and women with gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2004, 89, 5627–5633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriwijitkamol, A.; Christ-Roberts, C.; Berria, R.; Eagan, P.; Pratipanawatr, T.; DeFronzo, R.A.; Mandarino, L.J.; Musi, N. Reduced skeletal muscleinhibitor of κBβcontent is associated with insulin resistance insubjects with type 2 diabetes: Reversal by exercise training. Diabetes 2006, 55, 760–767. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Chang, Z.; Zhao, G.; Lu, H.; Xiong, W.; Liang, W.; Wang, H.; Villacorta, L.; Garcia-Barrio, M.T.; Zhu, T.; et al. Suppression of vascular macrophage activation by nitro-oleic acid and its implication for abdominal aortic aneurysm therapy. Cardiovasc. Drugs Ther. 2021, 35, 939–951. [Google Scholar] [CrossRef]
- Karin, M.; Cao, Y.; Greten, F.R.; Li, Z.-W. NF-κB in cancer: From innocent bystander to major culprit. Nat. Rev. 2002, 2, 301–310. [Google Scholar] [CrossRef]
- Grommes, C.; Landreth, G.E.; Heneka, M.T. Antineoplastic effects of peroxisome proliferator-activated receptor γ agonists. Lancet Oncol. 2004, 5, 419–429. [Google Scholar] [CrossRef]
- Theocharis, S.; Margeli, A.; Vielh, P.; Kouraklis, G. Peroxisome proliferator activated receptor-γ ligands as cell cycle modulators. Cancer Treat. Rev. 2004, 30, 545–554. [Google Scholar] [CrossRef]
- Tachibana, K.; Yamasaki, D.; Ishimoto, K.; Doi, T. The role of PPARs in cancer. PPAR Res. 2008, 2008, 102737. [Google Scholar] [CrossRef] [Green Version]
- Schopfer, F.J.; Cipollina, C.; Freeman, B.A. Formation and signaling actions of electrophilic lipids. Chem. Rev. 2012, 111, 5997–6021. [Google Scholar] [CrossRef] [Green Version]
- Ritossa, F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Matz, J.M.; Blake, M.J.; Tatelman, H.M.; Lavoi, K.P.; Holbrook, N.J. Characterization and regulation of cold-induced heat shock protein expression in mouse brown adipose tissue. Am. J. Physiol. 1995, 269, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ohwatari, N.; Matsumoto, T.; Kosaka, M.; Ohtsuru, A.; Yamashita, S. TGF-beta1 mediates 70-kDa heat shock protein induction due to ultraviolet irradiation in human skin fibroblasts. Pflüg. Arch. 1999, 438, 239–244. [Google Scholar] [CrossRef]
- Laplante, A.F.; Moulin, V.; Auger, F.A.; Landry, J.; Li, H.; Morrow, G.; Tanguay, R.M.; Germain, L. Expression of heat shock proteins in mouse skin during wound healing. J. Histochem. Cytochem. 1998, 46, 1291–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles, R.L.; Rudyk, O.; Prysyazhna, O.; Kamynina, A.; Yang, J.; Morisseau, C.; Hammock, B.D.; Freeman, B.A.; Eaton, P. Protection from hypertension in mice by the Mediterranean diet is mediated by nitro fatty acid inhibition of soluble epoxide hydrolase. Proc. Natl. Acad. Sci. USA 2014, 111, 8167–8172. [Google Scholar] [CrossRef] [Green Version]
- Kelley, E.E.; Batthyany, C.I.; Hundley, N.J.; Woodcock, S.R.; Bonacci, G.; Del Rio, J.M.; Schopfer, F.J.; Lancaster, J.R., Jr.; Freeman, B.A.; Tarpey, M.M. Nitro-oleic acid, a novel and irreversible inhibitor of xanthine oxidoreductase. J. Biol. Chem. 2008, 283, 36176–36184. [Google Scholar] [CrossRef] [Green Version]
- Kelley, E.E.; Khoo, N.K.; Hundley, N.J.; Malik, U.Z.; Freeman, B.A.; Tarpey, M.M. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 2010, 48, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, E.E.; Trostchansky, A.; Rubbo, H.; Freeman, B.A.; Radi, R.; Tarpey, M.M. Binding of xanthine oxidase to glycosaminoglycans limits inhibition by oxypurinol. J. Biol. Chem. 2004, 279, 37231–37234. [Google Scholar] [CrossRef] [Green Version]
- Freeman, A.B.; Pekarova, M.; Rubbo, H.; Trostchansky, A. Electrophilic nitro-fatty acids: Nitric oxide and nitrite-derived metabolic and inflammatory signaling mediators. Nitric Oxide 2017, 213–229. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxi. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Buchan, G.J.; Rühl, M.; Mukai, K.; Salvatore, S.R.; Ogawa, E.; Andersen, S.D.; Iversen, M.B.; Thielke, A.L.; Gunderstofte, C.; et al. Nitro-fatty acids are formed in response to virus infection and are potent inhibitors of STING palmitoylation and signaling. Proc. Natl. Acad. Sci. USA 2018, 115, 7768–7775. [Google Scholar] [CrossRef] [Green Version]
- Carreño, M.; Bresque, M.; Machado, M.R.; Santos, L.; Durán, R.; Vitturi, D.A.; Escande, C.; Denicola, A. Nitro-fatty acids as activators of hSIRT6 deacetylase activity. J. Biol. Chem. 2020, 295, 18355–18366. [Google Scholar] [CrossRef]
- Pereckova, J.; Pekarova, M.; Szamecova, N.; Hoferova, Z.; Kamarytova, K.; Falk, M.; Perecko, T. Nitro-oleic acid inhibits stemness maintenance and enhances neural differentiation of mouse embryonic stem cells via STAT3 signaling. Int. J. Mol. Sci. 2021, 22, 9981. [Google Scholar] [CrossRef]
- Wang, P.; Killeen, M.E.; Sumpter, T.L.; Ferris, L.K.; Falo, L.D., Jr.; Freeman, B.A.; Schopfer, F.J.; Mathers, A.R. Electrophilic nitro-fatty acids suppress psoriasiform dermatitis: STAT3 inhibition as a contributory mechanism. Redox Biol. 2021, 43, 101987. [Google Scholar] [CrossRef] [PubMed]
- Vitturi, D.A.; Chen, C.S.; Woodcock, S.R.; Salvatore, S.R.; Bonacci, G.; Koenitzer, J.R.; Stewart, N.A.; Wakabayashi, N.; Kensler, T.W.; Freeman, B.A.; et al. Modulation of nitro-fatty acid signaling: Prostaglandin reductase-1 is a nitroalkene reductase. J. Biol. Chem. 2013, 288, 25626–25637. [Google Scholar] [CrossRef] [Green Version]
- D’Ischia, M. Oxygen-dependent nitration of ethyl linoleate with nitric oxide. Tetrahedron Lett. 1996, 37, 5773–5774. [Google Scholar] [CrossRef]
- Pryor, W.A.; Lightsey, J.W.; Church, D.F. Reaction of nitrogen dioxide with alkenes and polyunsaturated fatty acids: Addition and hydrogen-abstraction mechanisms. J. Am. Chem. Soc. 1982, 104, 6685–6692. [Google Scholar] [CrossRef]
- Napotalino, A.; Camera, E.; Picardo, M.; D’Ischia, M. Acid-promoted reactions of ethyl linoleate with nitrite ions: Formation and structural characterization of isomeric nitroalkene, nitrohydroxy, and novel 3-Nitro-1,5-hexadiene and 1,5-dinitro-1,3-pentadiene products. J. Org. Chem. 2000, 65, 4583–4860. [Google Scholar]
- Manini, P.; Capelli, L.; Reale, S.; Arzillo, M.; Crescenzi, O.; Napolitano, A.; Barone, V.; D’Ischia, M. Chemistry of nitrated lipids: Remarkable instability of 9-nitrolinoleic acid in neutral aqueous medium and a novel nitronitrate ester product by concurrent autoxidation/nitric oxide-release pathways. J. Org. Chem. 2008, 73, 7517–7525. [Google Scholar] [CrossRef]
- Woodcock, S.R.; Bonacci, G.; Gelhaus, S.L.; Schopfer, F.J. Nitrated fatty acids: Synthesis and measurement. Free Radic. Biol. Med. 2013, 59, 14–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayama, T.; Tomoda, S.; Takeuchi, Y.; Nomura, Y. Synthesis of 2-nitroalkyl phenyl selenides and their conversion to nitroalkenes. Chem. Lett. 1982, 11, 1109–1112. [Google Scholar] [CrossRef]
- Acevedo, C.M.; Kogut, E.F.; Lipton, M.A. Synthesis and analysis of the sterically constrained L-glutamine analogues (3S,4R)-3,4-dimethyl-L-glutamine and (3S,4R)-3,4-dimethyl-L-pyroglutamic acid. Tetrahedron 2001, 57, 6353–6359. [Google Scholar] [CrossRef]
- Hassan, M.; Nde, C.N.; Manolikakes, G. Synthesis of nitroolefins via the direct nitration of alkenes. SynOpen 2021, 5, 229–231. [Google Scholar]
- Gorczynski, M.J.; Huang, J.; King, B. Regio- and Stereospecific syntheses and nitric oxide donor properties of (E)-9 and (E)-10-nitrooctadec-9-enoic acids. Org. Lett. 2006, 8, 2305–2308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodcock, S.R.; Marwitz, A.J.V.; Bruno, P.; Branchaud, B.P. Synthesis of nitrolipids. All four possible diastereomers of nitrooleic acids: (E)- and (Z)-, 9- and 10-nitro-octadec-9-enoic acids. Org. Lett. 2006, 8, 3931–3934. [Google Scholar] [CrossRef]
- Fioravanti, S.; Pellacani, L.; Tardella, P.A.; Vergari, M.C. Facile and highly stereoselective one-pot synthesis of either (E)- or (Z)-nitro alkenes. Org. Lett. 2008, 10, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, G.; Bendjeddou, M.V.L.; Porta, A.; Bruno, P.; Vidari, G. Improved synthesis of (E)-12-nitrooctadec-12-enoic acid, a potent PPARγ activator. Development of a “Buffer-free” enzymatic method for hydrolysis of methyl esters. J. Org. Chem. 2010, 75, 8311–8314. [Google Scholar] [CrossRef]
- Uppenberg, J.; Hansen, H.P.; Patkar, S.; Jones, T.A. The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida Antarctica. Structure 1994, 2, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Kirk, O.; Christensen, M.W. Lipases from Candida antarctica: Unique biocatalysts from a unique origin. Org. Proc. Res. Dev. 2002, 6, 446–451. [Google Scholar] [CrossRef]
- Branco, R.J.F.; Graber, M.; Denis, V.; Pleiss, J. Molecular mechanism of the hydration of Candida antarctica lipase B in gas phase: Water adsorption isotherms and molecular dynamics simulations. ChemBioChem 2009, 10, 2913–2919. [Google Scholar] [CrossRef] [Green Version]
- Hock, K.J.; Grimmer, J.; Göbel, D.; Gasaya, G.G.T.; Roos, J.; Maucher, I.V.; Kühn, B.; Fettel, J.; Maier, T.J.; Manolikakes, G. Modular regiospecific synthesis of nitrated fatty acids. Synthesis 2016, 49, 615–636. [Google Scholar]
- Hassan, M.; Krieg, S.-C.; Nde, C.-N.; Roos, J.; Maier, T.J.; El Rady, E.A.; Raslan, M.A.; Sadek, K.U.; Manolikakes, G. Streamlined One-pot synthesis of nitro fatty acids. Eur. J. Org. Chem. 2021, 15, 2239–2252. [Google Scholar] [CrossRef]
- Woodcock, S.R.; Salvatore, S.R.; Freeman, B.A.; Schopfer, F.J. Synthesis of 9- and 12-nitro conjugated linoleic acid: Regiospecific isomers of naturally occurring conjugated nitrodienes. Tetrahedron Lett. 2021, 81, 153371. [Google Scholar] [CrossRef]
- Fang, M.Y.; Huang, K.-H.; Tu, W.-J.; Chen, Y.-T.; Pan, P.-Y.; Hsiao, W.-C.; Ke, Y.Y.; Tsou, L.K.; Zhang, M.M. Chemoproteomic profiling reveals cellular targets of nitro-fatty acids. Redox Biol. 2021, 46, 102126. [Google Scholar] [CrossRef]










| Compound | IC50 (μM) | Ref. | |
|---|---|---|---|
| PPARγ | PPARα | ||
| Rosiglitazone | 0.25 | >15 | [75] |
| E-12-NO2-18:2 | 0.41 | 9.6 | [75] |
| E-9-NO2-18:1 | 0.98 | >15 | [74] |
| E-10-NO2-18:1 | 1.61 | >15 | [74] |
| Z-9/10-NO2-18:1 | 1.73 | 10.8 | [74] |
| E-9-NO2-16:1 | 0.83 | >15 | [74] |
| E-10-NO2-16:1 | 0.63 | >15 | [74] |
| E-5-NO2-18:1 | 1.68 | >15 | [74] |
| E-6-NO2-18:1 | 1.72 | >15 | [74] |
| E-12-NO2-18:1 | 0.039 | 1.6 | [74] |
| E-13-NO2-18:1 | 0.19 | 1.1 | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutoulogenis, G.S.; Kokotos, G. Nitro Fatty Acids (NO2-FAs): An Emerging Class of Bioactive Fatty Acids. Molecules 2021, 26, 7536. https://doi.org/10.3390/molecules26247536
Koutoulogenis GS, Kokotos G. Nitro Fatty Acids (NO2-FAs): An Emerging Class of Bioactive Fatty Acids. Molecules. 2021; 26(24):7536. https://doi.org/10.3390/molecules26247536
Chicago/Turabian StyleKoutoulogenis, Giorgos S., and George Kokotos. 2021. "Nitro Fatty Acids (NO2-FAs): An Emerging Class of Bioactive Fatty Acids" Molecules 26, no. 24: 7536. https://doi.org/10.3390/molecules26247536






