Total Syntheses of Pladienolide-Derived Spliceosome Modulators
Abstract
:1. Introduction
2. Synthesis of Pladienolides
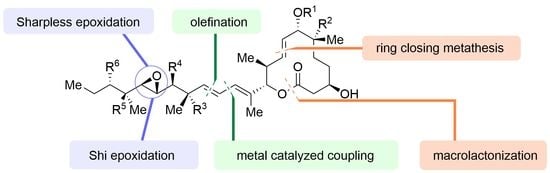
2.1. Synthetic Strategies Regarding Pladienolides
2.2. Macrocyclization via Ring-Closing Metathesis
2.2.1. Synthesis of Pladienolide B by Kotake and Coworkers (2007)
2.2.2. Synthesis of Pladienolide B by Ghosh and Anderson (2012)
2.2.3. Synthesis of FD-895 by Burkart and Coworkers (2012)
2.2.4. Synthesis of Pladienolide B by Kumar and Chandrasekhar (2013)
2.2.5. Synthesis of 6-Deoxyoladienolide D by Keaney and Coworkers (2014)
2.2.6. Synthesis of Pladeinolide B by Yoo and Krische (2021)
2.3. Synthesis via Macrolactonization
2.3.1. Synthesis of the Macrocyclic Core of ent-Pladienolide B by Skaanderup and Jensen (2008)
2.3.2. Synthesis of Pladienolide B by Maier and Coworkers (2014)
2.3.3. Total Synthesis of Pladienolide A, B, and H3B-8800 by Rhoades et al. (2021)
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boccaletto, P.; Machnicka, M.A.; Purta, E.; Piątkowski, P.; Baginski, B.; Wirecki, T.K.; De Crécy-Lagard, V.; Ross, R.; Limbach, P.A.; Kotter, A.; et al. Modomics: A database of RNA modification pathways. 2017 Update. Nucleic Acids Res. 2017, 46, D303–D307. [Google Scholar] [CrossRef] [PubMed]
- Di, C.; Syafrizayanti, Z.Q.; Chen, Y.; Wang, Y.; Zhang, X.; Liu, Y.; Sun, C.; Zhang, H.; Hoheisel, J.D. Function, clinical application, and strategies of Pre-mRNA splicing in cancer. Cell Death Differ. 2018, 26, 1181–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Will, C.L.; Lührmann, R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Qian, J.; Gu, C.; Yang, Y. Alternative splicing and cancer: A systematic review. Signal Transduct. Target. Ther. 2021, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Gong, Q.; Wang, Y.; Li, M.; Wang, L.; Ding, H.; Li, P. The biological function and clinical significance of SF3B1 mutations in cancer. Biomark. Res. 2020, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sanada, M.; Shiraishi, Y.; Nowak, D.; Nagata, Y.; Yamamoto, R.; Sato, Y.; Sato-Otsubo, A.; Kon, A.; Nagasaki, M.; et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 2011, 478, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, R.C.; Mar, B.G.; Mazzola, E.; Grauman, P.V.; Shareef, S.; Allen, S.L.; Pigneux, A.; Wetzler, M.; Stuart, R.K.; Erba, H.P.; et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015, 125, 1367–1376. [Google Scholar] [CrossRef] [Green Version]
- Eid, O.M.; Kader, R.M.A.A.; Fathalla, L.A.; Abdelrahman, A.H.; Rabea, A.; Mahrous, R.; Eid, M.M. Evaluation of MLPA as a comprehensive molecular cytogenetic tool to detect cytogenetic markers of chronic lymphocytic leukemia in Egyptian patients. J. Genet. Eng. Biotechnol. 2021, 19, 98–104. [Google Scholar] [CrossRef]
- Jiménez-Vacas, J.M.; Herrero-Aguayo, V.; Gómez-Gómez, E.; León-González, A.J.; Sáez-Martínez, P.; Alors-Pérez, E.; Fuentes-Fayos, A.C.; Martínez-López, A.; Sánchez-Sánchez, R.; González-Serrano, T.; et al. Spliceosome component SF3B1 as novel prognostic biomarker and therapeutic target for prostate cancer. Transl. Res. 2019, 212, 89–103. [Google Scholar] [CrossRef]
- Maguire, S.; Leonidou, A.; Wai, P.; Marchiò, C.; Ng, C.K.Y.; Sapino, A.; Salomon, A.; Reis-Filho, J.S.; Weigelt, B.; Natrajan, R.C. SF3B1 mutations constitute a novel therapeutic target in breast cancer. J. Pathol. 2014, 235, 571–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Huo, Y.; Yang, M.; Shen, Y.; Liu, D.; Fu, X.; Tao, L.; He, R.; Zhang, J.; Hua, R.; et al. SF3B1 mutation in pancreatic cancer contributes to aerobic glycolysis and tumor growth through a PP2A–c-Myc axis. Mol. Oncol. 2021, 1–15. [Google Scholar] [CrossRef]
- Donaldson, W.A. Syntheses of spliceostatins and thailanstatins: A review. Beilstein J. Org. Chem. 2020, 16, 1991–2006. [Google Scholar] [CrossRef]
- Lagisetti, C.; Pourpak, A.; Jiang, Q.; Cui, X.; Goronga, T.; Morris, S.W.; Webb, T.R. Antitumor compounds based on a natural product consensus pharmacophore. J. Med. Chem. 2008, 51, 6220–6224. [Google Scholar] [CrossRef] [Green Version]
- Lagisetti, C.; Pourpak, A.; Goronga, T.; Jiang, Q.; Cui, X.; Hyle, J.; Lahti, J.M.; Morris, S.W.; Webb, T.R. Synthetic mRNA splicing modulator compounds with In Vivo antitumor activity. J. Med. Chem. 2009, 52, 6979–6990. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Lagisetti, C.; Edwards, C.C.; Webb, T.R.; Potter, P.M. Sudemycins, novel small molecule analogues of FR901464, induce alternative gene splicing. ACS Chem. Biol. 2011, 6, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Thirupathi, B.; Zilla, M.K. Syntheses and biological importance of herboxidiene/GEX1A. Chem. Select. 2019, 4, 11944–11958. [Google Scholar] [CrossRef]
- Pokhrel, A.R.; Dhakal, D.; Jha, A.; Sohng, J.K. Herboxidiene biosynthesis, production, and structural modifications: Prospect for hybrids with related polyketide. Appl. Microbiol. Biotechnol. 2015, 99, 8351–8362. [Google Scholar] [CrossRef]
- Sakai, T.; Sameshima, T.; Matsufuji, M.; Kawamura, N.; Dobashi, K.; Mizui, Y. Pladienolides, new substances from culture of streptomyces platensis Mer-11107 I. Taxonomy, fermentation, isolation and screening. In Vitro and In Vivo antitumor activities. J. Antibiot. 2004, 57, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Sakai, T.; Asai, N.; Okuda, A.; Kawamura, N.; Mizui, Y. Pladienolides, new substances from culture of Streptomyces platensis mer-11107. II. Physico-chemical properties and structure elucidation. J. Antibiot. 2004, 57, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Mizui, Y.; Sakai, T.; Iwata, M.; Uenaka, T.; Okamoto, K.; Shimizu, H.; Yamori, T.; Yoshimatsu, K.; Asada, M. Pladienolides, new substances from culture of streptomyces platensis mer-11107 III. In Vitro and In Vivo antitumor activities. J. Antibiot. 2004, 57, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Kotake, Y.; Sagane, K.; Owa, T.; Mimori-Kiyosue, Y.; Shimizu, H.; Uesugi, M.; Ishihama, Y.; Iwata, M.; Mizui, Y. Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat. Chem. Biol. 2007, 3, 570–575. [Google Scholar] [CrossRef]
- Gundluru, M.K.; Pourpak, A.; Cui, X.; Morris, S.W.; Webb, T.R. Design, synthesis and initial biological evaluation of a novel pladienolide analog scaffold. Med. Chem. Comm. 2011, 2, 904–908. [Google Scholar] [CrossRef] [Green Version]
- Mandel, A.L.; Jones, B.D.; La Clair, J.J.; Burkart, M.D. A synthetic entry to pladienolide B and FD-895. Bioorganic Med. Chem. Lett. 2007, 17, 5159–5164. [Google Scholar] [CrossRef] [Green Version]
- Lagisetti, C.; Yermolina, M.V.; Sharma, L.K.; Palacios, G.; Prigaro, B.J.; Webb, T.R. Pre-mRNA splicing-modulatory pharmacophores: The total synthesis of herboxidiene, a pladienolide-herboxidiene hybrid analog and related derivatives. ACS Chem. Biol. 2014, 9, 643–648. [Google Scholar] [CrossRef]
- Booth, T.J.; Kalaitzis, J.A.; Vuong, D.; Crombie, A.; Lacey, E.; Piggott, A.M.; Wilkinson, B. Production of novel pladienolide analogues through native expression of a pathway-specific activator. Chem. Sci. 2020, 11, 8249–8255. [Google Scholar] [CrossRef] [PubMed]
- Machida, K.; Arisawa, A.; Takeda, S.; Tsuchida, T.; Aritoku, Y.; Yoshida, M.; Ikeda, H. Organization of the biosynthetic gene cluster for the polyketide antitumor macrolide, pladienolide, in Streptomyces platensis mer-11107. Biosci. Biotechnol. Biochem. 2008, 72, 2946–2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, N.; Kotake, Y.; Niijima, J.; Fukuda, Y.; Uehara, T.; Sakai, T. Stereochemistry of pladienolide B. J. Antibiot. 2007, 60, 364–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokoi, A.; Kotake, Y.; Takahashi, K.; Kadowaki, T.; Matsumoto, Y.; Minoshima, Y.; Sugi, N.H.; Sagane, K.; Hamaguchi, M.; Iwata, M.; et al. Biological validation that SF3b is a target of the antitumor macrolide pladienolide. FEBS J. 2011, 278, 4870–4880. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Muguruma, N.; Nakagawa, T.; Okamoto, K.; Kimura, T.; Kitamura, S.; Yano, H.; Sannomiya, K.; Goji, T.; Miyamoto, H.; et al. High antitumor activity of pladienolide B and its derivative in gastric cancer. Cancer Sci. 2014, 105, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Cretu, C.; Agrawal, A.A.; Cook, A.; Will, C.L.; Fekkes, P.; Smith, P.G.; Lührmann, R.; Larsen, N.; Buonamici, S.; Pena, V. Structural basis of splicing modulation by antitumor macrolide compounds. Mol. Cell 2018, 70, 265–273.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojkova, D.; Klann, K.; Koch, B.; Widera, M.; Krause, D.; Ciesek, S.; Cinatl, J.; Münch, C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 2020, 583, 469–472. [Google Scholar] [CrossRef]
- Kotake, Y.; Niijima, J.; Fukuda, Y.; Nagai, M.; Kanada, R.M.; Takeda, S.; Nakashima, T.; Yoshida, M.; Tsuchida, T.; Sameshima, T. Novel Physiologically Active Substances. U.S. Patent US/2007/7,256,178, 2007. [Google Scholar]
- Seki-Asano, M.; Okazaki, T.; Yamagishi, M.; Sakai, N.; Takayama, Y.; Hanada, K.; Morimoto, S.; Takatsuki, A.; Mizoue, K.; Morimoro, S. Isolation and characterization of a new 12-membered macrolide FD-895. J. Antibiot. 1994, 47, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Villa, R.; Mandel, A.L.; Jones, B.D.; La Clair, J.J.; Burkart, M.D. Structure of FD-895 revealed through total synthesis. Org. Lett. 2012, 14, 5396–5399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwata, M.; Ozawa, Y.; Uenaka, T.; Shimizu, H.; Niijima, J.; Kanada, R.M.; Fukuda, Y.; Nagai, M.; Kotake, Y.; Yoshida, M.; et al. E7107, a new 7-urethane derivative of pladienolide D, displays curative effect against several human tumor xenografts. Proc. Am. Assoc. Cancer Res. 2004, 45, 691. [Google Scholar]
- Hong, D.S.; Kurzrock, R.; Naing, A.; Wheler, J.J.; Falchook, G.S.; Schiffman, J.S.; Faulkner, N.; Pilat, M.J.; O’Brien, J.; LoRusso, P. A phase I, open-label, single-arm, dose-escalation study of E7107, a precursor messenger ribonucleic acid (pre-mRNA) splicesome inhibitor administered intravenously on days 1 and 8 every 21 days to patients with solid tumors. Invest. New Drugs 2014, 32, 436–444. [Google Scholar] [CrossRef]
- Eskens, F.; Ramos, F.J.; Burger, H.; O’Brien, J.; Piera, A.; De Jonge, M.; Mizui, Y.; Wiemer, E.; Carreras, M.; Maria, J.; et al. Phase I pharmacokinetic and pharmacodynamic study of the first-in-class spliceosome inhibitor E7107 in patients with advanced solid tumors. Clin. Cancer Res. 2013, 19, 6296–6304. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.M.; Zack, D.J. Alternative splicing and retinal degeneration. Clin. Genet. 2013, 84, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Steensma, D.P.; Wermke, M.; Klimek, V.M.; Greenberg, P.L.; Font, P.; Komrokji, R.S.; Yang, J.; Brunner, A.M.; Carraway, H.E.; Ades, L.; et al. Results of a clinical trial of H3B-8800, a splicing modulator, in patients with myelodysplastic syndromes (MDS), acute myeloid leukemia (AML) or chronic myelomonocytic leukemia (CMML). Blood 2019, 134, 673. [Google Scholar] [CrossRef]
- Webb, T.R.; Joyner, A.S.; Potter, P.M. The development and application of small molecule modulators of SF3b as therapeutic agents for cancer. Drug Discov. Today 2013, 18, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Salton, M.; Misteli, T. Small molecule modulators of pre-mRNA splicing in cancer therapy. Trends Mol. Med. 2016, 22, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Effenberger, K.A.; Urabe, V.K.; Jurica, M.S. Modulating splicing with small molecular inhibitors of the spliceosome. Wiley Interdiscip. Rev. RNA 2017, 8, e1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León, B.; Kashyap, M.; Chan, W.C.; Krug, K.; Castro, J.E.; La Clair, J.J.; Burkart, M.D. A challenging pie to splice: Drugging the spliceosome. Angew. Chem. Int. Ed. 2017, 56, 12052–12063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Meng, F. A comprehensive overview of structure-activity relationships of small-molecule splicing modulators targeting SF3B1 as anticancer agents. Chem. Med. Chem. 2020, 15, 2098–2120. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, T.; Sharpless, K.B. The first practical method for asymmetric epoxidation. J. Am. Chem. Soc. 1980, 102, 5974–5976. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, Z.-X.; Shi, Y. An efficient asymmetric epoxidation method for trans-olefins mediated by a fructose-derived ketone. J. Am. Chem. Soc. 2010, 28, 9806–9807. [Google Scholar] [CrossRef]
- Cossy, J.; Arseniyadis, S.; Meyer, C. Metathesis in Natural Product Synthesis: Strategies, Substrates and Catalysts; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Gradillas, A.; Pérez-Castells, J. Macrocyclization by ring-closing metathesis in the total synthesis of natural products: Reaction conditions and limitations. Angew. Chem. Int. Ed. 2006, 45, 6086–6101. [Google Scholar] [CrossRef]
- Kanada, R.M.; Itoh, D.; Nagai, M.; Niijima, J.; Asai, N.; Mizui, Y.; Abe, S.; Kotake, Y. Total synthesis of the potent antitumor macrolides pladienolide B and D. Angew. Chem. Int. Ed. 2007, 46, 4350–4355. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Anderson, D.D. Enantioselective total synthesis of pladienolide B: A potent spliceosome inhibitor. Org. Lett. 2012, 14, 4730–4733. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.P.; Chandrasekhar, S. Enantioselective synthesis of pladienolide B and truncated analogues as new anticancer agents. Org. Lett. 2013, 15, 3610–3613. [Google Scholar] [CrossRef]
- Arai, K.; Buonamici, S.; Chan, B.; Corson, L.; Endo, A.; Gerard, B.; Hao, M.-H.; Karr, C.; Kira, K.; Lee, L.; et al. Total synthesis of 6-deoxypladienolide d and assessment of splicing inhibitory activity in a mutant sf3b1 cancer cell line. Org. Lett. 2014, 16, 5560–5563. [Google Scholar] [CrossRef]
- Yoo, M.; Krische, M.J. Total synthesis of the spliceosome modulator pladienolide B via. asymmetric alcohol-mediated syn- and anti-diastereoselective carbonyl crotylation. Angew. Chem. Int. Ed. 2021, 60, 13923–13928. [Google Scholar] [CrossRef] [PubMed]
- Paterson, I.; Wallace, D.J.; Cowden, C.J. Polyketide synthesis using the boron-mediated, anti-aldol reactions of lactate-derived ketones: Total synthesis of (-)-ACRL toxin IIIB. Synthesis 1998, 29, 639–652. [Google Scholar] [CrossRef]
- Grieco, P.; Masaki, Y.; Boxler, D. Sesterterpenes. I. Stereospecific total synthesis of moenocinol. J. Am. Chem. Soc. 1975, 97, 1597–1599. [Google Scholar] [CrossRef]
- Fukuzawa, S.-I.; Matsuzawa, H.; Yoshimitsu, S.-I. Asymmetric samarium-Reformatsky reaction of chiral α-bromoacetyl-2-oxazolidinones with aldehydes. J. Org. Chem. 2000, 65, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Gage, J.R.; Evans, D.A. Diastereoselective aldol condensation using a chiral oxazolidinone auxiliary: (2S*,3S*)-3-hydroxy-3-phenyl-2-methylpropanoic acid. Org. Synth. 2003, 68, 83. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Choi, T.-L.; Sanders, A.D.P.; Grubbs, R.H. A general model for selectivity in olefin cross metathesis. J. Am. Chem. Soc. 2003, 125, 11360–11370. [Google Scholar] [CrossRef] [Green Version]
- Nagano, H.; Nakanishi, E.; Takajo, S.; Sakuma, M.; Kudo, K. Synthesis of 6-(poly)prenyl-substituted polyprenols and their phosphates. Tetrahedron 1999, 55, 2591–2608. [Google Scholar] [CrossRef]
- Brown, H.C.; Bhat, K.S. Chiral synthesis via. organoboranes. Part 7. Diastereoselective and enantioselective synthesis of erythro-and threo-β-methylhomoallyl alcohols via enantiomeric (Z)- and (E)-crotylboranes. J. Am. Chem. Soc. 1987, 108, 5919–5923. [Google Scholar] [CrossRef]
- Yatagai, M.; Ohnuki, T. Asymmetric reduction of functionalized ketones with a sodium borohydride-(L)-tartaric acid system. J. Chem. Soc. Perkin Trans. 1990, 6, 1826–1828. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Liu, H.; Reddy, M.V.R.; Brown, H.C. Synthesis of homoallylic chiral tertiary alcohols via. chelation-controlled diastereoselective nucleophilic addition on α-alkoxyketones: Application for the synthesis of the C1−C11 subunit of 8-epi-fostriecin. Org. Lett. 2003, 5, 3755–3757. [Google Scholar] [CrossRef]
- Still, W.C.; McDonald, J.H. Chelation-controlled nucleophilic additions. 1. A highly effective system for asymmetric induction in the reaction of organometallics with α-alkoxyketones. Tetrahedron Lett. 1980, 21, 1031–1034. [Google Scholar] [CrossRef]
- Zhang, Y.; Phillips, A.A.J.; Sammakia, T. Highly selective asymmetric acetate aldol reactions of an N-Acetyl thiazolidinethione reagent. Org. Lett. 2004, 6, 23–25. [Google Scholar] [CrossRef]
- Marshall, J.A. Chiral allylic and allenic metal reagents for organic synthesis. J. Org. Chem. 2007, 72, 8153–8166. [Google Scholar] [CrossRef]
- Crimmins, M.T.; King, B.W.; Tabet, E.A. Asymmetric aldol additions with titanium enolates of acyloxazolidinethiones: Dependence of selectivity on amine base and Lewis acid stoichiometry. J. Am. Chem. Soc. 1997, 119, 7883–7884. [Google Scholar] [CrossRef]
- Chan, W.C.; La Clair, J.J.; León, B.; Trieger, K.A.; Slagt, M.Q.; Verhaar, M.T.; Bachera, D.U.; Rispens, M.T.; Hofman, R.M.; de Boer, V.L.; et al. Scalable synthesis of 17S-FD-895 expands the structural understanding of splice modulatory activity. Cell Rep. Phys. Sci. 2020, 1, 100277. [Google Scholar] [CrossRef]
- Gonzalez, I.C.; Forsyth, C.J. Total synthesis of thyrsiferyl 23-acetate, a specific inhibitor of protein phosphatase 2A and an anti-leukemic inducer of apoptosis. J. Am. Chem. Soc. 2000, 122, 9099–9108. [Google Scholar] [CrossRef]
- Viti, S.M. Regioselective reductions of 2,3-epoxy alcohols. Tetrahedron Lett. 1982, 23, 4541–4544. [Google Scholar] [CrossRef]
- Jung, M.E.; Lee, W.S.; Sun, D. Synthesis of four diastereomeric 3,5-dialkoxy-2,4-dimethylalkanals by a simple extension of the non-aldol aldol process to bis (propionates). Org. Lett. 1999, 1, 307–310. [Google Scholar] [CrossRef]
- Ireland, R.E.; Mueller, R.H.; Willard, A.K. The ester enolate Claisen rearrangement. Stereochemical control through stereoselective enolate formation. J. Am. Chem. Soc. 1976, 98, 2868–2877. [Google Scholar] [CrossRef]
- Kajikawa, T.; Aoki, K.; Singh, R.S.; Iwashita, T.; Kusumoto, T.; Frank, H.A.; Hashimoto, H.; Katsumura, S. Syntheses of allene-modified derivatives of peridinin toward elucidation of the effective role of the allene function in high energy transfer efficiencies in photosynthesis. Org. Biomol. Chem. 2009, 7, 3723–3733. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.C.; Jadhav, P.K. Asymmetric carbon-carbon bond formation via β-allyldiisopinocampheylborane. Simple synthesis of secondary homoallylic alcohols with excellent enantiomeric purities. J. Am. Chem. Soc. 1983, 105, 2092–2093. [Google Scholar] [CrossRef]
- Kim, S.W.; Zhang, W.; Krische, M.J. Catalytic enantioselective carbonyl allylation and propargylation via. alcohol-mediated hydrogen transfer: Merging the chemistry of Grignard and Sabatier. Acc. Chem. Res. 2017, 50, 2371–2380. [Google Scholar] [CrossRef]
- Holmes, M.; Schwartz, L.A.; Krische, M.J. Intermolecular metal-catalyzed reductive coupling of dienes, allenes, and enynes with carbonyl compounds and imines. Chem. Rev. 2018, 118, 6026–6052. [Google Scholar] [CrossRef]
- Brito, G.A.; Jung, W.; Yoo, M.; Krische, M.J. Enantioselective iridium-catalyzed allylation of acetylenic ketones via 2-propanol-mediated reductive coupling of allyl acetate: C14-C23 of pladienolide D. Angew. Chem. Int. Ed. 2019, 58, 18803–18807. [Google Scholar] [CrossRef]
- Bertus, P.; Drouin, L.; Laroche, C.; Szymoniak, J. Pentadienyl transfer reagents based on zirconium: Preparation and reactions with carbonyl compounds. Tetrahedron 2004, 60, 1375–1383. [Google Scholar] [CrossRef]
- Overman, L.E.; Bell, K.L. Enantiospecific total synthesis of dendrobatid toxin 251D. A short chiral entry to the cardiac-active pumiliotoxin A alkaloids via. stereospecific iminium ion-vinylsilane cyclizations. J. Am. Chem. Soc. 1981, 103, 1851–1853. [Google Scholar] [CrossRef]
- Zhao, J.; Niu, Z.; Fu, H.; Li, Y. Ligand-free hydroboration of alkynes catalyzed by heterogeneous copper powder with high efficiency. Chem. Commun. 2013, 50, 2058–2060. [Google Scholar] [CrossRef]
- Parenty, A.; Moreau, X.; Campagne, J.-M. Macrolactonizations in the total synthesis of natural products. Chem. Rev. 2006, 106, 911–939. [Google Scholar] [CrossRef]
- Skaanderup, P.R.; Jensen, T. Synthesis of the macrocyclic core of (−)-pladienolide B. Org. Lett. 2008, 10, 2821–2824. [Google Scholar] [CrossRef]
- Müller, S.; Sasse, F.; Maier, M.E. Synthesis of pladienolide B and its 7-epimer with insights into the role of the allylic acetate. Eur. J. Org. Chem. 2014, 1025–1036. [Google Scholar] [CrossRef]
- Müller, S.; Mayer, T.; Sasse, F.; Maier, M.E. Synthesis of a pladienolide B analogue with the fully functionalized core structure. Org. Lett. 2011, 13, 3940–3943. [Google Scholar] [CrossRef]
- Rhoades, D.; Rheingold, A.L.; O’Malley, B.W.; Wang, J. Expedient total syntheses of pladienolide-derived spliceosome modulators. J. Am. Chem. Soc. 2021, 143, 4915–4920. [Google Scholar] [CrossRef]
- Abiko, A. Boron-mediated aldol reaction of carboxylic esters. Acc. Chem. Res. 2004, 37, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Menche, D.; Hassfeld, J.; Li, J.; Mayer, K.; Rudolph, S. Modular total synthesis of archazolid A and B. J. Org. Chem. 2009, 74, 7220–7229. [Google Scholar] [CrossRef]
- Nagao, Y.; Hagiwara, Y.; Kumagai, T.; Ochiai, M.; Inoue, T.; Hashimo, K.; Fujita, E. New C-4-chiral 1,3-thiazolidine-2-thiones: Excellent chiral auxiliaries for highly diastereo-controlled aldol-type reactions of acetic acid and α,β-unsaturated aldehydes. J. Org. Chem. 1986, 51, 2391–2393. [Google Scholar] [CrossRef]
- Nagao, Y.; Dai, W.M.; Ochiai, M.; Shiro, M. New general asymmetric synthesis of versatile γ-alkylated butenolides and its application to expeditious synthesis of the chiral Geissman-Waiss lactones useful for (+)-retronecine synthesis. J. Org. Chem. 1989, 54, 5211–5217. [Google Scholar] [CrossRef]
- Delaunay, D.; Toupet, L.; Corre, M.L. Reactivity of β-amino alcohols with carbon disulfide study on the synthesis of 2-oxazolidinethiones and 2-thiazolidinethiones. J. Org. Chem. 1995, 60, 6604–6607. [Google Scholar] [CrossRef]
- Shiina, I. Total synthesis of natural 8- and 9-membered lactones: Recent advancements in medium-sized ring formation. Chem. Rev. 2007, 107, 239–273. [Google Scholar] [CrossRef]
- Borisova, S.A.; Kim, H.J.; Pu, X.; Liu, H. Glycosylation of acyclic and cyclic aglycone substrates by macrolide glycosyltransferase DesVII/DesVIII: Analysis and implications. Chem. Bio. Chem. 2008, 9, 1554–1558. [Google Scholar] [CrossRef] [Green Version]
- Jain, N.F.; Cirillo, P.F.; Schaus, J.V.; Panek, J.S. An efficient procedure for the preparation of chiral β-substituted (E)-crotylsilanes: Application of a rhodium(II) catalyzed silylformylation of terminal alkynes. Tetrahedron Lett. 1995, 36, 8723–8726. [Google Scholar] [CrossRef]
- Kim, I.S.; Han, S.B.; Krische, M.J. Anti-diastereo- and enantioselective carbonyl crotylation from the alcohol or aldehyde oxidation level employing a cyclometallated iridium catalyst: α-methyl allyl acetate as a surrogate to preformed crotylmetal reagents. J. Am. Chem. Soc. 2009, 131, 2514–2520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakarian, A.; Batch, A.; Holton, R.A. A convergent total synthesis of hemibrevetoxin B. J. Am. Chem. Soc. 2003, 125, 7822–7824. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Zhugralin, A.R.; Lee, Y.; Hoveyda, A.H. Highly selective methods for synthesis of internal (α-) vinylboronates through efficient NHC–Cu-catalyzed hydroboration of terminal alkynes. Utility in chemical synthesis and mechanistic basis for selectivity. J. Am. Chem. Soc. 2011, 133, 7859–7871. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, W.P.; Vo, A. Dithiocarbamates: Reagents for the removal of transition metals from organic reaction media. Org. Process. Res. Dev. 2014, 19, 1369–1373. [Google Scholar] [CrossRef]
- Keaney, G.F.; Wang, J.; Gerard, B.; Arai, K.; Liu, X.; Zheng, G.Z.; Kira, K.; Tivitmahaisoon, P.; Prajapati, S.; Gearhart, N.C.; et al. Pladienolide Pyridine Compounds and Methods of Use. U.S. Patent 2,015,032,952,8A1, 1 November 2016. [Google Scholar]
































| Cyclization Method | Year | Group | Synthetic Target | Cycliz-ation Yield | LLS | Overall Yield (LLS) | Scheme | Refs. |
|---|---|---|---|---|---|---|---|---|
| RCM | 2007 | Kotake | pladienolide A | 93% | 21 | 3.5% | 1–4 | [49] |
| pladienolide B | 22 | 2.9% | ||||||
| pladienolide D | 20 | 3.1% | ||||||
| 2012 | Ghosh | pladienolide B | 83% | 17 | 1.4% | 5–7 | [50] | |
| 2012 | Burkart | FD-895 | 48% | 15 | 0.8% | 8–10 | [34] | |
| 2013 | Chandrasekhar | pladienolide B | 52% | 21 | 7.0% | 11–14 | [51] | |
| 2014 | Keaney | 6-deoxypladienolide D | 77% | 18 | 0.6% | 15, 16 | [52] | |
| 2021 | Krische | pladienolide B | 51% | 10 | 0.8% | 18–20 | [53] | |
| Macrolactonization | 2008 | Skaanderup | macrocycle of pladienolide B | 63% | 15 | 10.0% | 21, 22 | [81] |
| 2014 | Maier | pladienolide B | 93% | 17 | 2.0% | 23–25 | [82,83] | |
| 2021 | Rhoades–O’Malley–Wang | pladienolide A | 70% | 9 | 11.8% | 26–28 | [84] | |
| pladienolide B | 10 | 11.9% | ||||||
| H3B-8800 | 10 | 10.1% |
| Cyclization Method | Group | Synthetic Features |
|---|---|---|
| RCM | Kotake (Section 2.2.1) |
|
| Ghosh (Section 2.2.2) |
| |
| Burkart (Section 2.2.3) |
| |
| Chandrasekhar (Section 2.2.4) |
| |
| Keaney (Section 2.2.5) |
| |
| Krische (Section 2.2.6) |
| |
| Macro-lactonization | Skaanderup (Section 2.3.1) |
|
| Maier (Section 2.3.2) |
| |
| Rhoades–O’Malley–Wang (Section 2.3.3) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.; Jang, E.; Kim, H.J.; Jeon, H. Total Syntheses of Pladienolide-Derived Spliceosome Modulators. Molecules 2021, 26, 5938. https://doi.org/10.3390/molecules26195938
Sim J, Jang E, Kim HJ, Jeon H. Total Syntheses of Pladienolide-Derived Spliceosome Modulators. Molecules. 2021; 26(19):5938. https://doi.org/10.3390/molecules26195938
Chicago/Turabian StyleSim, Jaehoon, Eunbin Jang, Hyun Jin Kim, and Hongjun Jeon. 2021. "Total Syntheses of Pladienolide-Derived Spliceosome Modulators" Molecules 26, no. 19: 5938. https://doi.org/10.3390/molecules26195938