A Combined Experimental/Quantum-Chemical Study of Tetrel, Pnictogen, and Chalcogen Bonds of Linear Triatomic Molecules
Abstract
:1. Introduction
2. Methodology and Experimental and Computational Details
2.1. Infrared Spectroscopy
2.1.1. Methodology
2.1.2. Experimental Details
2.2. Theory and Computational Details
3. Results
3.1. Infrared Spectroscopy
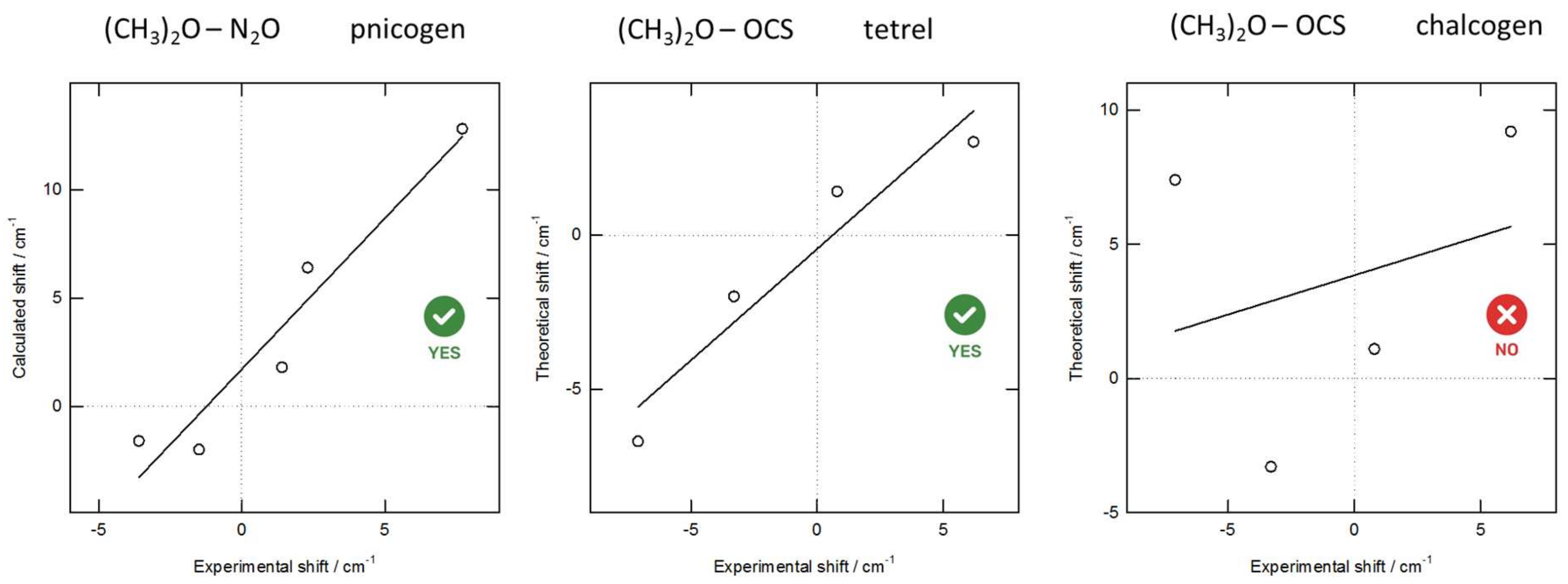
3.2. Ab Initio and DFA-Based Complexation Energies
4. Discussion
4.1. The Quadrupole-Dipole Model
4.2. Molecular Electrostatic Potential
4.3. Energy Decomposition Analysis
4.4. Bonding Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, H.J. Binding Mechanisms in Supramolecular Complexes. Angew. Chem. Int. Ed. 2009, 48, 3924–3977. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.S.; Rice, S.A.; Ross, J. Physical Chemistry. J. Chem. Educ. 1980. Available online: https://pubs.acs.org/doi/pdf/10.1021/ed058pA312.2 (accessed on 7 November 2021).
- Pauling, L. The Nature of the Chemical Bond; Cornell University Press: New York, NY, USA, 1960. [Google Scholar]
- Pimentel, G.C.; Mc Clellan, A.L. The Hydrogen Bond; W.H. Freeman: San Francisco, CA, USA, 1960; Available online: http://garfield.library.upenn.edu/classics1982/A1982PC81900001.pdf (accessed on 7 November 2021).
- Schuster, P.; Zundel, G.; Sandorfy, C. The Hydrogen Bond: Recent Developments in Theory and Experiments; North-Holland Publishing Company: Amsterdam, NY, USA, 1976; Available online: https://pubs.acs.org/doi/pdf/10.1021/ed055pA244.3 (accessed on 7 November 2021).
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond: Outline of a Comprehensive Hydrogen Bond.-Theory; Oxford Science Publications: Oxford, UK, 2009. [Google Scholar]
- Scheiner, S. Forty Years of Progress in the Study of the Hydrogen Bond. Struct. Chem. 2009, 30, 1119–1128. [Google Scholar] [CrossRef]
- Scheiner, S. The Hydrogen Bond: A Hundred Years and Counting. J. Indian Inst. Sci. 2020, 100, 61–76. [Google Scholar] [CrossRef]
- Alonso, M.; Woller, T.; Martin-Martinez, F.J.; Contreras-Garcia, J.; Geerlings, P.; De Proft, F. Understanding the Fundamental Role of π/π, σ/σ, and σ/π Dispersion Interactions in Shaping Carbon-Based Materials. Chem. Eur. J. 2014, 20, 4931–4941. [Google Scholar] [CrossRef]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.G.; Murray, J.S. An Overview of Halogen Bonding. J. Mol. Mod. 2007, 13, 305–311. [Google Scholar] [CrossRef]
- Metrangolo, P.; Resnati, G. Halogen Bonding: Fundamentals and Applications; Springer: Berlin, Germany, 2008. [Google Scholar]
- Legon, A.C. The Halogen Bond: An Interim Perspective. Phys. Chem. Chem. Phys. 2010, 12, 7736–7747. [Google Scholar] [CrossRef]
- Cavallo, C.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.J. The Halogen Bond: Nature and Applications. Chemical Synergies; De Gruyter: Berlin, Germany, 2017. [Google Scholar]
- Bonaccorsi, R.; Scrocco, E.; Tomasi, J. Molecular SCF Calculations for the Ground State of Some Three-Membered Ring Molecules: (CH2)3, (CH2)2NH, (CH2)2NH2+, (CH2)2O, (CH2)2S, (CH)2CH2, and N2CH2. J. Chem. Phys. 1970, 52, 5270–5284. [Google Scholar] [CrossRef]
- Brinck, T.; Murray, J.S.; Politzer, P. Surface Electrostatic Potentials of Halogenated Methanes as Indicators of Directional Intermolecular Interactions. Int. J. Quant. Chem. 1992, 44, 57–64. [Google Scholar] [CrossRef]
- Clark, T.; Henneman, M.; Murray, J.S.; Politzer, P. Halogen Bonding: The Sigma-Hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen Bonding: An Electrostatically-Driven Highly Directional Noncovalent Interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Halogen Bonding: An Interim Discussion. Chem. Phys. Chem. 2013, 14, 278–294. [Google Scholar] [CrossRef]
- Pelletier, P.; Caventou, J. Mémoire sur un Nouvel Alcali Vegetal (la Strychnine). Ann. Chim. Phys. 1819, 10, 142–177. [Google Scholar]
- Legon, A.C. π-Electron “Donor–Acceptor” Complexes B⋅⋅⋅ClF and the Existence of the “Chlorine Bond”. Chem. Eur. J. 1998, 4, 1890–1897. [Google Scholar] [CrossRef]
- Legon, A.C. Prereactive Complexes of Dihalogens XY with Lewis Bases B in the Gas Phase: A Systematic Case for the Halogen Analogue B···XY of the Hydrogen Bond B···HX. Angew. Chem. Int. Ed. 1999, 38, 2686–2714. [Google Scholar] [CrossRef]
- Hauchecorne, D.; Szostak, R.; Herrebout, W.A.; Van der Veken, B.J. C–X···O Halogen Bonding: Interactions of Trifluoromethyl Halides with Dimethyl Ether. Chem. Phys. Chem. 2009, 10, 2105–2115. [Google Scholar] [CrossRef]
- Hauchecorne, D.; Van de Veken, B.J.; Moiana, A.; Herrebout, W.A. The C–Cl···N Halogen Bond, the Weaker Relative of the C–I and C–Br⋯N Halogen Bonds, Finally Characterized in Solution. Chem. Phys. 2010, 374, 30–36. [Google Scholar] [CrossRef]
- Hauchecorne, D.; Molana, A.; Van der Veken, B.J.; Herrebout, W.A. Halogen Bonding to a Divalent Sulfur Atom: An Experimental Study of the Interactions of CF3X (X = Cl, Br, I) with Dimethyl Sulfide. Phys. Chem. Chem. Phys. 2011, 13, 10204–10213. [Google Scholar] [CrossRef]
- Hauchecorne, D.; Nagels, N.; Van der Veken, B.J.; Herrebout, W.A. C–X···π halogen and C–H···π Hydrogen Bonding: Interactions of CF3X (X = Cl, Br, I or H) with Ethene and Propene. Phys. Chem. Chem. Phys. 2012, 14, 681–690. [Google Scholar] [CrossRef]
- Nagels, N.; Hauchecorne, D.; Herrebout, W.A. Exploring the C-X···π Halogen Bonding Motif: An Infrared and Raman Study of the Complexes of CF3X (X = Cl, Br and I) with the Aromatic Model Compounds Benzene and Toluene. Molecules 2013, 18, 6829–6851. [Google Scholar] [CrossRef]
- Nagels, N.; Geboes, Y.; Pinter, B.; De Proft, F. Tuning the Halogen/Hydrogen Bond Competition: A Spectroscopic and Conceptual DFT Study of Some Model Complexes Involving CHF2I. Chem. Eur. J. 2014, 20, 8433–8443. [Google Scholar] [CrossRef]
- Geboes, Y.; Nagels, N.; Pinter, B.; De Proft, F.; Herrebout, W.A. Competition of C(sp2)–X···O Halogen Bonding and Lone Pair···π Interactions: Cryospectroscopic Study of the Complexes of C2F3X (X = F, Cl, Br, and I) and Dimethyl Ether. J. Phys. Chem. A 2015, 119, 2502–2516. [Google Scholar] [CrossRef]
- Geboes, Y.; De Proft, F.; Herrebout, W.A. Expanding Lone Pair···π Interactions to Nonaromatic Systems and Nitrogen Bases: Complexes of C2F3X (X = F, Cl, Br, I) and TMA-d9. J. Phys. Chem. A 2015, 119, 5597–5606. [Google Scholar] [CrossRef]
- Geboes, Y.; De Proft, F.; Herrebout, W.A. Lone pair⋯π interactions involving carbonyl π-systems: Experimental and theoretical study of the complexes of COF2 and COFCl with dimethyl ether. Chem. Phys. 2016, 476, 1–8. [Google Scholar] [CrossRef]
- Geboes, Y.; De Proft, F.; Herrebout, W.A. Effect of Fluorination on the Competition of Halogen Bonding and Hydrogen Bonding: Complexes of Fluoroiodomethane with Dimethyl Ether and Trimethylamine. J. Phys. Chem. A 2017, 121, 4180–4188. [Google Scholar] [CrossRef]
- Geboes, Y.; De Proft, F.; Herrebout, W.A. Taking the Halogen Bonding–Hydrogen Bonding Competition One Step Further: Complexes of Difluoro Iodo Methane with Tri Methyl Phosphine, Dimethyl Sulfide and Chloro Methane. Acta. Cryst. B 2017, 73, 168–178. [Google Scholar] [CrossRef]
- Geboes, Y.; De Proft, F.; Herrebout, W.A. Towards a Better Understanding of the Parameters Determining the Competition Between Bromine Halogen Bonding and Hydrogen Bonding: An FTIR Spectroscopic Study of the Complexes Between Bromodifluoromethane and Trimethylamine. J. Mol. Struct. 2018, 1165, 349–355. [Google Scholar] [CrossRef]
- De Vleeschouwer, F.; Denayer, M.; Pinter, B.; Geerlings, P.; De Proft, F. Characterization of Chalcogen Bonding Interactions via an In-Depth Conceptual Quantum Chemical Analysis. J. Comp. Chem. 2018, 39, 557–572. [Google Scholar] [CrossRef]
- Vogel, L.; Wonner, P.; Huber, S.M. Chalcogen Bonding: An Overview. Angew. Chem. Int. Ed. 2019, 58, 1880–1891. [Google Scholar] [CrossRef]
- Shuklaa, R.; Chopra, D. Chalcogen and Pnictogen Bonds: Insights and Relevance. Curr. Sci. 2021, 120, 1848–1853. [Google Scholar]
- Gromila, R.M.; Mooibroek, T.J.; Frontera, A. Hot Topics in Crystal Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Scheiner, S. The Pnicogen Bond: Its Relation to Hydrogen, Halogen, and Other Noncovalent bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. Pnicogen and Tetrel bonds–Tetrahedral Lewis Acid Centers. Struct. Chem. 2019, 30, 1141–1152. [Google Scholar] [CrossRef]
- Geboes, Y.; De Vleeschouwer, F.; De Proft, F.; Herrebout, W.A. Exploiting the σ-Hole Concept: An Infrared and Raman based Characterization of the S···O Chalcogen Bond Between 2,2,4,4-Tetrafluoro-1,3-Dithiethane and Dimethyl Ether. Chem. Eur. J. 2017, 23, 17384–17392. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Frontera, A. Not Only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Bauzá, A.; Mooibroek, T.J.; Frontera, A. Tetrel-Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef] [PubMed]
- Mani, D.; Arunan, E. The X–C⋯Y (X = O/F, Y = O/S/F/Cl/Br/N/P) ‘Carbon Bond’ and Hydrophobic Interactions. Phys. Chem. Chem. Phys. 2013, 15, 14377–14383. [Google Scholar] [CrossRef]
- Fraser, G.T.; Leopold, K.R.; Klemperer, W. Ammonia Dimer: A Surprising Structure. J. Chem. Phys. 1984, 81, 2577–2584. [Google Scholar] [CrossRef]
- Newby, J.J.; Peebles, R.A.; Peebles, S.A. The Dimethyl Ether-OCS Dimer: Rotational Spectrum, Structure and Ab Initio Calculations. J. Phys. Chem. A 2004, 108, 7372–7378. [Google Scholar] [CrossRef]
- Newby, J.J.; Peebles, R.A.; Peebles, S.A. Structure of the Dimethyl Ether−CO2 van der Waals Complex from Microwave Spectroscopy. J. Phys. Chem. A 2004, 108, 11234–11240. [Google Scholar] [CrossRef]
- Buckingham, A.D. Molecular Quadrupole Moments. Q. Rev. Chem. Soc. 1959, 13, 183–214. [Google Scholar] [CrossRef]
- Buckingham, A.D. Intermolecular Interactions: From Diatomics to Biopolymers; Pullmann, B., Ed.; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Esrafili, M.D.; Mousavian, P. Strong Tetrel Bonds: Theoretical Aspects and Experimental Evidence. Molecules 2018, 23, 2642. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Li, Q.; Scheiner, S. Comparative Strengths of Tetrel, Pnicogen, Chalcogen, and Halogen Bonds and Contributing Factors. Molecules 2018, 23, 1681. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, S. Origins and Properties of the Tetrel Bond. Phys. Chem. Chem. Phys. 2021, 23, 5702–5717. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. On the Calculation of Bonding Energies by the Hartree Fock Slater Method. I. The Transition State Method. Theor. Chim. Acta. 1977, 46, 1–10. [Google Scholar] [CrossRef]
- Ziegler, T.; Rauk, A. A Theoretical Study of the Ethylene-Metal Bond in Complexes Between Copper(1+), Silver(1+), Gold(1+), Platinum(0) or Platinum(2+) and Ethylene, Based on the Hartree-Fock-Slater Transition-State Method. Inorg. Chem. 1979, 18, 1558–1565. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of Chemical Bonds Based on Topological Analysis of Electron Localization Functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Legon, A.C. Tetrel, Pnictogen and Chalcogen Bonds Identified in the Gas Phase Before They Had Names: A Systematic Look at Non-Covalent Interactions. Phys. Chem. Chem. Phys. 2017, 19, 14884–14896. [Google Scholar] [CrossRef]
- Alkorta, I.; Legon, A.C. An Ab Initio Investigation of the Geometries and Binding Strengths of Tetrel-, Pnictogen-, and Chalcogen-Bonded Complexes of CO2, N2O, and CS2 with Simple Lewis Bases: Some Generalizations. Molecules 2018, 23, 2250. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Zhang, J.; Gou, Q.; Feng, G. Structure and C⋯N Tetrel-Bonding of the Isopropylamine–CO2 Complex Studied by Microwave Spectroscopy and Theoretical Calculations. Phys. Chem. Chem. Phys. 2020, 22, 8467–8475. [Google Scholar] [CrossRef]
- Van Ginderen, P.; Herrebout, W.A.; van der Veken, B.J. van der Waals Complex of Dimethyl Ether with Carbon Dioxide. J. Phys. Chem. A 2003, 107, 5391–5396. [Google Scholar] [CrossRef]
- Herrebout, W.A.; Nagels, N.; van der Veken, B.J. On the ν1 CO2/2 ν2 CO2 Resonance in the Complex of Carbon Dioxide with Dimethyl Ether. Chem. Phys. Chem. 2009, 10, 3054–3060. [Google Scholar] [CrossRef]
- Mitoraj, P.M.; Michalak, A. Natural Orbitals for Chemical Valence as Descriptors of Chemical Bonding in Transition Metal Complexes. J. Mol. Model. 2007, 13, 347–355. [Google Scholar] [CrossRef]
- Mitoraj, M.; Michalak, A.; Ziegler, T. A Combined Charge and Energy Decomposition Scheme for Bond Analysis. J. Chem. Theor. Comp. 2009, 5, 962–975. [Google Scholar] [CrossRef]
- Dyduch, K.; Mitoraj, M.P.; Michalak, A. ETS-NOCV Description of σ-Hole Bonding. J. Mol. Model. 2013, 19, 2747–2758. [Google Scholar] [CrossRef] [Green Version]
- Herrebout, W.A.; van der Veken, B.J. Physics and Chemistry at Low Temperatures; Khriachtchev, L., Ed.; Jenny Stanford Publishing: Singapore, 2011. [Google Scholar]
- Herrebout, W.A. Halogen Bonding I: Impact on Materials Chemistry and Life Sciences; Springer: New York, NY, USA, 2015. [Google Scholar]
- Wilcox, S.; Herrebout, W.A.; Erdélyi, M. Halogen Bonding in Solution; Hüber, S., Ed.; Wiley: Hoboken, NJ, USA, 2021; pp. 153–194. [Google Scholar]
- Van der Veken, B.J. Measurement of Enthalpy Differences in Cryosolutions: Influence of Thermal Expansion. J. Phys. Chem. 1996, 100, 17436–17438. [Google Scholar] [CrossRef]
- Hehre, W.J.; Radom, L.; Schleyer, P.V.R.; Pople, J.A. Ab Initio Molecular Orbital Theory; John Wiley & sons Ltd.: New York, NY, USA, 1986. [Google Scholar]
- Available online: https://s3-eu-west-1.amazonaws.com/pstorage-acs-6854636/3955375/jp500312r_si_001.pdf (accessed on 7 November 2021).
- Boys, S.F.; Bernardi, F. The Calculation of Small Molecular Interactions by the Differences of Separate Total Energies. Some Procedures with Reduced Errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Van Duijneveldt, F.B.; van Duijneveldt-van de Rijdt, J.G.C.M.; Van Lenthe, J.H. State of the Art in Counterpoise Theory. Chem. Rev. 1994, 94, 1873–1885. [Google Scholar] [CrossRef]
- Salvador, P.; Szczesniak, M.M. Counterpoise-Corrected Geometries and Harmonic Frequencies of N-body Clusters: Application to (HF)n (n = 3,4). J. Chem. Phys. 2003, 118, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.scirp.org/(S(lz5mqp453ed%20snp55rrgjct55))/reference/referencespapers.aspx?referenceid=2418053 (accessed on 7 November 2021).
- Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron Through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian-Basis Sets for Use in Correlated Molecular Calculations. 3. The Atoms Aluminum Through Argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, C. Methods in Computational Physics; Alder, B.J., Ed.; Academic: New York, NY, USA, 1963; Volume 2. [Google Scholar]
- Martin, J.M.L. Energetics of Stable Molecules and Reactive Intermediates; Minas da Piedade, M.E., Ed.; Kluwer Academic Publishers: New York, NY, USA, 1999. [Google Scholar]
- Feller, D. Application of Systematic Sequences of Wave Functions to the Water Dimer. J. Chem. Phys. 1992, 96, 6104–6114. [Google Scholar] [CrossRef]
- Feller, D. The Use of Systematic Sequences of Wave Functions for Estimating the Complete Basis Set, Full Configuration Interaction Limit in Water. J. Chem. Phys. 1993, 98, 7059–7071. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parameterization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods Without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comp. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- ADF 2020.102, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: http://www.scm.com (accessed on 7 November 2021).
- Van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic Regular Two-Component Hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. [Google Scholar] [CrossRef]
- Van Lenthe, E.; van Leeuwen, R.; Baerends, E.J.; Snijders, J.G. Relativistic Regular Two-Component Hamiltonians. Int. J. Quant. Chem. 1996, 57, 281–293. [Google Scholar] [CrossRef]
- Jorgensen, W.L. BOSS-Biochemical and Organic Simulation System. Encycl. Comput. Chem. 1998, 5, 3281–3285. [Google Scholar]
- NIST Chemistry Webbook. Available online: https://webbook.nist.gov/chemistry (accessed on 8 November 2021).
- Graham, C.; Imrie, D.A.; Raab, R.E. Measurement of the electric quadrupole moments of CO2, CO, N2, Cl2 and BF3. Mol. Phys. 1998, 93, 49–56. [Google Scholar] [CrossRef]
- Watson, J.; Craven, I.E.; Ritchie, G.L.D. Temperature Dependence of Electric Field-Gradient Induced Birefringence in Carbon Dioxide and Carbon Disulfide. Chem. Phys. Lett. 1997, 274, 1–6. [Google Scholar] [CrossRef]
- Reinartz, J.M.L.J.; Meerts, W.L.; Dymanus, A. Hyperfine Structure, Electric and Magnetic Properties of 14N216O. Chem. Phys. 1978, 31, 19–29. [Google Scholar] [CrossRef]
- NIST Triatomic Spectral Database. Available online: http://www.physics.nist.gov/PhysRefData/MolSpec/Triatomic/index.html (accessed on 7 November 2021).
- De Leeuw, F.H.; Dymanus, A. Electric and Magnetic Properties of OCS Measured by Molecular-Beam Electric-Resonance Spectroscopy. Chem. Phys. Lett. 1970, 7, 288–292. [Google Scholar] [CrossRef]
- Zolandz, Z.; Yaron, D.; Peterson, K.I.; Klemperer, W. Water in Weak Interactions: The Structure of the Water-Nitrous Oxide Complex. J. Chem. Phys. 1992, 97, 2861–2868. [Google Scholar] [CrossRef]
- Fraser, G.T.; Nelson, D.D., Jr.; Gerfen, G.J.; Klemperer, W. The Rotational spectrum, Barrier to Internal Rotation, and Structure of NH3-N2O. J. Chem. Phys. 1985, 83, 5442–5449. [Google Scholar] [CrossRef]
- Gougoula, E.; Medcraft, C.; Alkorta, I.; Walker, N.R.; Legon, A.C. A Chalcogen-Bonded Complex H3N⋯S=C=S Formed by Ammonia and Carbon Disulfide Characterised by Chirped-Pulse, Broadband Microwave Spectroscopy. J. Chem. Phys. 2019, 150, 084307. [Google Scholar] [CrossRef] [Green Version]
- Benkova, Z.; Sadlej, A.J. Electric Moments of Carbon Dichalcogenides. Mol. Phys. 2004, 102, 687–699. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S.; Wothers, P. Organic Chemistry; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Gougoula, E.; Moxon, J.A.; Walker, N.R.; Legon, A.C. A Chalcogen-Bonded Complex (CH3)3N⋯ SCO Characterised by Rotational Spectroscopy. Chem. Phys. Lett. 2020, 743, 137177. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Del Bene, J.E. Complexes of O=C=S with Nitrogen Bases: Chalcogen Bonds, Tetrel Bonds, and Other Secondary Interactions. Chem. Phys. Chem. 2018, 19, 1886–1894. [Google Scholar] [CrossRef]
- Bhattarai, S.; Sutradhar, D.; Chandra, A.K.; Zeegers-Huyskens, T. Nature of the Interaction of Pyridines with OCS. A Theoretical Investigation. Molecules 2020, 25, 416. [Google Scholar] [CrossRef] [Green Version]
- Montana, A.M. The σ and π Holes. The Halogen and Tetrel Bondings: Their Nature, Importance and Chemical, Biological and Medicinal Implications. Chem. Sel. 2017, 2, 9094–9112. [Google Scholar] [CrossRef]
- Hou, M.-C.; Yang, S.-B.; Li, Q.-Z.; Cheng, J.-B.; Li, H.-B.; Liu, S.-F. Tetrel Bond between 6-OTX3-Fulvene and NH3: Substituents and Aromaticity. Molecules 2019, 24, 10. [Google Scholar] [CrossRef] [Green Version]








| Lewis Base | Lewis Acid | Bond Type | |||
|---|---|---|---|---|---|
| DME | CO2 | tetrel | −8.0(3) | −14.4(8) | −16.6(8) |
| DME | N2O | pnictogen | −8.0(15) | −13.0(17) | −15.3(17) |
| DME | OCS | tetrel | −6.5(11) | −11.7(12) | −13.6(12) |
| TMA | CO2 | tetrel | −11.2(3) | −17.7(8) | −19.8(8) |
| TMA | N2O | pnictogen | −5.5(8) | −8.3(11) | −10.3(11) |
| TMA | OCS | tetrel | −4.2(3) | −12.4(9) | −14.1(9) |
| Complex | Bond Type | MP2/aug-cc-pVTZ a | CCSD(T)/CBS b | M06-2X-D3/cc-pVTZ a | |
|---|---|---|---|---|---|
| DME···CO2 | tetrel | −16.7 | −15.3 | −17.1 | −18.0 |
| DME···N2O | pnictogen | −15.3 | −14.6 | −13.4 | −13.6 |
| DME···OCS | tetrel | −13.6 | −11.2 | −12.1 | −11.9 |
| DME···OCS | chalcogen | - | −10.3 | −10.4 | −9.0 |
| TMA···CO2 | tetrel | −19.9 | −16.9 | −17.7 | −19.9 |
| TMA···N2O | pnictogen | −10.3 | −15.2 | −13.3 | −14.9 |
| TMA···OCS | tetrel | −14.2 | −13.9 | −12.9 | −14.5 |
| TMA···OCS | chalcogen | - | −13.6 | −12.9 | −12.2 |
| MUE c | 2.1 (1.5) | 1.7 (1.5) | 1.6 (1.0) | ||
| RMSE c | 2.6 (1.8) | 1.9 (1.6) | 2.2 (1.2) | ||
| MAX c | 4.9 (3.0) | 3.0 (2.2) | 4.6 (1.7) | ||
| Monomer | Q | |||
|---|---|---|---|---|
| Calculated | Experimental | Calculated | Experimental | |
| TMA | 0.664 | 0.612 [89] | ||
| DME | 1.345 | 1.30 [89] | ||
| CO2 | 0 | 0 | −4.14 | −4.28 [90] |
| CS2 | 0 | 0 | +3.18 | +3.45 [91] |
| CSe2 | 0 | 0 | +5.00 | - |
| N2O | 0.172 | 0.167 a [89] | −3.61 | −3.30 [92] |
| OCS | 0.710 | 0.715 b [93] | −0.77 | −0.78 [94] |
 | ||||||
|---|---|---|---|---|---|---|
| Complex | Bond Type | Atom Type | dA2···B | dA3···B | ||
| DME···CO2 | tetrel | A1 = O, A2 = C, A3 = O, B = O | 2.61 | 91.3 | - | - |
| DME···N2O | pnictogen | A1 = N, A2 = N, A3 = O, B = O | 2.69 | 92.1 | - | - |
| DME···OCS | tetrel | A1 = O, A2 = C, A3 = S, B = O | 2.75 | 85.2 | - | - |
| DME···OCS | chalcogen | A1 = O, A2 = C, A3 = S, B = O | - | - | 2.92 | 170.9 |
| TMA···CO2 | tetrel | A1 = O, A2 = C, A3 = O, B = N | 2.66 | 92.3 | - | - |
| TMA···N2O | pnictogen | A1 = N, A2 = N, A3 = O, B = N | 2.82 | 92.4 | - | - |
| TMA···OCS | tetrel | A1 = O, A2 = C, A3 = S, B = N | 2.81 | 86.9 | - | - |
| TMA···OCS | chalcogen | A1 = O, A2 = C, A3 = S, B = N | - | - | 2.93 | 170.2 |
| Complex | Bond Type | ΔEcomplex | ΔEstrain | ΔEint | ΔEPauli | ΔVelst | ΔEoi | Edisp |
|---|---|---|---|---|---|---|---|---|
| DME···CO2 | tetrel | −16.0 | 0.6 | −16.7 | 26.3 | −29.2 (68) | −8.5 (20) | −5.3 (12) |
| DME···N2O | pnictogen | −12.0 | 0.1 | −12.1 | 20.6 | −22.5 (69) | −5.1 (15) | −5.1 (16) |
| DME···OCS | tetrel | −9.7 | 0.3 | −10.0 | 23.1 | −19.7 (59) | −6.8 (20) | −6.7 (20) |
| DME···OCS | chalcogen | −9.9 | 0.1 | −10.0 | 17.2 | −15.3 (56) | −7.3 (27) | −4.6 (17) |
| TMA···CO2 | tetrel | −19.6 | 1.7 | −21.2 | 45.4 | −42.9 (64) | −15.0 (23) | −8.7 (13) |
| TMA···N2O | pnictogen | −12.9 | 0.1 | −13.0 | 29.4 | −27.5 (65) | −6.6 (15) | −8.3 (20) |
| TMA···OCS | tetrel | −12.6 | 0.7 | −13.3 | 40.5 | −30.7 (57) | −11.9 (22) | −11.3 (21) |
| TMA···OCS | chalcogen | −14.1 | 0.2 | −14.2 | 35.3 | −27.5 (56) | −14.2 (29) | −7.8 (16) |
| Complex | Bond Type | Δρ | ΔEoi,NOCV | ΔEoi,NOCV/ΔEoi,total (in %) |
|---|---|---|---|---|
| DME···CO2 | tetrel | 0.09 | −5.1 | 60 |
| DME···N2O | pnictogen | 0.06 | −2.2 | 43 |
| DME···OCS | tetrel | 0.08 | −3.4 | 50 |
| DME···OCS | chalcogen | 0.08 | −3.9 | 53 |
| TMA···CO2 | tetrel | 0.16 | −10.8 | 72 |
| TMA···N2O | pnictogen | 0.07 | −2.6 | 40 |
| TMA···OCS | tetrel | 0.15 | −7.2 | 60 |
| TMA···OCS | chalcogen | 0.14 | −9.8 | 69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vleeschouwer, F.; De Proft, F.; Ergün, Ö.; Herrebout, W.; Geerlings, P. A Combined Experimental/Quantum-Chemical Study of Tetrel, Pnictogen, and Chalcogen Bonds of Linear Triatomic Molecules. Molecules 2021, 26, 6767. https://doi.org/10.3390/molecules26226767
De Vleeschouwer F, De Proft F, Ergün Ö, Herrebout W, Geerlings P. A Combined Experimental/Quantum-Chemical Study of Tetrel, Pnictogen, and Chalcogen Bonds of Linear Triatomic Molecules. Molecules. 2021; 26(22):6767. https://doi.org/10.3390/molecules26226767
Chicago/Turabian StyleDe Vleeschouwer, Freija, Frank De Proft, Özge Ergün, Wouter Herrebout, and Paul Geerlings. 2021. "A Combined Experimental/Quantum-Chemical Study of Tetrel, Pnictogen, and Chalcogen Bonds of Linear Triatomic Molecules" Molecules 26, no. 22: 6767. https://doi.org/10.3390/molecules26226767