Present and Perspectives of Photoactive Porous Composites Based on Semiconductor Nanocrystals and Metal-Organic Frameworks
Abstract
:1. Introduction
1.1. Photoactive Semiconductor Nanocrystals
1.2. Metal-Organic Frameworks (MOFs)
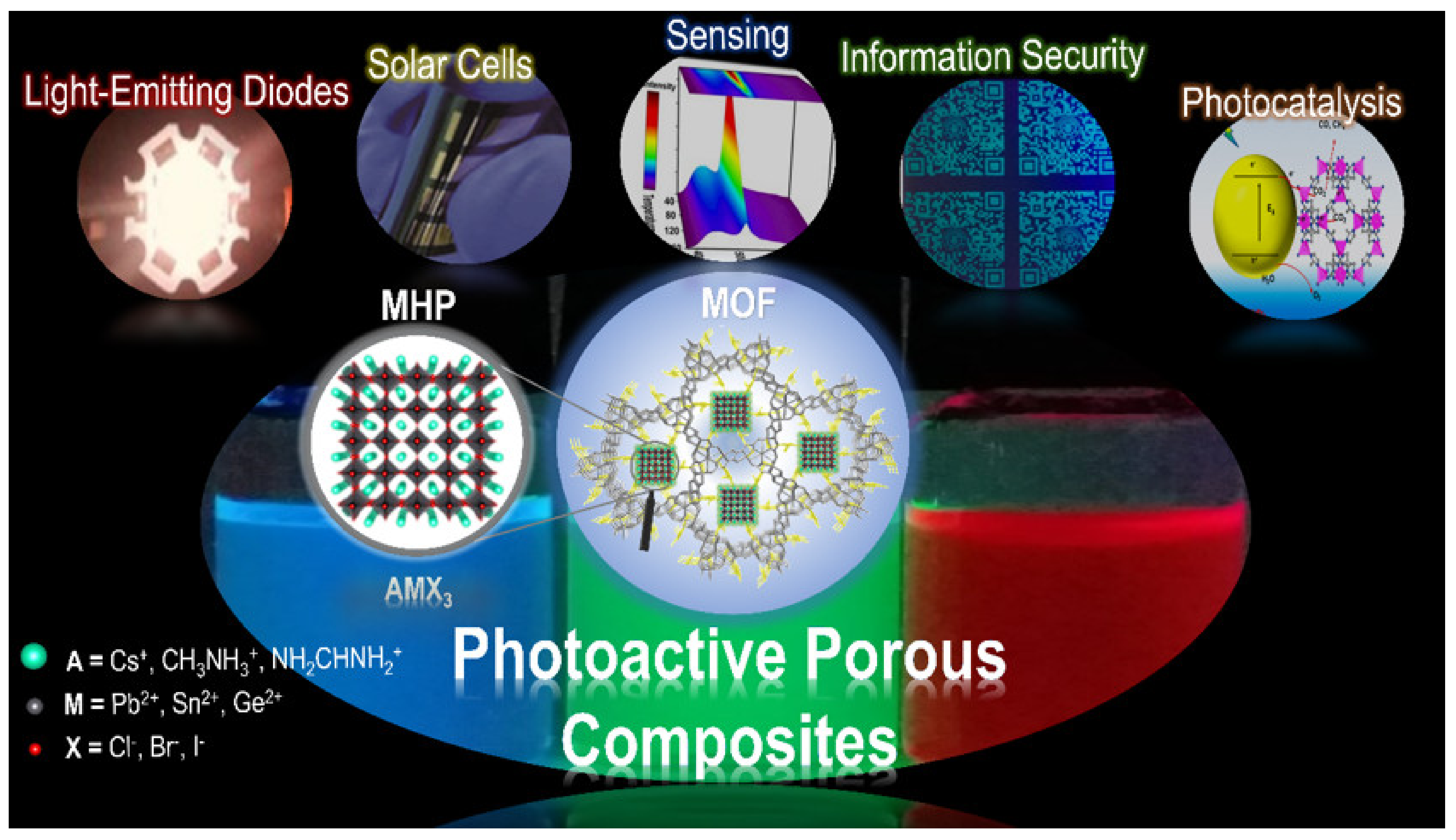
1.3. Photoactive Porous Composites Based on Semiconductor Nanocrystals and Metal-Organic Frameworks
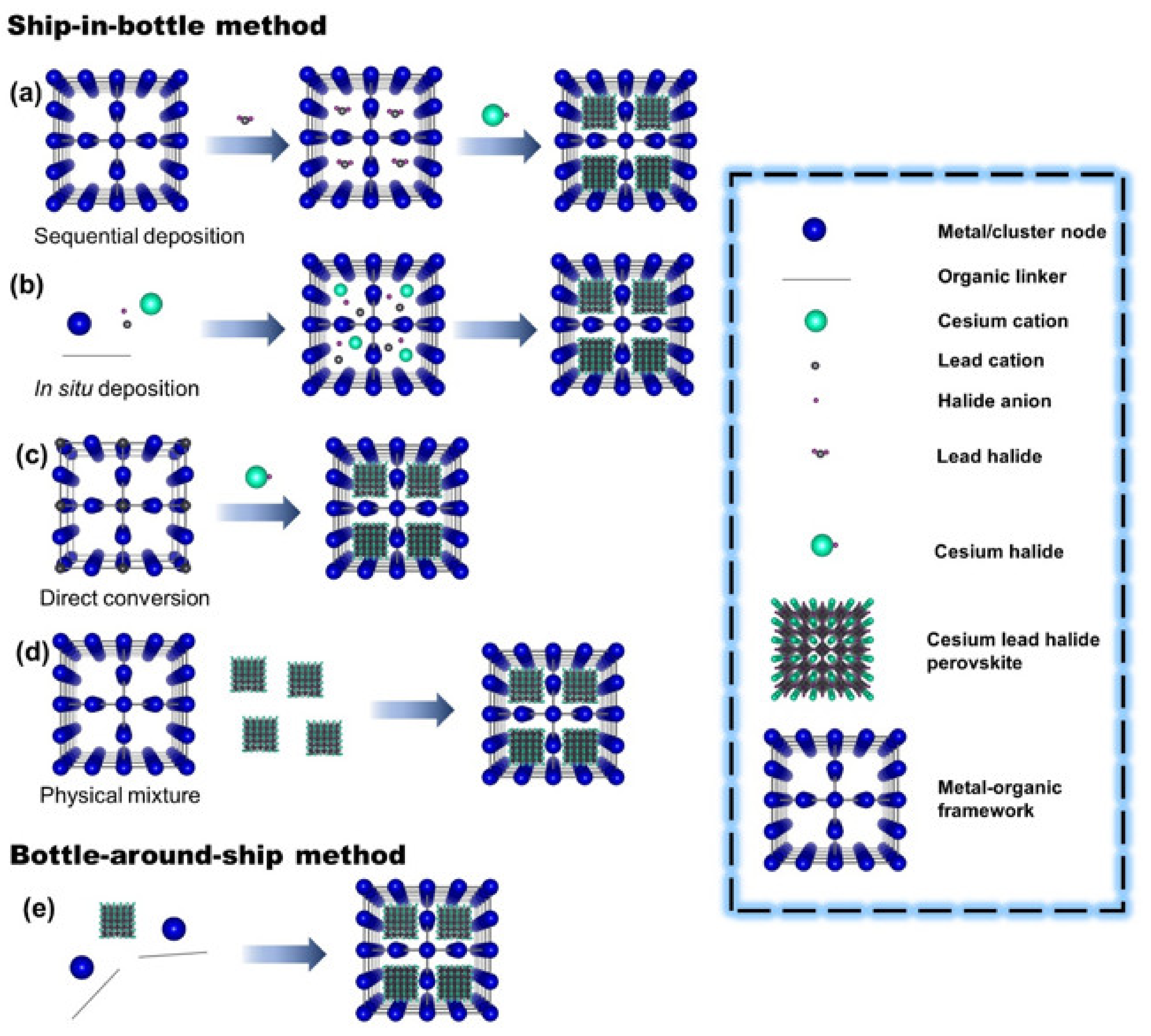
2. Synthesis Methodology of Photoactive Porous Composites
2.1. Ship-in-Bottle Method
2.2. Bottle-around-Ship Method
3. Properties and Applications of MHP@MOF Composites
3.1. Light-Emitting Diodes
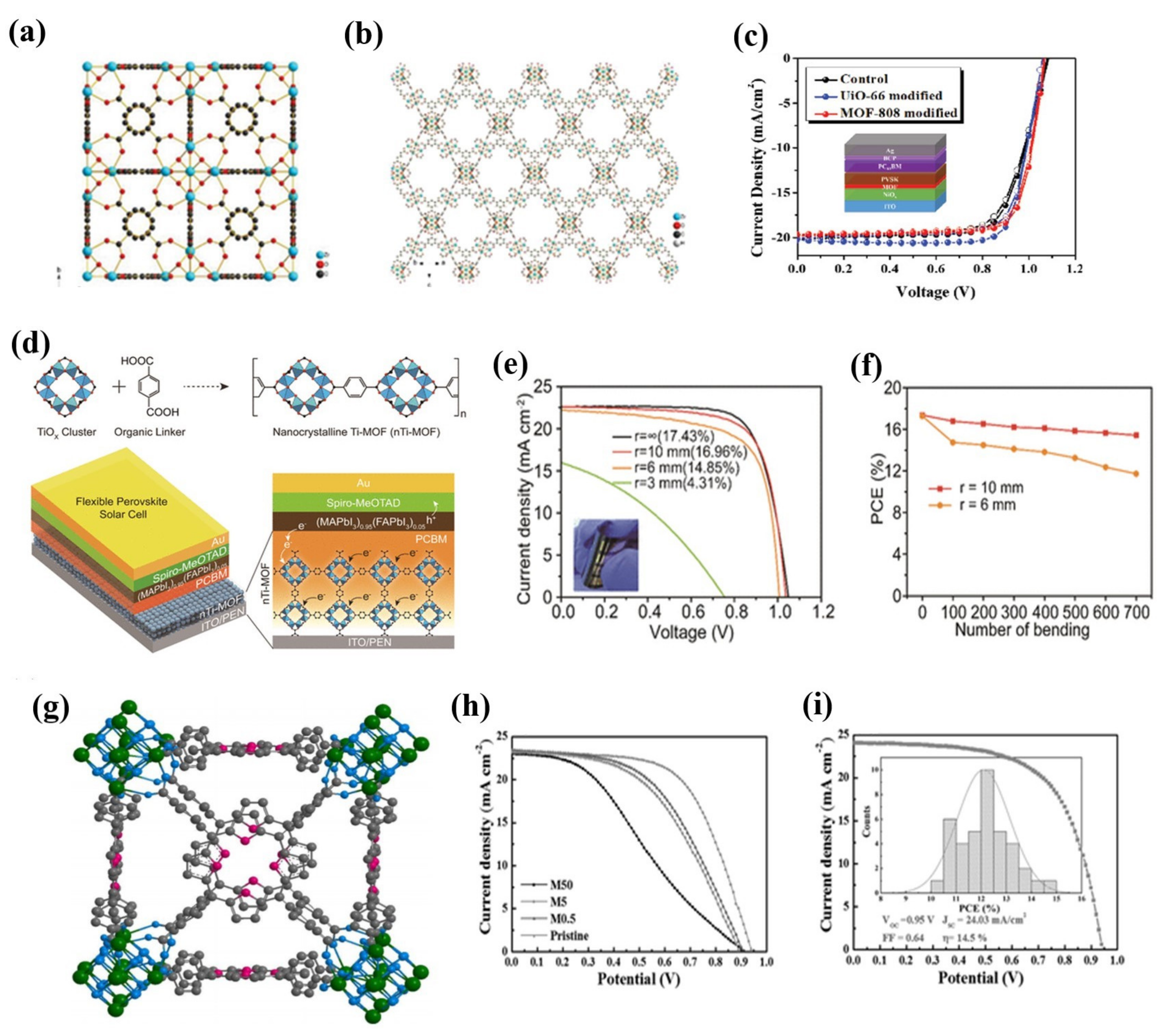
3.2. Perovskite Solar Cells
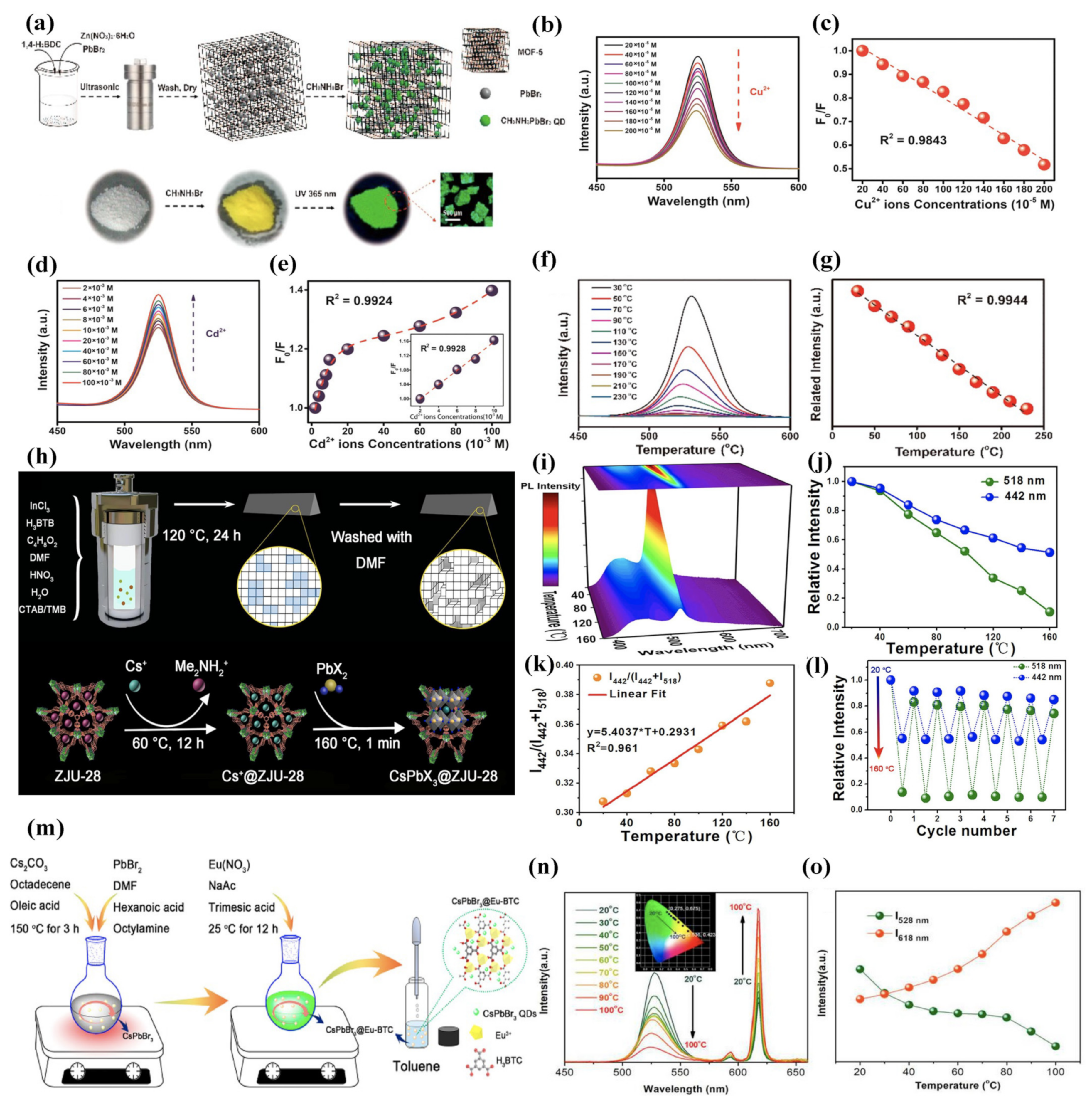
3.3. Sensing Applications
3.4. Information Security
3.5. Photocatalysis
4. Conclusions, Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raquel Eugenia, G.; Julia, P.-P. Synergism at the Nanoscale: Photoactive Semiconductor Nanoparticles and their Organic Ligands. In Research Perspectives on Functional Micro- and Nanoscale Coatings; Ana, Z., Maria Carmen, M.-M., Eds.; IGI Global: Hershey, PA, USA, 2016; pp. 42–77. [Google Scholar]
- Wei, Y.; Cheng, Z.; Lin, J. An overview on enhancing the stability of lead halide perovskite quantum dots and their applications in phosphor-converted LEDs. Chem. Soc. Rev. 2019, 48, 310–350. [Google Scholar] [CrossRef] [PubMed]
- Blay, V.; Galian, R.E.; Muresan, L.M.; Pankratov, D.; Pinyou, P.; Zampardi, G. Research Frontiers in Energy-Related Materials and Applications for 2020–2030. Adv. Sustain. Syst. 2020, 4, 1900145. [Google Scholar] [CrossRef]
- Corsini, F.; Griffini, G. Recent progress in encapsulation strategies to enhance the stability of organometal halide perovskite solar cells. J. Phys. Energy 2020, 2, 031002. [Google Scholar] [CrossRef]
- Wang, D.; Wright, M.; Elumalai, N.K.; Uddin, A. Stability of perovskite solar cells. Sol. Energy Mater. Sol. Cells 2016, 147, 255–275. [Google Scholar] [CrossRef]
- Gonzalez-Carrero, S.; Francés-Soriano, L.; González-Béjar, M.; Agouram, S.; Galian, R.E.; Pérez-Prieto, J. The Luminescence of CH3NH3PbBr3 Perovskite Nanoparticles Crests the Summit and Their Photostability under Wet Conditions is Enhanced. Small 2016, 12, 5245–5250. [Google Scholar] [CrossRef]
- Huang, H.; Chen, B.; Wang, Z.; Hung, T.F.; Susha, A.S.; Zhong, H.; Rogach, A.L. Water resistant CsPbX3 nanocrystals coated with polyhedral oligomeric silsesquioxane and their use as solid state luminophores in all-perovskite white light-emitting devices. Chem. Sci. 2016, 7, 5699–5703. [Google Scholar] [CrossRef] [Green Version]
- Hills-Kimball, K.; Yang, H.; Cai, T.; Wang, J.; Chen, O. Recent Advances in Ligand Design and Engineering in Lead Halide Perovskite Nanocrystals. Adv. Sci. 2021, 8, 2100214. [Google Scholar] [CrossRef]
- Wang, H.-C.; Lin, S.-Y.; Tang, A.-C.; Singh, B.P.; Tong, H.-C.; Chen, C.-Y.; Lee, Y.-C.; Tsai, T.-L.; Liu, R.-S. Mesoporous Silica Particles Integrated with All-Inorganic CsPbBr3 Perovskite Quantum-Dot Nanocomposites (MP-PQDs) with High Stability and Wide Color Gamut Used for Backlight Display. Angew. Chem. Int. Ed. 2016, 55, 7924–7929. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Rabouw, F.T.; Yang, X.-F.; Huang, X.-Y.; Jing, X.-P.; Ye, S.; Zhang, Q.-Y. Facile Two-Step Synthesis of All-Inorganic Perovskite CsPbX3 (X = Cl, Br, and I) Zeolite-Y Composite Phosphors for Potential Backlight Display Application. Adv. Funct. Mater. 2017, 27, 1704371. [Google Scholar] [CrossRef]
- Lee, C.-C.; Chen, C.-I.; Liao, Y.-T.; Wu, K.C.W.; Chueh, C.-C. Perovskite Solar Cells: Enhancing Efficiency and Stability of Photovoltaic Cells by Using Perovskite/Zr-MOF Heterojunction Including Bilayer and Hybrid Structures. Adv. Sci. 2019, 6, 1970030. [Google Scholar] [CrossRef]
- Liu, M.; Matuhina, A.; Zhang, H.; Vivo, P. Advances in the Stability of Halide Perovskite Nanocrystals. Materials 2019, 12, 3733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bag, P.P.; Singh, G.P.; Singha, S.; Roymahapatra, G. Synthesis of Metal-Organic Frameworks (MOFs) and Their Biological, Catalytic and Energetic Applications: A Mini Review. Eng. Sci. 2021, 13, 1–10. [Google Scholar]
- Reed, M.A. Quantum Dots. Sci. Am. 1993, 268, 118–123. [Google Scholar] [CrossRef]
- Chen, F.; Gerion, D. Fluorescent CdSe/ZnS Nanocrystal−Peptide Conjugates for Long-term, Nontoxic Imaging and Nuclear Targeting in Living Cells. Nano Lett. 2004, 4, 1827–1832. [Google Scholar] [CrossRef] [Green Version]
- Alivisatos, P. The use of nanocrystals in biological detection. Nat. Biotechnol. 2004, 22, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.J.; Lim, J.; Roh, J.; Neo, D.C.J.; Law, M.; Klimov, V.I. Solution-processable integrated CMOS circuits based on colloidal CuInSe2 quantum dots. Nat. Commun. 2020, 11, 5280. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Rao, H.; Mora-Seró, I.; Bisquert, J.; Zhong, X. Quantum dot-sensitized solar cells. Chem. Soc. Rev. 2018, 47, 7659–7702. [Google Scholar] [CrossRef]
- Li, B.; Lu, M.; Feng, J.; Zhang, J.; Smowton, P.M.; Sohn, J.I.; Park, I.-K.; Zhong, H.; Hou, B. Colloidal quantum dot hybrids: An emerging class of materials for ambient lighting. J. Mater. Chem. C 2020, 8, 10676–10695. [Google Scholar] [CrossRef]
- Park, Y.-S.; Roh, J.; Diroll, B.T.; Schaller, R.D.; Klimov, V.I. Colloidal quantum dot lasers. Nat. Rev. Mater. 2021, 6, 382–401. [Google Scholar] [CrossRef]
- McJunkin, T.W. Heterostructure Modifications, Fabrication Improvements, and Measurements Automation of Si/SiGe Quantum Dots for Quantum Computation. Ph.D. Dissertation, The University of Wisconsin-Madison, Madison, WI, USA, 2021. [Google Scholar]
- Konstantatos, G.; Sargent, E.H. Nanostructured materials for photon detection. Nat. Nanotechnol. 2010, 5, 391–400. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, S.; Fan, D.; Reilly, J.; Zhang, H.; Yao, W.; Huang, J. Carbon Quantum Dot/TiO2 Nanohybrids: Efficient Photocatalysts for Hydrogen Generation via Intimate Contact and Efficient Charge Separation. ACS Appl. Nano Mater. 2019, 2, 1027–1032. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, In Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Zhang, H.; Zhong, X. Facile synthesis of red- to near-infrared-emitting CdTexSe1-x alloyed quantum dots via noninjection one-pot route. J. Lumin. 2011, 131, 322–327. [Google Scholar] [CrossRef]
- Troparevsky, M.C.; Kronik, L.; Chelikowsky, J.R. Optical properties of CdSe quantum dots. J. Chem. Phys. 2003, 119, 2284–2287. [Google Scholar] [CrossRef]
- Borchert, H.; Talapin, D.V.; McGinley, C.; Adam, S.; Lobo, A.; de Castro, A.R.B.; Möller, T.; Weller, H. High resolution photoemission study of CdSe and CdSe/ZnS core-shell nanocrystals. J. Chem. Phys. 2003, 119, 1800–1807. [Google Scholar] [CrossRef]
- Carrillo-Carrión, C.; Cárdenas, S.; Simonet, B.M.; Valcárcel, M. Quantum dots luminescence enhancement due to illumination with UV/Vis light. Chem. Commun. 2009, 5214–5226. [Google Scholar] [CrossRef]
- de Mello Donegá, C.; Liljeroth, P.; Vanmaekelbergh, D. Physicochemical Evaluation of the Hot-Injection Method, a Synthesis Route for Monodisperse Nanocrystals. Small 2005, 1, 1152–1162. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Joo, J.; Kwon, S.G.; Jang, Y.; Hyeon, T. Synthesis of Monodisperse Spherical Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 4630–4660. [Google Scholar] [CrossRef]
- Heo, D.Y.; Do, H.H.; Ahn, S.H.; Kim, S.Y. Metal-Organic Framework Materials for Perovskite Solar Cells. Polymers 2020, 12, 2061. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Van Le, Q.; Jang, H.W.; Kim, S.Y. Recent Advances toward High-Efficiency Halide Perovskite Light-Emitting Diodes: Review and Perspective. Small Methods 2018, 2, 1700419. [Google Scholar] [CrossRef]
- Wang, X.; Bao, Z.; Chang, Y.-C.; Liu, R.-S. Perovskite Quantum Dots for Application in High Color Gamut Backlighting Display of Light-Emitting Diodes. ACS Energy Lett. 2020, 5, 3374–3396. [Google Scholar] [CrossRef]
- Stylianakis, M.M.; Maksudov, T.; Panagiotopoulos, A.; Kakavelakis, G.; Petridis, K. Inorganic and Hybrid Perovskite Based Laser Devices: A Review. Materials 2019, 12, 859. [Google Scholar] [CrossRef] [Green Version]
- Huynh, K.A.; Nguyen, D.L.T.; Nguyen, V.-H.; Vo, D.-V.N.; Trinh, Q.T.; Nguyen, T.P.; Kim, S.Y.; Le, Q.V. Halide perovskite photocatalysis: Progress and perspectives. J. Chem. Technol. Biotechnol. 2020, 95, 2579–2596. [Google Scholar] [CrossRef]
- Dey, A.; Ye, J.; De, A.; Debroye, E.; Ha, S.K.; Bladt, E.; Kshirsagar, A.S.; Wang, Z.; Yin, J.; Wang, Y.; et al. State of the Art and Prospects for Halide Perovskite Nanocrystals. ACS Nano 2021, 15, 10775. [Google Scholar] [CrossRef]
- Watthage, S.C.; Song, Z.; Phillips, A.B.; Heben, M.J. Evolution of Perovskite Solar Cells. In Perovskite Photovoltaics; Thomas, S., Thankappan, A., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 43–88. [Google Scholar]
- Zhou, C.; Lin, H.; He, Q.; Xu, L.; Worku, M.; Chaaban, M.; Lee, S.; Shi, X.; Du, M.-H.; Ma, B. Low dimensional metal halide perovskites and hybrids. Mater. Sci. Eng. R Rep. 2019, 137, 38–65. [Google Scholar] [CrossRef]
- Yadav, S.K.; Grandhi, G.K.; Dubal, D.P.; de Mello, J.C.; Otyepka, M.; Zbořil, R.; Fischer, R.A.; Jayaramulu, K. Metal Halide Perovskite@Metal-Organic Framework Hybrids: Synthesis, Design, Properties, and Applications. Small 2020, 16, 2004891. [Google Scholar] [CrossRef]
- González-Carrero, S.; Galian, R.E.; Pérez-Prieto, J. Organometal Halide Perovskites: Bulk Low-Dimension Materials and Nanoparticles. Part. Part. Syst. Charact. 2015, 32, 709–720. [Google Scholar] [CrossRef]
- Huang, H.; Polavarapu, L.; Sichert, J.A.; Susha, A.S.; Urban, A.S.; Rogach, A.L. Colloidal lead halide perovskite nanocrystals: Synthesis, optical properties and applications. NPG Asia Mater. 2016, 8, e328. [Google Scholar] [CrossRef] [Green Version]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Snaith, H.J. Present status and future prospects of perovskite photovoltaics. Nat. Mater. 2018, 17, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Y.; Tang, J.; Tang, W. Surface ligands engineering of semiconductor quantum dots for chemosensory and biological applications. Mater. Today 2017, 20, 360–376. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Y.; Cai, B.; Ma, Q.; Zheng, X.; Zhang, W.-H. Solution-Processable Perovskite Solar Cells toward Commercialization: Progress and Challenges. Adv. Funct. Mater. 2019, 29, 1807661. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.-G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Aldakov, D.; Reiss, P. Safer-by-Design Fluorescent Nanocrystals: Metal Halide Perovskites vs Semiconductor Quantum Dots. J. Phys. Chem. C 2019, 123, 12527–12541. [Google Scholar] [CrossRef] [Green Version]
- Lyu, M.; Yun, J.-H.; Chen, P.; Hao, M.; Wang, L. Addressing Toxicity of Lead: Progress and Applications of Low-Toxic Metal Halide Perovskites and Their Derivatives. Adv. Energy Mater. 2017, 7, 1602512. [Google Scholar] [CrossRef]
- Ke, W.; Kanatzidis, M.G. Prospects for low-toxicity lead-free perovskite solar cells. Nat. Commun. 2019, 10, 965. [Google Scholar] [CrossRef]
- Kong, X.-J.; Li, J.-R. An Overview of Metal–Organic Frameworks for Green Chemical Engineering. Engineering 2021, in press. [Google Scholar] [CrossRef]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous metal–organic framework materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yagui, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Hamisu, A.M.; Ariffin, A.; Wibowo, A.C. Cation exchange in metal-organic frameworks (MOFs): The hard-soft acid-base (HSAB) principle appraisal. Inorg. Chim. Acta 2020, 511, 119801. [Google Scholar] [CrossRef]
- Devic, T.; Serre, C. High valence 3p and transition metal based MOFs. Chem. Soc. Rev. 2014, 43, 6097–6115. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascanu, V.; González Miera, G.; Inge, A.K.; Martín-Matute, B. Metal–Organic Frameworks as Catalysts for Organic Synthesis: A Critical Perspective. J. Am. Chem. Soc. 2019, 141, 7223–7234. [Google Scholar] [CrossRef] [Green Version]
- Viciano-Chumillas, M.; Mon, M.; Ferrando-Soria, J.; Corma, A.; Leyva-Pérez, A.; Armentano, D.; Pardo, E. Metal–Organic Frameworks as Chemical Nanoreactors: Synthesis and Stabilization of Catalytically Active Metal Species in Confined Spaces. Acc. Chem. Res. 2020, 53, 520–531. [Google Scholar] [CrossRef]
- Bilanin, C.; Tiburcio, E.; Ferrando-Soria, J.; Armentano, D.; Leyva-Pérez, A.; Pardo, E. Cover Feature: Crystallographic Visualization of a Double Water Molecule Addition on a Pt1-MOF during the Low-temperature Water-Gas Shift Reaction. ChemCatChem 2021, 13, 1038. [Google Scholar] [CrossRef]
- Escamilla, P.; Viciano-Chumillas, M.; Bruno, R.; Armentano, D.; Pardo, E.; Ferrando-Soria, J. Photodegradation of Brilliant Green Dye by a Zinc bioMOF and Crystallographic Visualization of Resulting CO2. Molecules 2021, 26, 4098. [Google Scholar] [CrossRef]
- Li, D.-Z.; Chen, L.; Liu, G.; Yuan, Z.-Y.; Li, B.-F.; Zhang, X.; Wei, J.-Q. Porous metal–organic frameworks for methane storage and capture: Status and challenges. New Carbon Mater. 2021, 36, 468–496. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef]
- Negro, C.; Martínez Pérez-Cejuela, H.; Simó-Alfonso, E.F.; Herrero-Martínez, J.M.; Bruno, R.; Armentano, D.; Ferrando-Soria, J.; Pardo, E. Highly Efficient Removal of Neonicotinoid Insecticides by Thioether-Based (Multivariate) Metal–Organic Frameworks. ACS Appl. Mater. Interfaces 2021, 13, 28424. [Google Scholar] [CrossRef]
- Udourioh, G.A.; Solomon, M.M.; Epelle, E.I. Metal Organic Frameworks as Biosensing Materials for COVID-19. Cell. Mol. Bioeng. 2021, 1–19. [Google Scholar] [CrossRef]
- Kaur, H.; Sundriyal, S.; Pachauri, V.; Ingebrandt, S.; Kim, K.-H.; Sharma, A.L.; Deep, A. Luminescent metal-organic frameworks and their composites: Potential future materials for organic light emitting displays. Coord. Chem. Rev. 2019, 401, 213077. [Google Scholar] [CrossRef]
- Bieniek, A.; Terzyk, A.P.; Wiśniewski, M.; Roszek, K.; Kowalczyk, P.; Sarkisov, L.; Keskin, S.; Kaneko, K. MOF materials as therapeutic agents, drug carriers, imaging agents and biosensors in cancer biomedicine: Recent advances and perspectives. Prog. Mater. Sci. 2021, 117, 100743. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. J. Am. Chem. Soc. 1989, 111, 5962–5964. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. A reappraisal of the zinc cyanide and cadmium cyanide structures and the synthesis and structure of the diamond-related frameworks [N(CH3)4][CuIZnII(CN)4] and CuI[4,4′,4″,4‴-tetracyanotetraphenylmethane]BF4.xC6H5NO2. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar]
- Yaghi, O.M.; Li, G.; Li, H. Selective binding and removal of guests in a microporous metal–organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Kondo, M.; Yoshitomi, T.; Matsuzaka, H.; Kitagawa, S.; Seki, K. Three-Dimensional Framework with Channeling Cavities for Small Molecules: {[M2(4, 4′-bpy)3(NO3)4]·xH2O}n (M Co, Ni, Zn). Angew. Chem. Int. Ed. Engl. 1997, 36, 1725–1727. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; Groy, T.L.; Yaghi, O.M. Establishing Microporosity in Open Metal−Organic Frameworks: Gas Sorption Isotherms for Zn(BDC) (BDC = 1,4-Benzenedicarboxylate). J. Am. Chem. Soc. 1998, 120, 8571–8572. [Google Scholar] [CrossRef]
- Livage, C.; Egger, C.; Férey, G. Hybrid Open Networks (MIL 16): Synthesis, Crystal Structure, and Ferrimagnetism of Co4(OH)2(H2O)2(C4H4O4)3·2H2O, a New Layered Cobalt(II) Carboxylate with 14-Membered Ring Channels. Chem. Mater. 1999, 11, 1546–1550. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar] [CrossRef] [PubMed]
- Remya, V.R.; Kurian, M. Synthesis and catalytic applications of metal–organic frameworks: A review on recent literature. Int. Nano Lett. 2019, 9, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Pachfule, P.; Das, R.; Poddar, P.; Banerjee, R. Solvothermal Synthesis, Structure, and Properties of Metal Organic Framework Isomers Derived from a Partially Fluorinated Link. Cryst. Growth Des. 2011, 11, 1215–1222. [Google Scholar] [CrossRef]
- Ni, Z.; Masel, R.I. Rapid Production of Metal−Organic Frameworks via Microwave-Assisted Solvothermal Synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395. [Google Scholar] [CrossRef]
- Cui, J.; Gao, N.; Wang, C.; Zhu, W.; Li, J.; Wang, H.; Seidel, P.; Ravoo, B.J.; Li, G. Photonic metal–organic framework composite spheres: A new kind of optical material with self-reporting molecular recognition. Nanoscale 2014, 6, 11995–12001. [Google Scholar] [CrossRef]
- Lv, D.; Chen, Y.; Li, Y.; Shi, R.; Wu, H.; Sun, X.; Xiao, J.; Xi, H.; Xia, Q.; Li, Z. Efficient Mechanochemical Synthesis of MOF-5 for Linear Alkanes Adsorption. J. Chem. Eng. Data 2017, 62, 2030–2036. [Google Scholar] [CrossRef]
- Son, W.-J.; Kim, J.; Kim, J.; Ahn, W.-S. Sonochemical synthesis of MOF-5. Chem. Commun. 2008, 6336–6338. [Google Scholar] [CrossRef]
- Campagnol, N.; Van Assche, T.; Boudewijns, T.; Denayer, J.; Binnemans, K.; De Vos, D.; Fransaer, J. High pressure, high temperature electrochemical synthesis of metal–organic frameworks: Films of MIL-100 (Fe) and HKUST-1 in different morphologies. J. Mater. Chem. A 2013, 1, 5827–5830. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, S.; Kondo, M. Functional Micropore Chemistry of Crystalline Metal Complex-Assembled Compounds. Bull. Chem. Soc. Jpn. 1998, 71, 1739–1753. [Google Scholar] [CrossRef]
- Moghadam, P.Z.; Li, A.; Liu, X.-W.; Bueno-Perez, R.; Wang, S.-D.; Wiggin, S.B.; Wood, P.A.; Fairen-Jimenez, D. Targeted classification of metal–organic frameworks in the Cambridge structural database (CSD). Chem. Sci. 2020, 11, 8373–8387. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.W.; Lee, D.T.; Barton, H.F.; Epps, T.H.; Parsons, G.N. Fibre-based composites from the integration of metal–organic frameworks and polymers. Nat. Rev. Mater. 2021, 6, 605–621. [Google Scholar] [CrossRef]
- Han, S.; Ding, Y.; Teng, F.; Yao, A.; Leng, Q. Determination of chloropropanol with an imprinted electrochemical sensor based on multi-walled carbon nanotubes/metal–organic framework composites. RSC Adv. 2021, 11, 18468–18475. [Google Scholar] [CrossRef]
- Shi, X.; Xu, Y.; Zhao, B.; Li, P.; Song, M.; Jia, J.; Yu, H.; Lu, G. Integrating Conductive Metal–Organic Framework with Graphene Oxide to Highly Sensitive Platform for Electrochemical Sensing. Adv. Mater. Interfaces 2021, 8, 2100586. [Google Scholar] [CrossRef]
- Ali, M.M.; Zhu, Z.; Hussain, D.; Shen, Z.; He, Y.; Du, Z. Flexible and hierarchical metal-organic framework composite as solid-phase media for facile affinity-tip fabrication to selectively enrich glycopeptides and phosphopeptides. Talanta 2021, 233, 122576. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Ying, Y. Recent advances in sensing applications of metal nanoparticle/metal–organic framework composites. TrAC Trends Anal. Chem. 2021, 143, 116395. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Guo, Q. CdSe QDs@ Fe-based metal organic framework composites for improved photocatalytic RhB degradation under visible light. Microporous Mesoporous Mater. 2021, 324, 111291. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Li, L. Metal Halide Perovskite Nanocrystals in Metal–Organic Framework Host: Not Merely Enhanced Stability. Angew. Chem. Int. Ed. 2021, 60, 7488–7501. [Google Scholar] [CrossRef]
- Kumagai, K.; Uematsu, T.; Torimoto, T.; Kuwabata, S. Direct surface modification of semiconductor quantum dots with metal–organic frameworks. CrystEngComm 2019, 21, 5568–5577. [Google Scholar] [CrossRef]
- Aguilera-Sigalat, J.; Bradshaw, D. Synthesis and applications of metal-organic framework–quantum dot (QD@MOF) composites. Coord. Chem. Rev. 2016, 307, 267–291. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Liu, X.; Liu, Y.; Cheng, M.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; Zhang, W.; He, Q.; et al. Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation. Coord. Chem. Rev. 2020, 403, 213097. [Google Scholar] [CrossRef]
- Xu, H.-Q.; Yang, S.; Ma, X.; Huang, J.; Jiang, H.-L. Unveiling Charge-Separation Dynamics in CdS/Metal–Organic Framework Composites for Enhanced Photocatalysis. ACS Catal. 2018, 8, 11615–11621. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, Z.-G.; Fu, W.-Q.; Wang, F.; Zhang, J. A Confined Fabrication of Perovskite Quantum Dots in Oriented MOF Thin Film. ACS Appl. Mater. Interfaces 2016, 8, 28737–28742. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Cui, Y.; Li, B.; Wang, B.; Jin, C.; Yu, J.; Yao, L.; Yang, Y.; Chen, B.; Qian, G. Confinement of Perovskite-QDs within a Single MOF Crystal for Significantly Enhanced Multiphoton Excited Luminescence. Adv. Mater. 2019, 31, 1806897. [Google Scholar] [CrossRef]
- Cha, J.-H.; Noh, K.; Yin, W.; Lee, Y.; Park, Y.; Ahn, T.K.; Mayoral, A.; Kim, J.; Jung, D.-Y.; Terasaki, O. Formation and Encapsulation of All-Inorganic Lead Halide Perovskites at Room Temperature in Metal–Organic Frameworks. J. Phys. Chem. Lett. 2019, 10, 2270–2277. [Google Scholar] [CrossRef]
- Chen, W.; Liu, H.; Fan, R.; Wang, P.; Sun, T.; Yang, Y. Formation and Encapsulation of Lead Halide Perovskites in Lanthanide Metal–Organic Frameworks for Tunable Emission. ACS Appl. Mater. Interfaces 2020, 12, 9851–9857. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, J.; Liu, Q.; Xia, Z. Synthesis and Luminescence Properties of CsPbX3@Uio-67 Composites toward Stable Photoluminescence Convertors. Inorg. Chem. 2019, 58, 1690–1696. [Google Scholar] [CrossRef]
- Ren, J.; Li, T.; Zhou, X.; Dong, X.; Shorokhov, A.V.; Semenov, M.B.; Krevchik, V.D.; Wang, Y. Encapsulating all-inorganic perovskite quantum dots into mesoporous metal organic frameworks with significantly enhanced stability for optoelectronic applications. Chem. Eng. J. 2019, 358, 30–39. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, H.; Lin, W.; Wang, J.; Chi, Y. Enhancing air-stability of CH3NH3PbBr3 perovskite quantum dots by in-situ growth in metal-organic frameworks and their applications in light emitting diodes. J. Solid State Chem. 2019, 272, 221–226. [Google Scholar] [CrossRef]
- Mollick, S.; Mandal, T.N.; Jana, A.; Fajal, S.; Ghosh, S.K. A hybrid blue perovskite@metal–organic gel (MOG) nanocomposite: Simultaneous improvement of luminescence and stability. Chem. Sci. 2019, 10, 10524–10530. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Xie, C.; Zhang, X.; Yang, P. CsPbX3 Quantum Dots Embedded in Zeolitic Imidazolate Framework-8 Microparticles for Bright White Light-Emitting Devices. ACS Appl. Nano Mater. 2021, 4, 5478–5485. [Google Scholar] [CrossRef]
- Vinogradov, A.V.; Zaake-Hertling, H.; Hey-Hawkins, E.; Agafonov, A.V.; Seisenbaeva, G.A.; Kessler, V.G.; Vinogradov, V.V. The first depleted heterojunction TiO2–MOF-based solar cell. Chem. Commun. 2014, 50, 10210–10213. [Google Scholar] [CrossRef]
- Chang, T.-H.; Kung, C.-W.; Chen, H.-W.; Huang, T.-Y.; Kao, S.-Y.; Lu, H.-C.; Lee, M.-H.; Boopathi, K.M.; Chu, C.-W.; Ho, K.-C. Planar Heterojunction Perovskite Solar Cells Incorporating Metal–Organic Framework Nanocrystals. Adv. Mater. 2015, 27, 7229–7235. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Pang, A.; Li, Y.; Dou, J.; Wei, M. Metal–organic frameworks at interfaces of hybrid perovskite solar cells for enhanced photovoltaic properties. Chem. Commun. 2018, 54, 1253–1256. [Google Scholar] [CrossRef]
- Ryu, U.; Jee, S.; Park, J.-S.; Han, I.K.; Lee, J.H.; Park, M.; Choi, K.M. Nanocrystalline Titanium Metal–Organic Frameworks for Highly Efficient and Flexible Perovskite Solar Cells. ACS Nano 2018, 12, 4968–4975. [Google Scholar] [CrossRef]
- Lee, C.-C.; Chen, C.-I.; Liao, Y.-T.; Wu, K.C.W.; Chueh, C.-C. Enhancing Efficiency and Stability of Photovoltaic Cells by Using Perovskite/Zr-MOF Heterojunction Including Bilayer and Hybrid Structures. Adv. Sci. 2019, 6, 1801715. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhang, J.; Yang, Y.; Qiu, L.; Xia, D.; Lin, K.; Wang, J.; Fan, X.; Fan, R. Self-Assembly of Hybrid Oxidant POM@Cu-BTC for Enhanced Efficiency and Long-Term Stability of Perovskite Solar Cells. Angew. Chem. Int. Ed. 2019, 58, 17610–17615. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.H.; Bark, C.W. Synthesis of Cobalt-Doped TiO2 Based on Metal–Organic Frameworks as an Effective Electron Transport Material in Perovskite Solar Cells. ACS Omega 2020, 5, 2280–2286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadian-Yazdi, M.-R.; Gholampour, N.; Eslamian, M. Interface Engineering by Employing Zeolitic Imidazolate Framework-8 (ZIF-8) as the Only Scaffold in the Architecture of Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 3134–3143. [Google Scholar] [CrossRef]
- Qiu, L.; Xing, K.; Zhang, J.; Yang, Y.; Cao, W.; Zhou, X.; Zhu, K.; Xia, D.; Fan, R. Two-Dimensional Metal–Organic Frameworks-Based Grain Termination Strategy Enables High-Efficiency Perovskite Photovoltaics with Enhanced Moisture and Thermal Stability. Adv. Funct. Mater. 2021, 31, 2010368. [Google Scholar] [CrossRef]
- Sowmehesaraee, M.S.; Ranjbar, M.; Abedi, M.; Mozaffari, S.A. Fabrication of lead iodide perovskite solar cells by incorporating zirconium, indium and zinc metal-organic frameworks. Sol. Energy 2021, 214, 138–148. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, Y.; Liu, Q.; Xia, Z. Encapsulation of CH3NH3PbBr3 Perovskite Quantum Dots in MOF-5 Microcrystals as a Stable Platform for Temperature and Aqueous Heavy Metal Ion Detection. Inorg. Chem. 2018, 57, 4613–4619. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Li, X.; Wu, J.; Han, Y.; Zhang, X.; Xu, Y. Dual-Emissive CsPbBr3@Eu-BTC Composite for Self-Calibrating Temperature Sensing Application. Cryst. Growth Des. 2020, 20, 454–459. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Li, W.; Huang, S.; Kong, L.; Li, Z.; Li, L. Conversion of invisible metal-organic frameworks to luminescent perovskite nanocrystals for confidential information encryption and decryption. Nat. Commun. 2017, 8, 1138. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Zhou, W.; Liu, Q.; Xia, Z. CH3NH3PbBr3 Perovskite Nanocrystals Encapsulated in Lanthanide Metal–Organic Frameworks as a Photoluminescence Converter for Anti-Counterfeiting. ACS Appl. Mater. Interfaces 2018, 10, 27875–27884. [Google Scholar] [CrossRef]
- Shi, L.; Wang, J.; Zhou, L.; Chen, Y.; Yan, J.; Dai, C. Facile in-situ preparation of MAPbBr3@UiO-66 composites for information encryption and decryption. J. Solid State Chem. 2020, 282, 121062. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Rambabu, D.; Maji, T.K. Mechanochemical synthesis of a processable halide perovskite quantum dot–MOF composite by post-synthetic metalation. J. Mater. Chem. A 2019, 7, 21106–21111. [Google Scholar] [CrossRef]
- Ren, J.; Zhou, X.; Wang, Y. Dual-emitting CsPbX3@ZJU-28 (X = Cl, Br, I) composites with enhanced stability and unique optical properties for multifunctional applications. Chem. Eng. J. 2020, 391, 123622. [Google Scholar] [CrossRef]
- Kong, Z.-C.; Liao, J.-F.; Dong, Y.-J.; Xu, Y.-F.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Core@Shell CsPbBr3@Zeolitic Imidazolate Framework Nanocomposite for Efficient Photocatalytic CO2 Reduction. ACS Energy Lett. 2018, 3, 2656–2662. [Google Scholar] [CrossRef]
- Mollick, S.; Mandal, T.N.; Jana, A.; Fajal, S.; Desai, A.V.; Ghosh, S.K. Ultrastable Luminescent Hybrid Bromide Perovskite@MOF Nanocomposites for the Degradation of Organic Pollutants in Water. ACS Appl. Nano Mater. 2019, 2, 1333–1340. [Google Scholar] [CrossRef]
- Wan, S.; Ou, M.; Zhong, Q.; Wang, X. Perovskite-type CsPbBr3 quantum dots/UiO-66(NH2) nanojunction as efficient visible-light-driven photocatalyst for CO2 reduction. Chem. Eng. J. 2019, 358, 1287–1295. [Google Scholar] [CrossRef]
- Wu, L.-Y.; Mu, Y.-F.; Guo, X.-X.; Zhang, W.; Zhang, Z.-M.; Zhang, M.; Lu, T.-B. Encapsulating Perovskite Quantum Dots in Iron-Based Metal–Organic Frameworks (MOFs) for Efficient Photocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2019, 58, 9491–9495. [Google Scholar] [CrossRef]
- Rambabu, D.; Bhattacharyya, S.; Singh, T.; M L, C.; Maji, T.K. Stabilization of MAPbBr3 Perovskite Quantum Dots on Perovskite MOFs by a One-Step Mechanochemical Synthesis. Inorg. Chem. 2020, 59, 1436–1443. [Google Scholar] [CrossRef]
- Protesescu, L.; Calbo, J.; Williams, K.; Tisdale, W.; Walsh, A.; Dincă, M. Colloidal nano-MOFs nucleate and stabilize ultra-small quantum dots of lead bromide perovskites. Chem. Sci. 2021, 12, 6129–6135. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luque, R.; Li, Y. Controllable design of tunable nanostructures inside metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 4614–4630. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Z.; Chen, P.; Chen, V.; Cheetham, A.K.; Wang, L. Intermarriage of Halide Perovskites and Metal-Organic Framework Crystals. Angew. Chem. Int. Ed. 2020, 59, 19434–19449. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ling, L.; Zhang, W.; Guo, J.; Ding, K.; Duan, W.; Liu, B. “Ship-in-Bottle” Strategy to Encapsulate Shape-Controllable Metal Nanocrystals into Metal–Organic Frameworks: Internal Space Matters. Chem. Mater. 2019, 31, 9546–9553. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, Y.; Lin, H.; Liu, C.-J. Nanoparticle/Metal–Organic Framework Composites for Catalytic Applications: Current Status and Perspective. Molecules 2017, 22, 2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal halide perovskites for light-emitting diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Yoon, B.; Lee, J.; Park, I.S.; Jeon, S.; Lee, J.; Kim, J.-M. Recent functional material based approaches to prevent and detect counterfeiting. J. Mater. Chem. C 2013, 1, 2388–2403. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Li, J.; Wei, B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat. Commun. 2021, 12, 1343. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qian, K.; Xu, B.; Li, Z.; Zheng, J.; Zhao, S.; Ding, F.; Sun, Y.; Xu, Z. Recent advances in visible-light-driven conversion of CO2 by photocatalysts into fuels or value-added chemicals. Carbon Resour. Convers. 2020, 3, 46–59. [Google Scholar] [CrossRef]
- Han, G.; Sun, Y. Visible-light-driven organic transformations on semiconductors. Mater. Today Phys. 2021, 16, 100297. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Park, S.; Chang, W.J.; Lee, C.W.; Park, S.; Ahn, H.-Y.; Nam, K.T. Photocatalytic hydrogen generation from hydriodic acid using methylammonium lead iodide in dynamic equilibrium with aqueous solution. Nat. Energy 2016, 2, 16185. [Google Scholar] [CrossRef]
- Rosa-Pardo, I.; Casadevall, C.; Schmidt, L.; Claros, M.; Galian, R.E.; Lloret-Fillol, J.; Pérez-Prieto, J. The synergy between the CsPbBr3 nanoparticle surface and the organic ligand becomes manifest in a demanding carbon–carbon coupling reaction. Chem. Commun. 2020, 56, 5026–5029. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Pradhan, B.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A. Solar-Driven Metal Halide Perovskite Photocatalysis: Design, Stability, and Performance. ACS Energy Lett. 2020, 5, 1107–1123. [Google Scholar] [CrossRef]

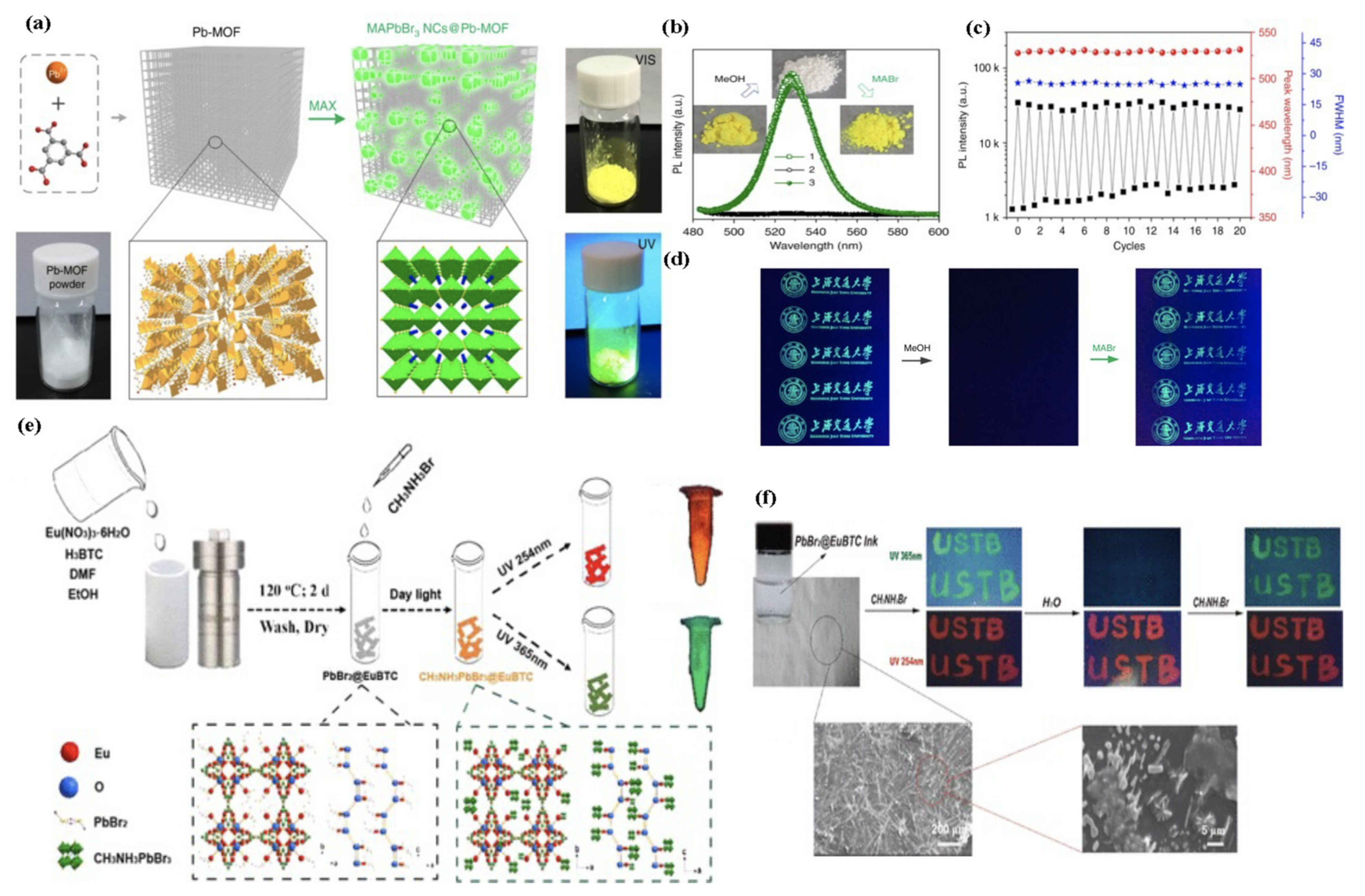
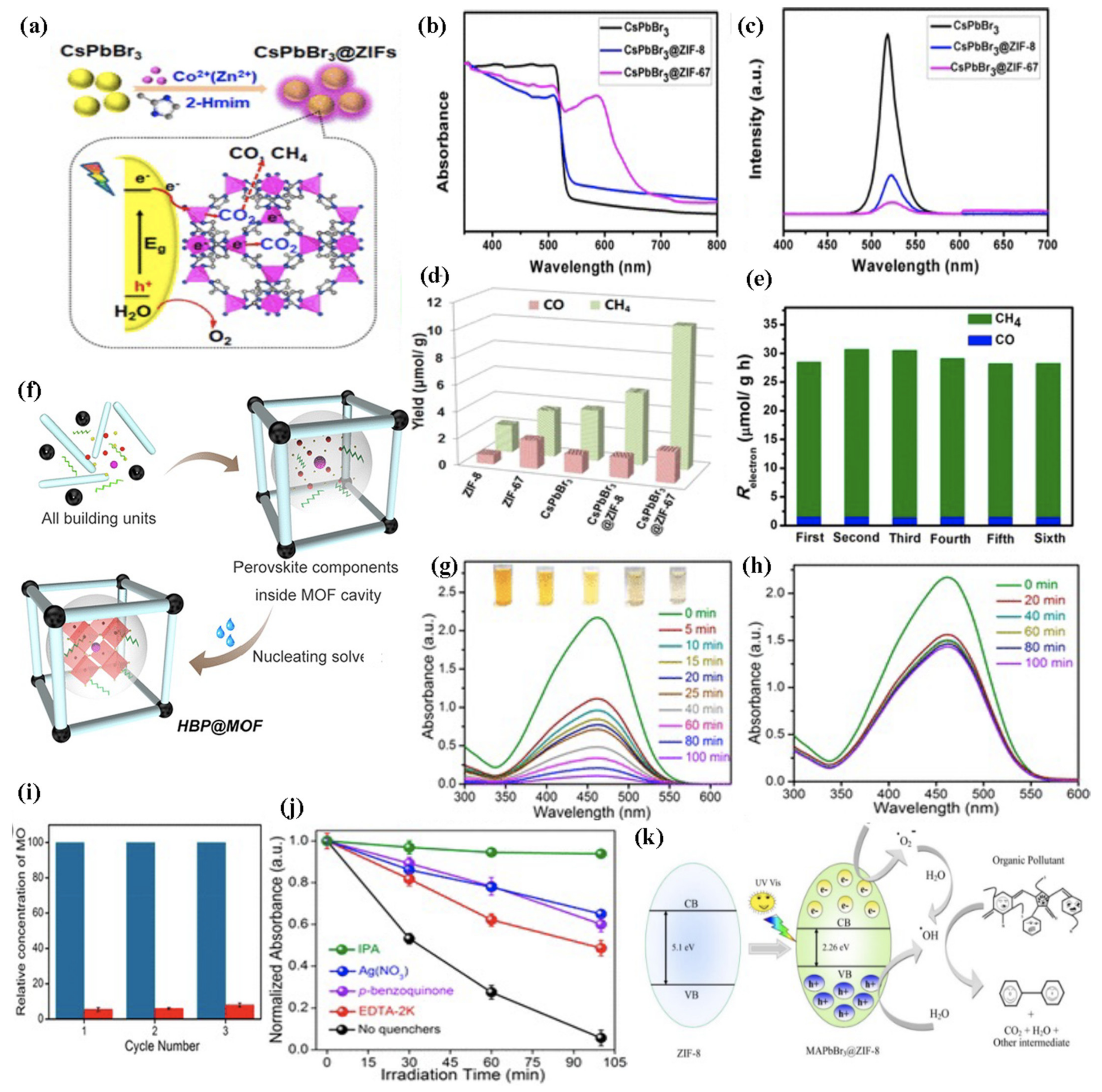
| MHP@MOF Composite | Synthesis Strategy | MOFs | Pore Size (nm) a | MHP | MHP Size (nm) | λem (nm) b | ΦPL (%) c | Application [Ref] |
|---|---|---|---|---|---|---|---|---|
| MAPbX3@HKUST-1 | Ship-in-Bottle (Sequential Deposition) | HKUST-1 [Cu3(BTC)2, BTC = 1,3,5-benzene tricarboxylate] | 1.66 | MAPbI2Cl MAPbI2Br MAPbI3 | 1.5–2 | 536 655 715 | - - - | Luminescence [101] |
| MAPbBr3@ZJU-28 | Ship-in-Bottle (Sequential Deposition) | ZJU-28 (ZJU-28 = [In3(BTB)4](Me2NH2)3) | - | MAPbBr3 | 7.7 11 | 509 530 | 51 | Multiphoton Exciton Luminescence [102] |
| CsPbX3@MIL-101 | Ship-in-Bottle (Sequential Deposition) | MIL-101(Cr) | 3 | CsPbCl3 CsPbCl2Br CsPbCl1Br2 CsPbBr3 CsPbBr2I CsPbBr1I2 CsPbI3 | 1.14 | 417 426 454 520 569 633 698 | - - - - - - - | Tunable Emission [103] |
| MAPbBr3@LnMOF | Ship-in-Bottle (Sequential Deposition) | LnMOF [Ln(tpob)(MDF)(H2O)]n, (Lntpob, Ln = Nd, Sm, Eu, Gd, Tb, Dy, H3tpob = 1,3,5-tris(4-carbonylphenyloxy)benzene) | - | MAPbBr3 | - | 515 | - | Tunable Emission [104] |
| CsPbX3@UiO-67 | Ship-in-Bottle (Sequential Deposition) | UiO-67 | 12 16 | CsPbBr3 CsPbBr1.2I1.8 | - - | 521 634 | 39 30 | Light-Emitting Diodes [105] |
| CsPbX3@MOF-5 | Ship-in-Bottle (Physical Mixing) | MOF-5 | 25 | CsPbBr3 CsPbBr0.6I2.4 | 9.49 | 519 655 | 52 56 | White Light-Emitting Diodes [106] |
| MAPbBr3@Bio-MOF-1 | Ship-in-Bottle (Sequential Deposition) | Bio-MOF-1 (Zn8(Ad)4(BPDC)6O·2Me2NH2, Ad = adeninate; BPDC = biphenyl-dicarboxylate) | - | MAPbBr3 | - | 519 (417,465) | 16 | Light-Emitting Diodes [107] |
| EAPbBr3@MIL-100(Al) | Ship-in-Bottle (in situ Deposition) | MIL-100(Al) (MOG) | 2.5–10 | EAPbBr3 | 3–11 | 436 | 53 | Light-Emitting Diodes [108] |
| CsPbX3@ZIF-8 | Ship-in-Bottle (Sequential Deposition) d | ZIF-8 | 5.7 | CsPbCl3 CsPbBr3 CsPbI3 | 5–10 | - 520 - | 51 72 57 | Light-Emitting Diodes [109] |
| MAPbI3/MIL-125 | - | MIL-125 | - | MAPbI3 | Bulk | - | - | Photovoltaic [110] |
| MAPbClxI3-x/MOF-525 | - | MOF-525 | 1.8 | MAPbClxI3-x | Bulk | - | - | Photovoltaic [111] |
| MAPbI3/ZIF-8 | - | ZIF-8 | - | MAPbI3 | Bulk | - | - | Photovoltaic [112] |
| MA0.95FA0.05PbI3/MIL-125(Ti) | - | MIL-125(Ti) | - | MA0.95FA0.05PbI3 | Bulk | - | - | Photovoltaic [113] |
| MAPbI3/UiO-66 MAPbI3/MOF-808 | Ship-in-Bottle (Sequential Deposition) | UiO-66 (Zr6O4(OH)4(BDC)6) MOF-808 (Zr6O4(OH)4(BTC)2(HCOO)6) | 1.3 1.9 | MAPbI3 | 720 e 640 e | - | - | Photovoltaic [114] |
| Cs0.1FA0.747MA0.153PbI2.49Br0.51/[Cu2(BTC)4/3(H2O)2]6 | - | [Cu2(BTC)4/3(H2O)2]6 | - | Cs0.1FA0.747MA0.153 PbI2.49Br0.51 | Bulk | - | - | Photovoltaic [115] |
| MAPbI3/Co-doped-Ti-MOF | - | Co-Doped-Ti-MOF | 6.79 | MAPbI3 | Bulk | - | - | Photovoltaic [116] |
| APbI3/ZIF-8 | - | ZIF-8 | - | (Cs/MA/FA)PbI3 | Bulk | - | - | Photovoltaic [117] |
| APbI3/Zn-cbpp | - | 2D Zn-cbpp [Zn(cbpp)(HCOO)]n, [hcbpp = 1-[4-carboxylbenzyl]-3-[pyrzin-2-yl]pyrazole] | - | (Cs/MA/FA)PbI3 | Bulk | 744 | - | Photovoltaic [118] |
| MAPbBr3/X-LH2 | Ship-in-Bottle (Sequential Deposition) | Zr-LH2 In-LH2 Zn-LH2 [(pydaH2)2+(pydc)2−, pyda = 2,6-pyridinediamine; pydcH2 = 2,6-pyridinedicarboxylic acid] | - | MAPbBr3 | 49 34 46 | - | - | Photovoltaic [119] |
| MAPbBr3@MOF-5 | Ship-in-Bottle (Sequential Deposition) | MOF-5 | 1.28 | MAPbBr3 | - | 533 (428) | 38 | Temperature Sensing, Metal Ion Detection [120] |
| CsPbBr3@Eu-BTC | Ship-in-Bottle (in situ Deposition) | Eu-BTC | - | CsPbBr3 | - | 528 (593,618) | - | Temperature Sensing [121] |
| MAPbX3@Pb-MOF | Ship-in-Bottle (Direct Conversion) | Pb-MOF (Pb2(1,3,5-HBTC)2-(H2O)4) | - | MAPbCl3 MAPbCl2Br1 MAPbClBr2 MAPbBr3 MAPbBr2I MAPbBrI2 MAPbI3 | - - - 10–20 - - - | 406 443 487 527 582 687 746 | - - - 40 - - - | Information Security [122] |
| MAPbBr3@Eu-BTC | Ship-in-Bottle (Sequential Deposition) | Eu-BTC | - | MAPbBr3 | - | 513 (593,617) | 42 | Information Security [123] |
| MAPbBr3@UiO-66 | Ship-in-Bottle (Sequential Deposition) | UiO-66 | 0.69 | MAPbBr3 | - | 505 | - | Information Security [124] |
| CsPbX3@AMOF-1 | Ship-in-Bottle (Sequential Deposition) d | AMOF-1 (L = 5,5′-(1,4-phenylenebis(methylene))bis(oxy)diisophthalate) | - | CsPbCl3 CsPbBr3 CsPbI3 | 2–9 2–9 5–8 | 412 515 695 | 2 14 5 | Information Security [125] |
| CsPbX3@ZJU-28 | Ship-in-Bottle (Sequential Deposition) | ZJU-28 | - - 23.56 - - - | CsPbCl3 CsPbCl1.5Br1.5 CsPbBr3 CsPbBr1.5I1.5 CsPbBr0.6I2.4 CsPbI3 | 7.92 9.21 11.85 12.87 13.74 15.65 | - - 518 (445,478) - - | - - 62 - - - | Information Security, Temperature Sensing, Light-Emitting Diodes [126] |
| CsPbBr3@ZIF-8 CsPbBr3@ZIF-67 | Bottle-Around-Ship | ZIF-8 ZIF-67 | - - | CsPbBr3 | 5 | 524 | - | Photocatalysis [127] |
| APbBr3@ZIF-8 | Ship-in-Bottle (in situ Deposition) | ZIF-8 | 1.16 | MAPbBr3 MA0.75EA0.25PbBr3 MA0.5EA0.5PbBr3 MA0.25EA0.75PbBr3 EAPbBr3 | 6–8 - - - - | 527 493 481 455 < 440 | 80 - - - - | Photocatalysis [128] |
| CsPbBr3@UiO-66(NH2) | Ship-in-Bottle (Physical Mixing) | UiO-66(NH2) | - | CsPbBr3 | 10 | - | - | Photocatalysis [129] |
| MAPbI3@PCN-221(Fex) | Ship-in-Bottle (Sequential Deposition) | PCN-221(Fex) (x = 0–1) | 2 | MAPbI3 | 1.8 | 610 | - | Photocatalysis [130] |
| MAPbBr3@ MA-M(HCOO)3 | Ship-in-Bottle (Sequential Deposition) d | MA-M(HCOO)3 [M = Mn, Co] | - | MAPbBr3 | 5–10 | 520 | <3 | Photo-Electrochemical Activity [131] |
| APbBr3@Cr-MIL-101 | Ship-in-Bottle (Sequential Deposition) | Cr-MIL-101 [(Cr3O(OH)(H2O)2(terephthalate)3] | 2.9 3.4 | CsPbBr3 MAPbBr3 FAPbBr3 | 3 | 440 446 450 | <5 | - [132] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Villena, A.; Galian, R.E. Present and Perspectives of Photoactive Porous Composites Based on Semiconductor Nanocrystals and Metal-Organic Frameworks. Molecules 2021, 26, 5620. https://doi.org/10.3390/molecules26185620
Cortés-Villena A, Galian RE. Present and Perspectives of Photoactive Porous Composites Based on Semiconductor Nanocrystals and Metal-Organic Frameworks. Molecules. 2021; 26(18):5620. https://doi.org/10.3390/molecules26185620
Chicago/Turabian StyleCortés-Villena, Alejandro, and Raquel E. Galian. 2021. "Present and Perspectives of Photoactive Porous Composites Based on Semiconductor Nanocrystals and Metal-Organic Frameworks" Molecules 26, no. 18: 5620. https://doi.org/10.3390/molecules26185620






