Shear-Induced Crystallization of Star and Linear Poly(L-lactide)s
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
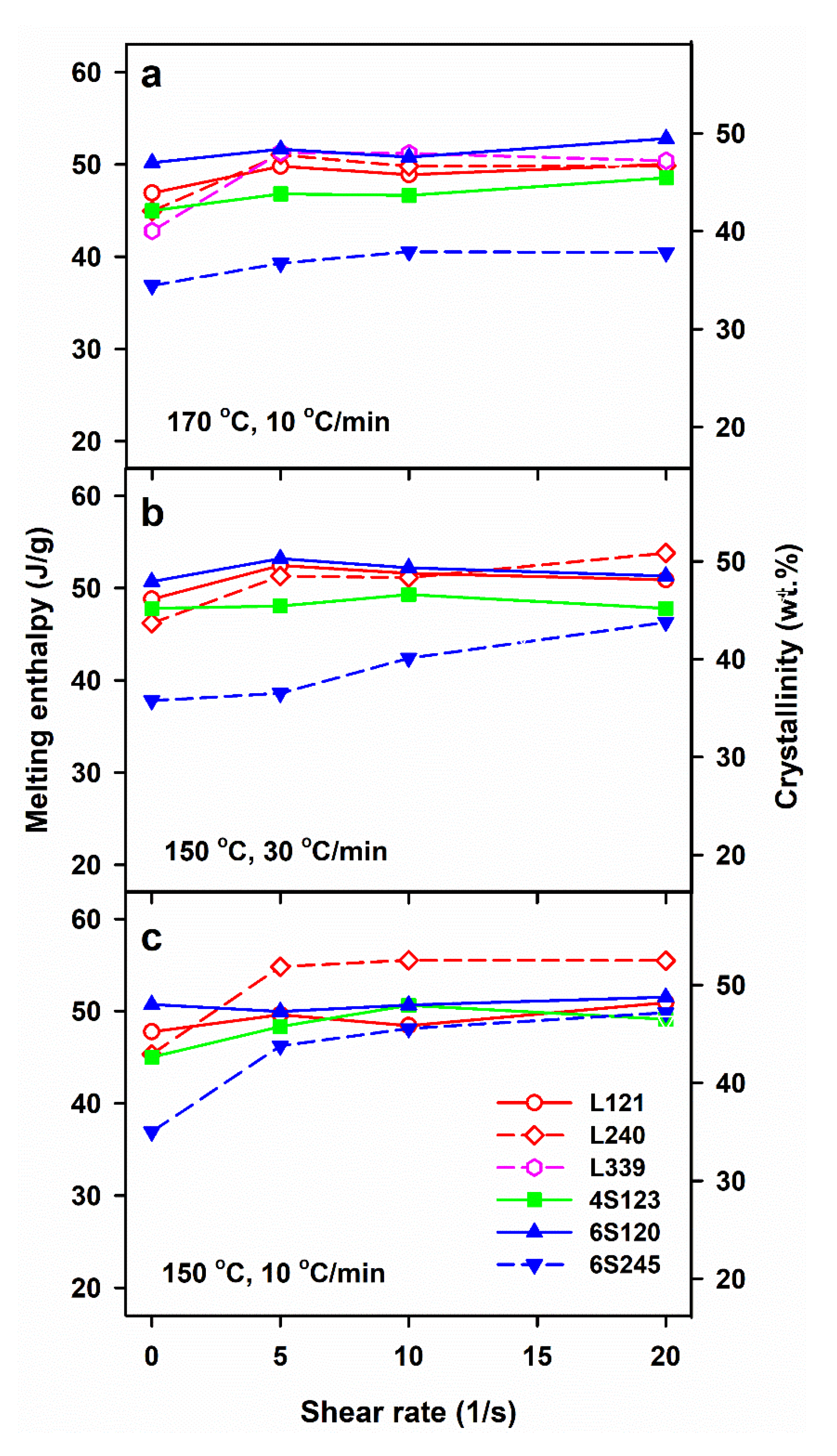
3.1. Crystallization
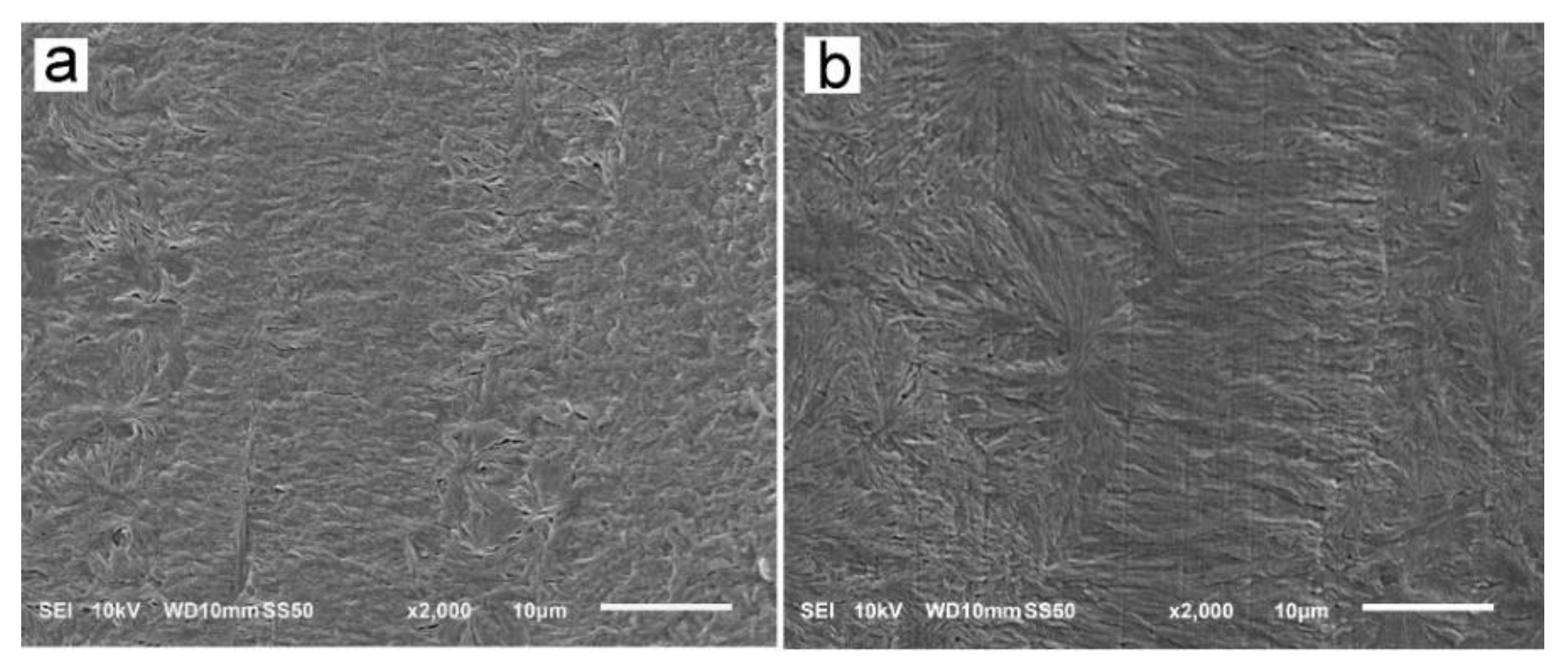
3.2. Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Piorkowska, E. Overview of biobased polymers. Adv. Polym. Sci. 2019, 283, 1–35. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. Present situation and future perspectives of poly(lactic acid). Adv. Polym. Sci. 2017, 279, 1–25. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Castro-Aguirre, E.; Inigues-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(lactic acid)-mass production, processing, industrial applications, and end of life. Adv. Drug. Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef] [Green Version]
- Kost, B.; Svyntkivska, M.; Brzeziński, M.; Makowski, T.; Piorkowska, E.; Rajkowska, K.; Kunicka-Styczyńska, A.; Biela, T. PLA/β-CD-based fibres loaded with quercetin as potential antibacterial dressing materials. Colloids Surf. B Biointerfaces 2020, 190, 110949. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Muller, A.J.; Avila, M.; Saenz, G.; Salazar, J. Crystallization of PLA-based materials. In Poly(Lactic Acid) Science and Technology: Processing, Properties, Additives and Applications; Jimenez, A., Peltzer, M., Ruseckaite, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 66–98. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(L-lactide) Investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Kalish, J.P.; Aou, K.; Yang, X.; Hsu, S.H. Spectroscopic and thermal analyses of α′ and α crystalline forms of poly(L-lactic acid). Polymer 2011, 52, 814–821. [Google Scholar] [CrossRef]
- Pan, P.; Yang, J.; Shan, G.; Bao, Y.; Weng, Z.; Cao, A.; Yazawa, K.; Inoue, Y. Temperature-variable FTIR and solid-state 13C NMR investigations on crystalline structure and molecular dynamics of polymorphic poly(L-lactide) and poly(L-lactide)/poly(D-lactide) stereocomplex. Macromolecules 2012, 45, 189–197. [Google Scholar] [CrossRef]
- Michalski, A.; Brzezinski, M.; Lapienis, G.; Biela, T. Star-shaped and branched polylactides: Synthesis, characterization, and properties. Prog. Polym. Sci. 2019, 89, 159–212. [Google Scholar] [CrossRef]
- Cameron, D.J.A.; Shaver, M.P. Aliphatic polyester polymer stars: Synthesis, properties and applications in biomedicine and nanotechnology. Chem. Soc. Rev. 2011, 40, 1761–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srisa-ard, M.; Baimark, Y. Effect of arm number and arm length on thermal properties of linear and star-shaped poly(D,L-lactides)s. J. Appl. Sci. 2010, 10, 1937–1943. [Google Scholar] [CrossRef]
- Kim, S.H.; Han, Y.K.; Kim, Y.H.; Hong, S.I. Multifunctional initiation of lactide polymerization by stannous octoate/pentaerythritol. Makromol. Chem. 1992, 193, 1323–1631. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, S. Synthesis and characterization of dendritic star poly(L-lactide)s. Polym. Bull. 2007, 58, 767–775. [Google Scholar] [CrossRef]
- Zhang, C.X.; Wang, B.; Chen, Y.; Cheng, F.; Jiang, S.C. Amphiphilic multiarm star polylactide with hyperbranched polyethylenimine as core: A systematic reinvestigation. Polymer 2012, 53, 3900–3909. [Google Scholar] [CrossRef]
- Biela, T.; Duda, A.; Penczek, S.; Rode, K.; Pasch, H. Well-defined star polylactides and their behavior in two-dimensional chromatography. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2884–2887. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Kinetics and mechanism of cyclic esters polymerization initiated with tin(II) octoate. 3. Polymerization of L,L-dilactide. Macromolecules 2000, 33, 7359–7370. [Google Scholar] [CrossRef]
- Biela, T.; Kowalski, A.; Libiszowski, J.; Duda, A.; Penczek, S. Progress in polymerization of cyclic esters: Mechanisms and synthetic applications. Macromol. Symp. 2006, 240, 47–55. [Google Scholar] [CrossRef]
- Tsuji, H. Quiescent crystallization of poly(lactic acid) and its copolymers-based materials. Adv. Polym. Sci. 2019, 283, 37–86. [Google Scholar] [CrossRef]
- Tsuji, H.; Miyase, T.; Tezuka, Y.; Saha, S.K. Physical properties, crystallization, and spherulite growth of linear and 3-arm poly(L-lactide)s. Biomacromolecules 2005, 6, 244–254. [Google Scholar] [CrossRef]
- Hao, Q.; Li, F.; Li, Q.; Li, Y.; Jia, L.; Yang, J.; Fang, Q.; Cao, A. Preparation and crystallization kinetics of new structurally well-defined star-shaped biodegradable poly(L-lactide)s initiated with diverse natural sugar alcohols. Biomacromolecules 2005, 6, 2236–2247. [Google Scholar] [CrossRef]
- Wang, L.; Dong, C.M. Synthesis, crystallization kinetics, and spherulitic growth of linear and star-shaped poly(L-lactide)s with different numbers of arms. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2226–2236. [Google Scholar] [CrossRef]
- Bojda, J.; Piorkowska, E.; Lapienis, G.; Michalski, A. Crystallization of star-shaped and linear poly(L-lactide)s. Eur. Polym. J. 2018, 105, 126–134. [Google Scholar] [CrossRef]
- Lamberti, G. Flow induced crystallisation of polymers. Chem. Soc. Rev. 2014, 43, 2240–2252. [Google Scholar] [CrossRef]
- Peters, G.W.M.; Balzano, L.; Steenbakkers, R.J.A. Flow-induced crystallization. In Handbook of Polymer Crystallization; Piorkowska, E., Rutledge, G.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 399–432. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Z.; Li, L. Flow-induced crystallization of polymers: Molecular and thermodynamic considerations. Macromolecules 2016, 49, 1505–1517. [Google Scholar] [CrossRef]
- van Meerveld, J.; Peters, G.W.M.; Hutter, M. Towards a rheological classification of flow induced crystallization experiments of polymer melts. Rheol. Acta 2004, 44, 119–134. [Google Scholar] [CrossRef] [Green Version]
- Janeschitz-Kriegl, H.; Ratajski, E.; Stadlbauer, M. Flow as an effective promotor of nucleation in polymer melts: A quantitative evaluation. Rheol. Acta 2003, 42, 355–364. [Google Scholar] [CrossRef]
- Mykhaylyk, O.O.; Chambon, P.; Graham, R.S.; Fairclough, J.P.A.; Olmsted, P.D.; Ryan, A.J. The specific work of flow as a criterion for orientation in polymer crystallization. Macromolecules 2008, 41, 1901–1904. [Google Scholar] [CrossRef]
- Hsiao, B.S. Role of chain entanglement network on formation of flow-induced crystallization precursor structure. In Progress in Understanding of Polymer Crystallization; Reiter, G., Strobl, G.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 133–149. [Google Scholar] [CrossRef]
- Somani, R.H.; Yang, L.; Zhu, L.; Hsiao, B.S. Shish-kebab precursor structures in entangled polymer melts. Polymer 2005, 46, 8587–8623. [Google Scholar] [CrossRef] [Green Version]
- Rhoades, A.; Pantani, R. Poly(Lactic Acid): Flow-Induced Crystallization. Adv. Polym. Sci. 2019, 283, 87–117. [Google Scholar] [CrossRef]
- Zhong, Y.; Fang, H.; Zhang, Y.; Wang, Z.; Yang, J.; Wang, Z. Rheologically determined critical shear rates for shear-induced nucleation rate enhancements of poly(lactic acid). ACS Sustain. Chem. Eng. 2013, 1, 663–672. [Google Scholar] [CrossRef]
- Xu, H.; Xie, L.; Hakkarainen, M. Beyond a model of polymer processing-triggered shear: Reconciling shish-kebab formation and control of chain degradation in sheared poly(L-lactic acid). ACS Sustain. Chem. Eng. 2015, 3, 1443–1452. [Google Scholar] [CrossRef]
- Bojda, J.; Piorkowska, E. Shear-induced nonisothermal crystallization of two grades of PLA. Polym. Test. 2016, 50, 172–181. [Google Scholar] [CrossRef]
- Li, X.J.; Li, Z.M.; Zhong, G.J.; Li, L.B. Steady- shear- induced isothermal crystallization of poly(L-lactide) (PLLA). J. Macrom. Sci. Part B Polym. Phys. 2008, 47, 511–522. [Google Scholar] [CrossRef]
- Kim, E.S.; Kim, B.C.; Kim, S.H. Structural effect of linear and star-shaped poly(L-lactic acid) on physical properties. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 939–946. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, Y.; Bai, J.; Wang, Z. Shear-induced nucleation and morphological evolution for bimodal long chain branched polylactide. Macromolecules 2013, 46, 6555–6565. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Zhang, Y.; Fang, H.; Wang, Z. Shear-induced enhancements of crystallization kinetics and morphological transformation for long chain branched polylactides with different branching degrees. Sci. Rep. 2016, 6, 26560. [Google Scholar] [CrossRef] [PubMed]
- Michalski, A.; Łapienis, G. Synthesis and characterization of high-molar-mass star-shaped poly(L-lactide)s. Polimery 2018, 63, 488–494. [Google Scholar] [CrossRef]
- Biela, T.; Duda, A.; Penczek, S. Enhanced melt stability of star-shaped stereocomplexes as compared with linear stereocomplexes. Macromolecules 2006, 39, 3710–3713. [Google Scholar] [CrossRef]
- He, Y.; Wu, T.; Wie, J.; Fan, Z.; Li, S. Morphological investigation on melt crystallized polylactide homo- and stereocomplex by enzymatic degradation with proteinase K. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 959–970. [Google Scholar] [CrossRef]
- Huang, S.; Li, H.; Jiang, S.; Chen, X.; An, L. Crystal structure and morphology influenced by shear effect of poly(L-lactide) and its melting behaviour revealed by WAXS, DSC and in-situ POM. Polymer 2011, 52, 3478–3487. [Google Scholar] [CrossRef]
- Righetti, M.C.; Gazzano, M.; Di Lorenzo, M.L.; Androsch, R. Enthalpy of melting of α’- and α- crystals of poly(L-lactic acid). Eur. Polym. J. 2015, 70, 215–220. [Google Scholar] [CrossRef]
- Sarasua, J.R.; Prud’Homme, R.E.; Wisniewski, M.; Le Borgne, A.; Spassky, N. Crystallization and melting behaviour of polylactides. Macromolecules 1998, 31, 3895–3905. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Piorkowska, E.; Galeski, A. Overall crystallization kinetics. In Handbook of Polymer Crystallization; Piorkowska, E., Rutledge, G.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 215–236. [Google Scholar] [CrossRef]
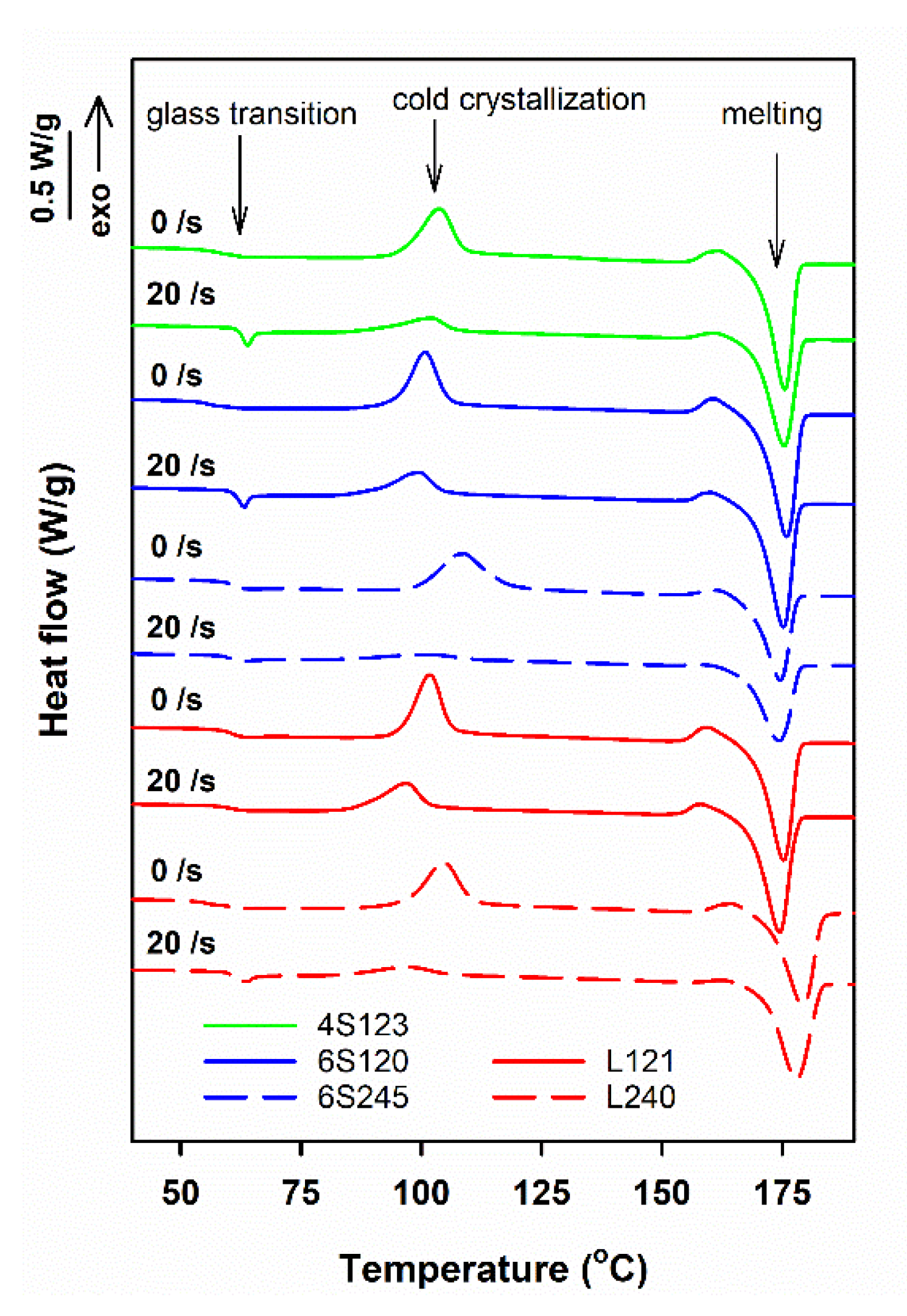
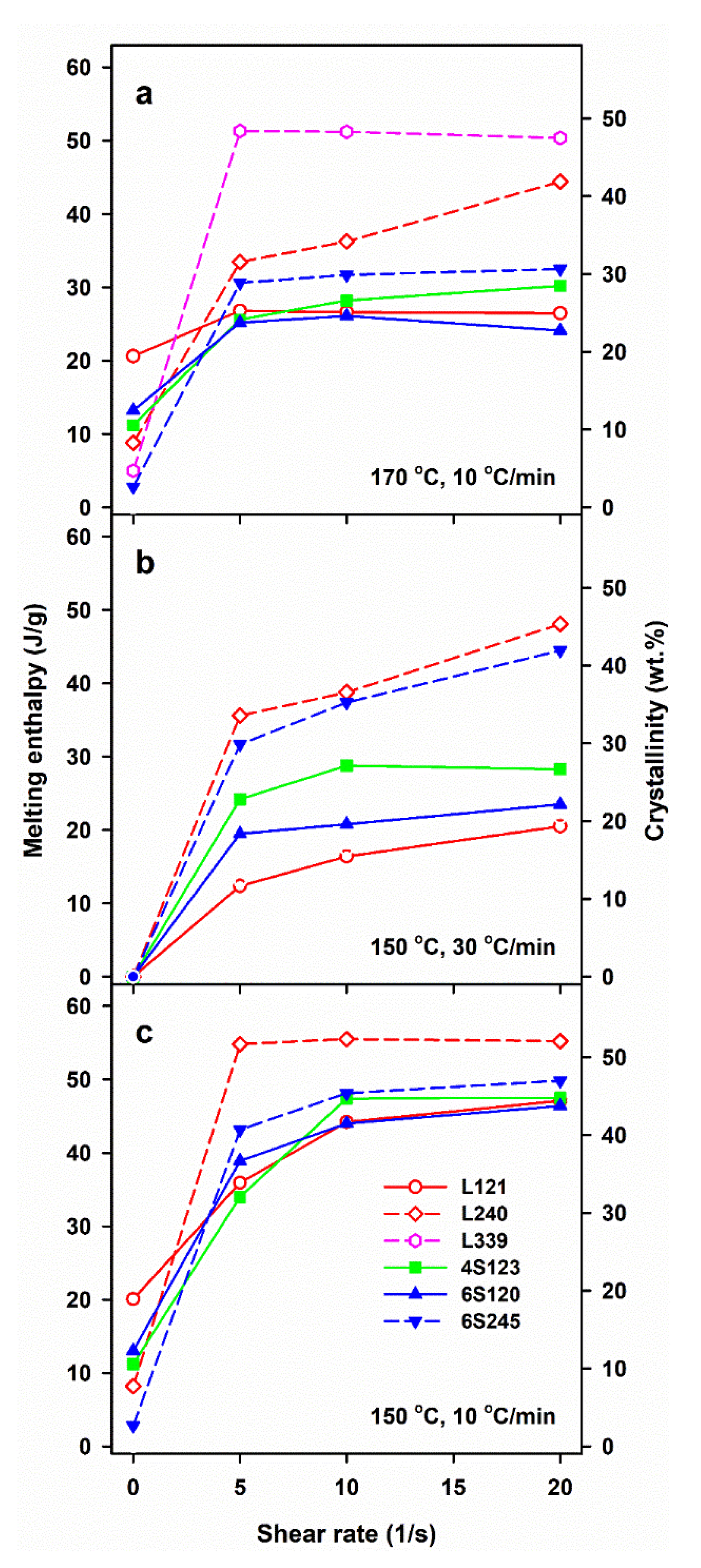
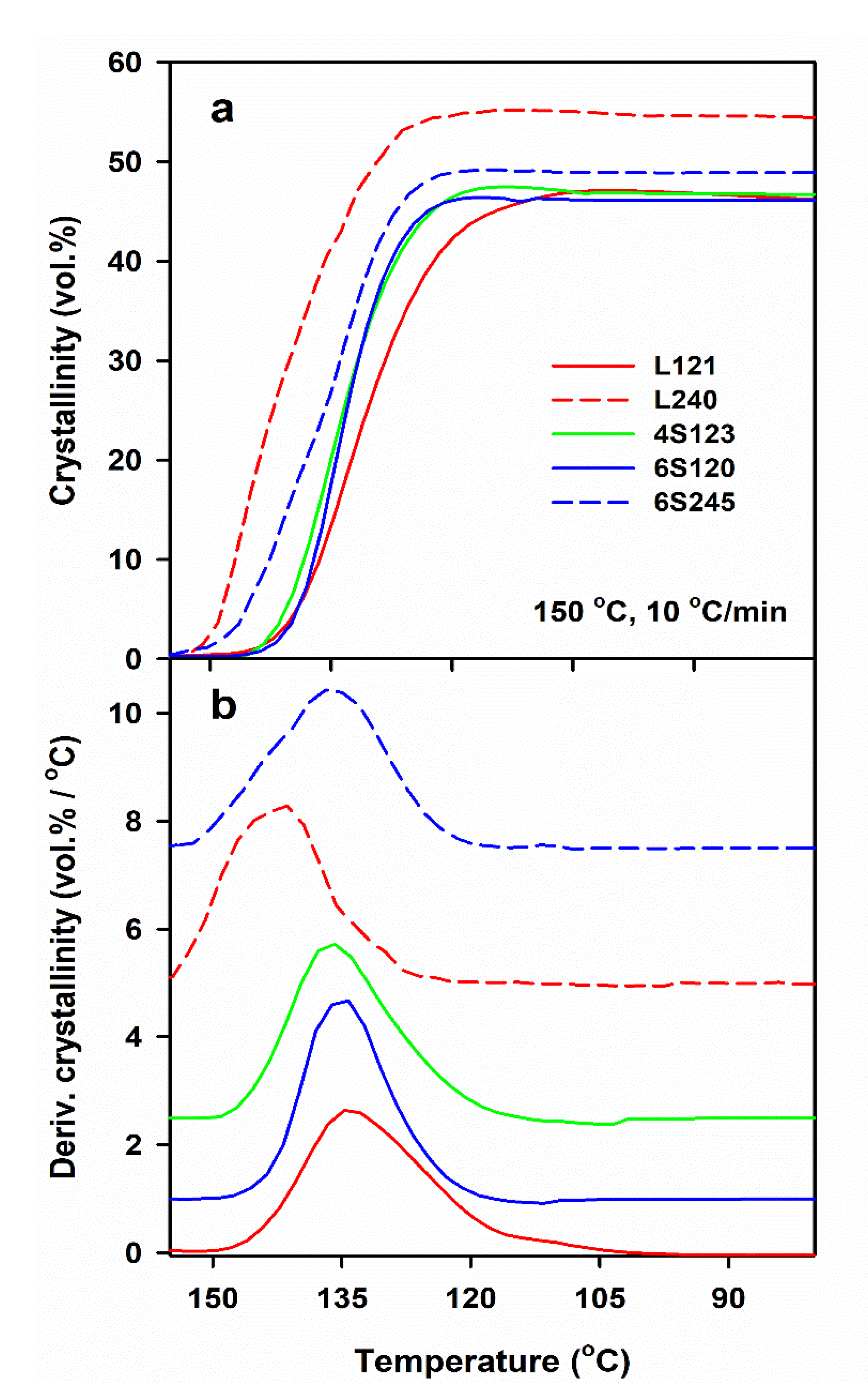
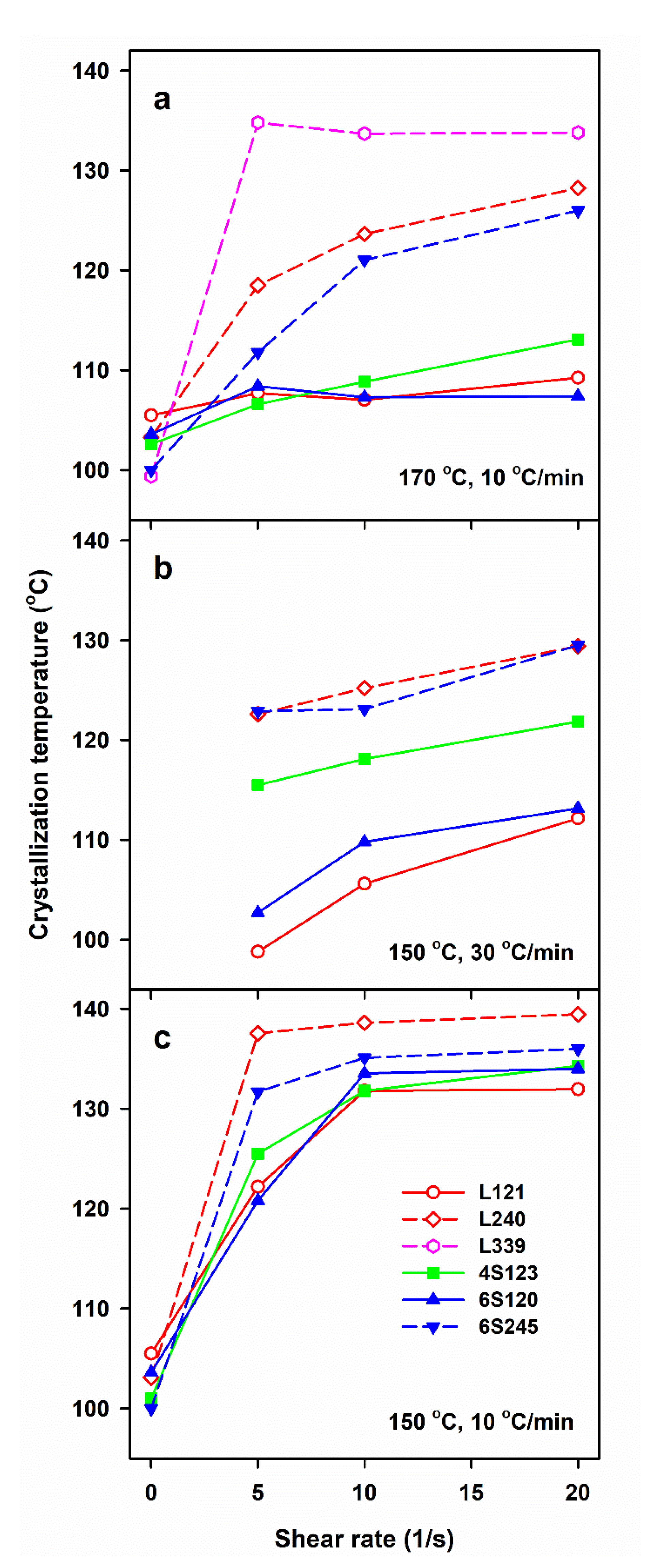





| Sample Code | Mw (kg/mol) | Mn (kg/mol) | Mz (kg/mol) | Mw/Mn |
|---|---|---|---|---|
| L121 | 121 | 81 | 194 | 1.5 |
| L240 | 240 | 157 | 414 | 1.3 |
| L339 | 339 | 257 | 495 | 1.3 |
| 4S123 | 123 | 97 | 152 | 1.3 |
| 6S120 | 120 | 80 | 162 | 1.5 |
| 6S245 | 245 | 183 | 294 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojda, J.; Piorkowska, E.; Lapienis, G.; Michalski, A. Shear-Induced Crystallization of Star and Linear Poly(L-lactide)s. Molecules 2021, 26, 6601. https://doi.org/10.3390/molecules26216601
Bojda J, Piorkowska E, Lapienis G, Michalski A. Shear-Induced Crystallization of Star and Linear Poly(L-lactide)s. Molecules. 2021; 26(21):6601. https://doi.org/10.3390/molecules26216601
Chicago/Turabian StyleBojda, Joanna, Ewa Piorkowska, Grzegorz Lapienis, and Adam Michalski. 2021. "Shear-Induced Crystallization of Star and Linear Poly(L-lactide)s" Molecules 26, no. 21: 6601. https://doi.org/10.3390/molecules26216601






