In Vitro Tests for a Rapid Evaluation of Antidiabetic Potential of Plant Species Containing Caffeic Acid Derivatives: A Validation by Two Well-Known Antidiabetic Plants, Ocimum gratissimum L. Leaf and Musanga cecropioides R. Br. ex Tedlie (Mu) Stem Bark
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis of Oc and Mu
2.1.1. LC-MS Analysis
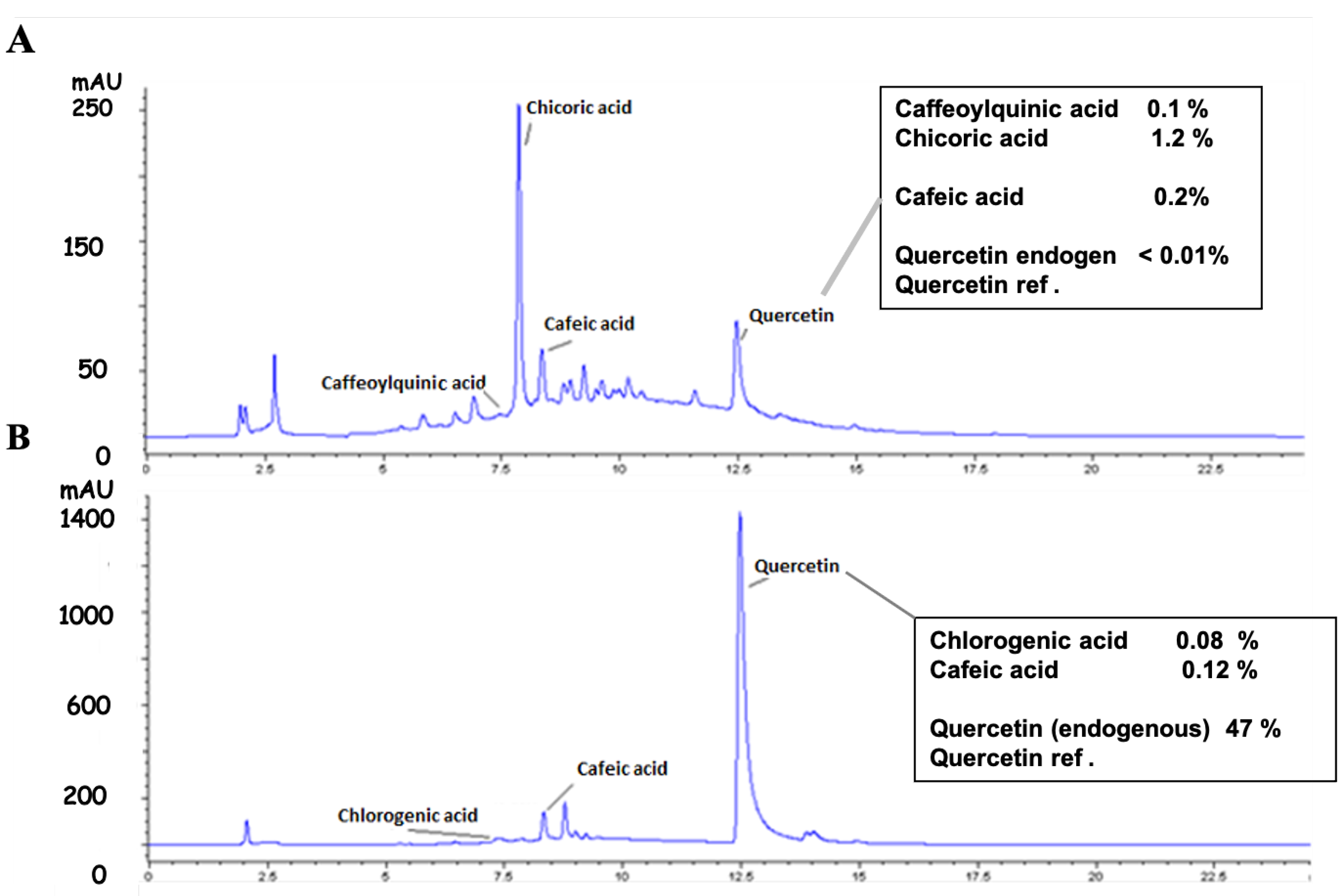
2.1.2. HPLC Analysis
2.1.3. DPPH Free-Radical Scavenging Capacities of Oc and Mu Extracts
2.2. Oc and Mu In Vitro Effects
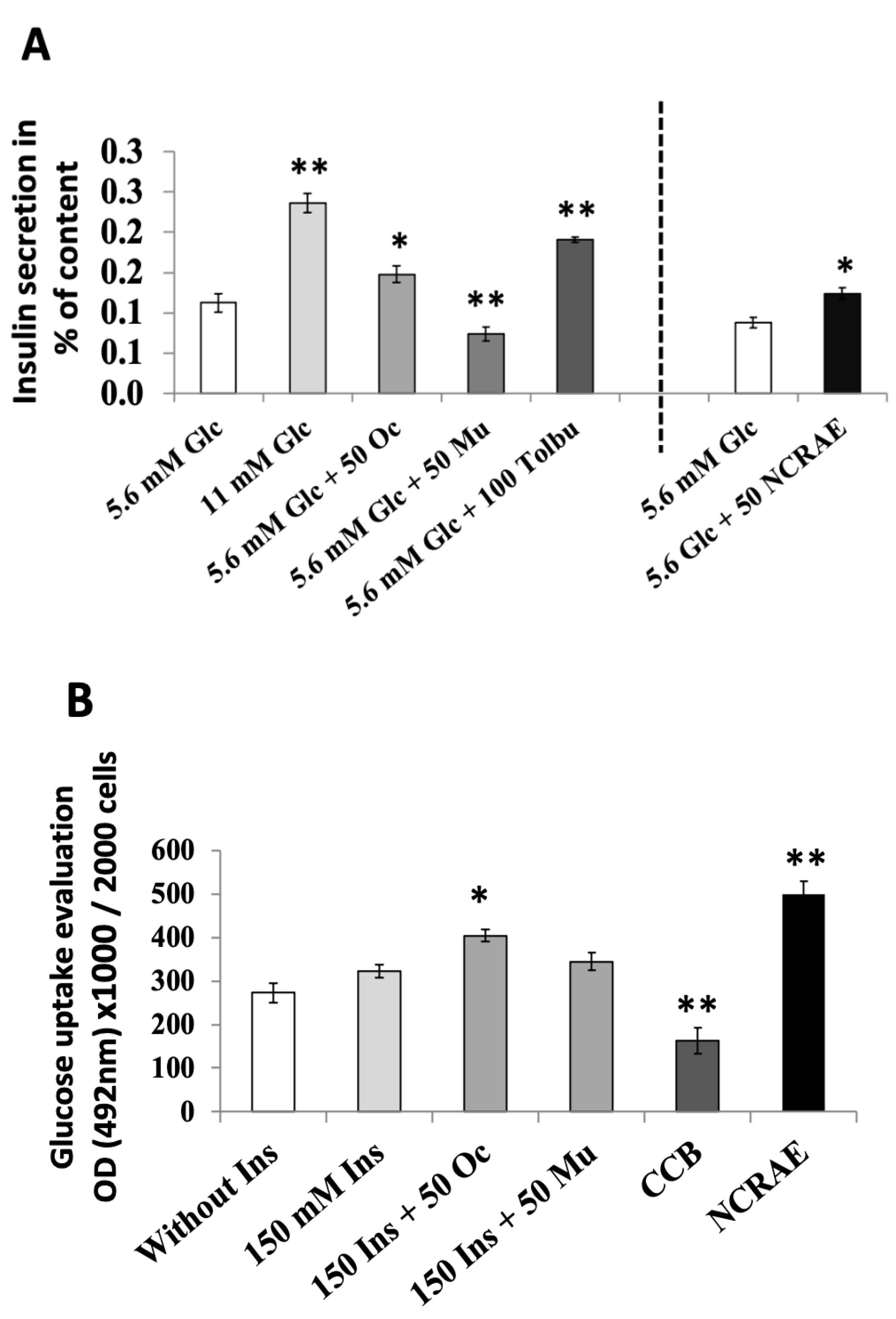
2.2.1. Insulin Secretion Evaluations
2.2.2. Insulin Sensitizing Evaluations
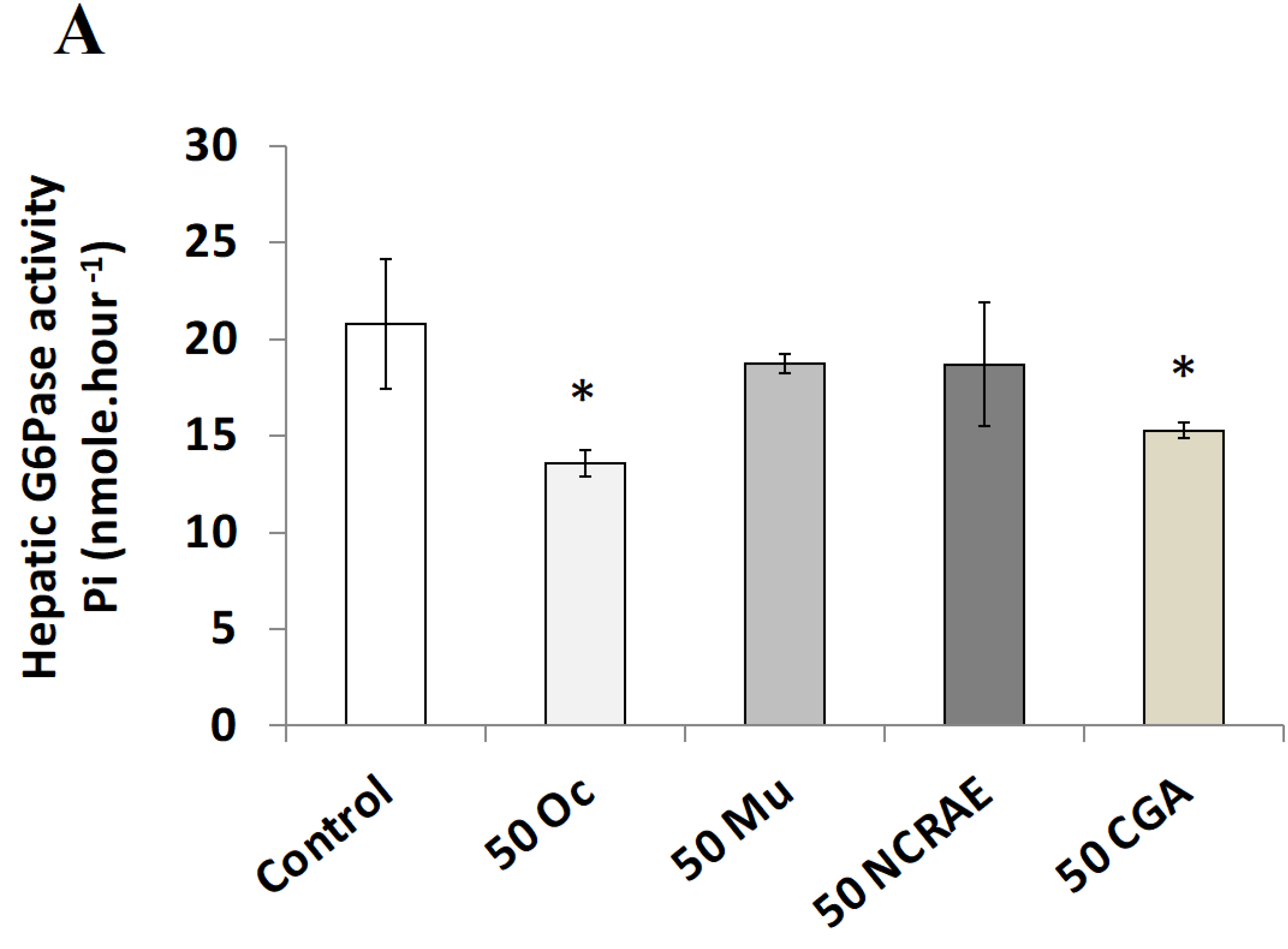
2.2.3. Hepatic Glucose 6-Phosphatase Activity
2.2.4. Protective Effect on L6 Cells against H2O2 Stress
3. Discussion
4. Materials and Methods
4.1. Biological Material and Drugs
4.2. Chemical Analysis
4.2.1. LC-MS Analysis
4.2.2. HPLC Analysis
4.3. DPPH Free-Radical Scavenging Capacity
4.4. In Vitro Tests
4.4.1. Insulin Secretion Investigations
4.4.2. Insulin-Sensitizing Evaluations
4.4.3. Hepatic Glucose 6-Phosphatase (G6Pase) Activity Evaluation on Rat Microsomes
4.4.4. Protective Evaluation of L6 Cells against H2O2 Oxidative Stress
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Poitout, V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004, 53 (Suppl. 1), S119–S124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiq, H.; Uribe, K.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Awwad, A.; Poucheret, P.; Idres, A.Y.; Bidel, L.; Tousch, D. The bitter Asteraceae: An interesting approach to delay the metabolic syndrome progression. NFS J. 2020, 18, 29–38. [Google Scholar] [CrossRef]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J.-L. Caffeoyl derivatives: Major antioxidant compounds of some wild herbs of the Asteraceae family. Food Nutr. Sci. 2011, 2, 181–192. [Google Scholar] [CrossRef]
- Schlernitzauer, A.; Oiry, C.; Hamad, R.; Galas, S.; Cortade, F.; Chabi, B.; Casas, F.; Pessemesse, L.; Fouret, G.; Feillet-Coudray, C.; et al. Chicoric acid is an antioxidant molecule that stimulates AMP kinase pathway in L6 myotubes and extends lifespan in Caenorhabditis elegans. PLoS ONE 2013, 8, e78788. [Google Scholar]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef]
- Tousch, D.; Lajoix, A.D.; Hosy, E.; Azay-Milhau, J.; Ferrare, K.; Jahannault, C.; Cros, G.; Petit, P. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem. Biophys. Res. Commun. 2008, 377, 131–135. [Google Scholar] [CrossRef]
- Tousch, D.; Bidel, L.P.; Cazals, G.; Ferrare, K.; Leroy, J.; Faucanié, M.; Chevassus, H.; Tournier, M.; Lajoix, A.D.; Azay-Milhau, J. Chemical analysis and antihyperglycemic activity of an original extract from burdock root (Arctium lappa). J. Agric. Food. Chem. 2014, 62, 7738–7745. [Google Scholar] [CrossRef]
- Azay-Milhau, J.; Ferrare, K.; Leroy, J.; Aubaterre, J.; Tournier, M.; Lajoix, A.D.; Tousch, D. Antihyperglycemic effect of a natural chicoric acid extract of chicory (Cichorium intybus L.): A comparative in vitro study with the effects of caffeic and ferulic acids. J. Ethnopharmacol. 2013, 150, 755–760. [Google Scholar] [CrossRef]
- Ferrare, K.; Bidel, L.P.R.; Awwad, A.; Poucheret, P.; Cazals, G.; Lazennec, F.; Azay-Milhau, J.; Tournier, M.; Lajoix, A.D.; Tousch, D. Increase in insulin sensitivity by the association of chicoric acid and chlorogenic acid contained in a natural chicoric acid extract (NCRAE) of chicory (Cichorium intybus) for an antidiabetic effect. J. Ethnopharmacol. 2018, 215, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguiyi, J.C.; Obi, C.I.; Gang, S.S.; Igweh, A.C. Hypoglycaemic activity of Ocimum gratissimum in rats. Fitoterapia 2000, 71, 444–446. [Google Scholar] [CrossRef]
- Egesie, U.G.; Adelaiye, A.B.; Ibu, J.O.; Egesie, O.J. Safety and hypoglycaemic properties of aqueous leaf extract of Ocimum gratissimum in streptozotocin induced diabetic rats. Niger J. Physiol. Sci. 2006, 21, 31–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, A.; Tanko, Y.; Okasha, M.A.; Magaji, R.A.; Yaro, A.H. Effects of aqueous leaves extract of Ocimum gratissimum on blood glucose levels of streptozocin induced diabetic wistar rats. Afr. J. Biotechnol. 2007, 6, 2087–2090. [Google Scholar]
- Okon, U.A.; Owo, D.U.; Udokang, N.E.; Udobang, J.A.; Ekpenyong, C.E. Oral administration of aqueous leaf extract of Ocimum gratissimum ameliorates polyphagia, polydipsia and weight loss in streptozotocin-induced diabetic rats. Am. J. Med. Med. Sci. 2012, 2, 45–49. [Google Scholar]
- Nwogor, U.A. Effects of prolonged administration of aqueous extract of Ocimum gratissimum (Scent Leaf) on blood glucose and lipid profile in alloxan induced diabetic albino rats. Am. J. Biomed. Life Sci. 2016, 4, 30–34. [Google Scholar] [CrossRef]
- Okoduwa, S.I.R.; Umar, I.A.; James, D.B.; Inuwa, H.M. Anti-diabetic potential of Ocimum gratissimum leaf fractions in fortified diet-fed streptozotocin treated rat model of type-2 diabetes. Medicines 2017, 11, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Ajayi, A.M.; Umukoro, S.; Ben-Azu, B.; Adzu, B.; Ademowo, O.G. Toxicity and protective effect of phenolic-enriched ethylacetate fraction of Ocimum gratissimum (Linn.) leaf against acute inflammation and oxidative stress in rats. Drug Dev. Res. 2017, 78, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Casanova, L.M.; da Silva, D.; Sola-Penna, M.; de Magalhães Camargo, L.M.; de Moura Celestrini, D.; Luzineide, W.T.; Soares, C.S. Identification of chicoric acid as a hypoglycemic agent from Ocimum gratissimum leaf extract in a biomonitoring in vivo study. Fitoterapia 2014, 93, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Ajayi, G.O.; Igboekwe, N.A. Evaluation of anti-diabetic potential of the leaves of Musanga cecropioides R. Brown. Planta Med. 2013, 79, PE7. [Google Scholar] [CrossRef]
- Nyunaï, N.; Yaya, A.J.; Tabi, T.G.; Tchamgoue, A.; Ngondé, M.; Minka, C. Anti-hyperglycemic and antioxidant potential of water-ethanol extract of Musanga cecropioides stem bark. Int. J. Pharma. Sci. Drug Res. 2016, 8, 01. [Google Scholar] [CrossRef] [Green Version]
- Adeneye, A.A.; Ajagbonna, O.P.; Ayodele, O.W. Hypoglycemic and antidiabetic activities on the stem bark aqueous and ethanol extracts of Musanga cecropioides in normal and alloxan-induced diabetic rats. Fitoterapia 2007, 78, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Adeneye, A.A.; Ajagbonna, O.P.; Adeleke, T.I.; Bello, S.O. Preliminary toxicity and phytochemical studies of the stem bark aqueous extract of Musanga cecropioides in rats. J. Ethnopharmacol. 2006, 105, 374–379. [Google Scholar] [CrossRef]
- Adeneye, A.A.; Ajagbonna, O.P.; Mojiminiyi, F.B.; Odigie, I.P.; Ojobor, P.D.; Etarrh, R.R.; Adeneye, A.K. The hypotensive mechanisms for the aqueous stem bark extract of Musanga cecropioides in Sprague-Dawley rats. J. Ethnopharmacol. 2006, 106, 203–207. [Google Scholar] [CrossRef]
- Sowemimoa, A.; Okwuchukua, E.; Samuel, F.M.; Ayoolaa, O.; Mutiata, I. Musanga cecropioides leaf extract exhibits anti-inflammatory and anti-nociceptive activities in animal models. Rev. Brasil. Farmacog. 2015, 25, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Salawu, S.O.; Ogundare, A.O.; Ola-Salawu, B.B.; Akindahunsi, A.A. Antimicrobial activities of phenolic containing extracts of some tropical vegetables. Afric. J. Pharm. Pharmacol. 2011, 5, 486–492. [Google Scholar]
- Lacaille-Dubois, M.A.; Franck, U.; Wagner, H. Search for potential angiotensin converting enzyme (ACE)-inhibitors from plants. Phytomedicine 2001, 8, 47–52. [Google Scholar] [CrossRef]
- Ayinde, B.; Onwukaeme, D.N.; Omogbay, E.K. Isolation and characterization of two phenolic compounds from the stem bark of Musanga cecropioides R. Brown (Moraceae). Acta Pol. Pharma. 2017, 64, 183–185. [Google Scholar]
- Venuprasad, M.P.; Hemanth, K.K.; Sakina, R.; Farhath, K. Phytochemical analysis of Ocimum gratissimum by LC-ESI–MS/MS and its antioxidant and anxiolytic effects. S. Afr. J. Bot. 2014, 92, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; Guido, F.; Kilinçarslan, O.; Mammado, R.; Bardaweel, R.S. Chemical composition and bioactivity of essential oils and Ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complementary Altern. Med. 2019, 19, 146. [Google Scholar]
- Pereira, C.A.M.; Yariwake, J.H.; McCullagh, M. Distinction of the C-glycosylflavone isomer pairs orientin/isoorientin and vitexin/isovitexin using HPLC-MS exact mass. Phytochem. Anal. 2005, 16, 295–301. [Google Scholar] [CrossRef]
- Arion, W.J.; Canfield, W.K.; Ramos, F.C.; Su, M.L.; Burger, H.J.; Hemmerle, H.; Schubert, G.; Below, P.; Herling, A.W. Chlorogenic acid analogue S 3483: A potent competitive inhibitor of the hepatic and renal glucose-6-phosphatase systems. Arch. Biochem. Biophys. 1998, 351, 279–285. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid.-Based Complementary Altern. Med. 2013, 201, 801457. [Google Scholar] [CrossRef]
- Chiu, Y.W.; Lo, H.J.; Huang, H.Y.; Chao, P.Y.; Hwang, J.M.; Huang, P.Y.; Huang, S.J.; Liu, J.Y.; Lai, T.J. The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J. Food Drug Anal. 2013, 21, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Madunić, I.V.; Madunić, J.; Antunović, M.; Paradžik, M.; Garaj-Vrhovac, V.; Breljak, D.; Marijanović, I.; Gajski, G. Apigenin, a dietary flavonoid, induces apoptosis, DNA damage, and oxidative stress in human breast cancer MCF-7 and MDA MB-231 cells. Arch. Pharmacol. 2018, 391, 537–550. [Google Scholar] [CrossRef]
- Seydi, E.; Rasekh, H.R.; Salimi, A.; Mohsenifar, Z.; Pourahmad, J. Selective toxicity of Apigenin on cancerous hepatocytes by directly targeting their mitochondria. Anti-Cancer Agents Med. Chem. 2016, 16, 11–22. [Google Scholar] [CrossRef]
- Martucciello, S.; Masulo, M.; Cerulli, A.; Piacente, S. Natural products targeting ER stress, and the functional link to mitochondria. Int. J. Mol. Sci. 2020, 21, 1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babich, H.; Sedletcaia, A.; Kenigsberg, B. In Vitro cytotoxicity of protocatechuic acid to cultured human cells from oral tissue: Involvement in oxidative stress. Pharmacol. Toxicol. 2002, 91, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villano, D.; Fernandez-Pachon, M.S.; Moya, M.L.; Troncoso, A.M.; Garcıa-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Mezghenna, K.; Leroy, J.; Azay-Milhau, J.; Tousch, D.; Castex, F.; Gervais, S.; Delgado-Betancourt, V.; Gross, R.; Lajoix, A.D. Counteracting neuronal NO synthase proteasomal degradation improves glucose transport in insulin resistant skeletal muscle. Diabetologia 2014, 57, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Khayat, Z.; Kishi, K.; Ebina, Y.; Klip, A. GLUT4 translocation by insulin in intact muscle cells: Detection by a fast and quantitative assay. FEBS Lett. 1998, 427, 193–197. [Google Scholar] [CrossRef] [Green Version]


| UV λmax (nm) | Retention Time | Molecular Formula before Ionization | Monoisotopic Ratio m/z | Mass Deviation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | Tentative Identification | Theoretical | Observed | Δ (ppm) | Major Fragments | Extract | ||||
| (nm) | (min) | [M − H]− | [M − H]− | |||||||
| 260, 294 | P1 | 1.30 | 3,4-Dihydroxybenzoic acid (protocatechuic acid) | C7H6O4 | 153,0188 | 153,0185 | 2.0 | 109 | Ocimum | Musanga |
| 256 | P2 | 1.90 | 4-Hydroxybenzoic acid | C7H6O3 | 137,0239 | 137,0235 | 2.9 | Ocimum | ||
| 330 | P3 | 2.13 | 2,5-Dihydroxybenzoic acid (gentisic acid) | C7H6O4 | 153,0188 | 153,0187 | 0.7 | 109 | Ocimum | Musanga |
| 306 | P4 | 2.25 | 2,3-Dihydroxybenzoic acid | C7H6O4 | 153,0188 | 153,0189 | −0.7 | 109 | Musanga | |
| 309 | P5 | 2.30 | 3,4-Dihydroxybenzaldehyde | C7H6O3 | 137,0239 | 137,0237 | 1.5 | 109 | Musanga | |
| 252, 306sh, 328 | P6 | 2.42 | Luteolin-6-C-hexosyl-7-O-hexoside | C27H30O16 | 609,1456 | 609,1452 | 0.7 | 447, 357, 329, 327, 299 | Ocimum | |
| 251, 267sh, 287 | P7 | 2.45 | Vanillic acid hexoside | C14H18O9 | 329,0873 | 329,0875 | −0.6 | 167, 152, 123, 108 | Musanga | |
| 252, 267sh, 287 | P8 | 2.54 | Vanillic acid = 4-Hydroxy-3-methoxybenzoic acid | C8H8O4 | 167,0344 | 167,0341 | 1.8 | 108 | Ocimum | |
| 230, 301 | P9 | 2.65 | Dihydroxybenzoic acid hexoside | C13H16O9 | 315,0716 | 315,0718 | −0.6 | 153, 109, 108 | Musanga | |
| 246, 310sh, 324 | P10 | 2.80 | 5-O-Caffeoylquinic acid | C16H18O9 | 353,0878 | 353,0877 | 0.3 | 191, 85 | Ocimum | Musanga |
| 302, 348 | P11 | 3.40 | Esculetin 6-O-glucoside | C15H16O9 | 339,0716 | 339,0719 | -0,9 | 177 | Ocimum | |
| 234, 272sh, 334 | P12 | 5.34 | Vicenin-2 (Apigenin 6,8-di-C-glucoside) | C27H30O15 | 593,1506 | 593,1502 | 0.7 | 431, 269, 235, 209, 153, 135 | Ocimum | Musanga |
| 232, 268sh, 335 | P13 | 5.87 | Apigenin-C-hexosyl-C-hexoside | C27H30O15 | 593,1506 | 593,1509 | −0.5 | 473, 383, 353 | Musanga | |
| 232, 270sh, 338 | P14 | 5.77 | Apigenin-C-pentosyl-C-hexoside | C26H28O14 | 563,1401 | 563,1404 | −0.5 | 545, 503, 473, 443, 431, 383 | Ocimum | |
| 232, 270sh, 316 | P15 | 5.95 | Apigenin-C-hexosyl-C-hexoside | C27H30O15 | 593,1506 | 593,1504 | 0.3 | 473, 383, 353 | Musanga | |
| 269, 286sh, 337 | P16 | 6.09 | Apigenin-8C-hexoside | C21H20O10 | 431,0978 | 431,0975 | 0,7 | 341, 311, 283 | Ocimum | |
| 232, 270sh, 344 | P17 | 6.13 | Luteolin-6-C-Xylosyl-8-C-Glucoside | C26H28O15 | 579,1350 | 579,1353 | −0,5 | 519, 489, 459, 429, 399, 369, 401 | Musanga | |
| 232, 269sh, 336 | P18 | 6.27 | Apigenin-C-pentosyl-C-hexosyl | C26H28O14 | 563,1401 | 563,1407 | −1.1 | 503, 473, 443, 383, 293 | Musanga | |
| 269, 286sh, 334 | P19 | 6.60 | Apigenin-C-hexose-C-xylose p-coumaroyl-ester | C35H34O16 | 709,1769 | 709,1766 | 0.4 | 563, 545, 420 | Musanga | |
| 232, 270sh, 337 | P20 | 6.53 | Apigenin-C-pentosyl-C-hexosyl | C26H28O14 | 563,1401 | 563,1402 | −0.2 | 545, 503, 473, 443, 383 | Musanga | |
| 269, 286sh, 337 | P21 | 6.76 | Apigenin-8C-glucoside (vitexin) | C21H20O10 | 431,0978 | 431,0973 | 1.2 | 341, 311, 283 | Musanga | |
| 269, 285sh, 336 | P22 | 6.94 | Apigenin-6C-glucoside (isovitexin) | C21H20O10 | 431,0978 | 431,0980 | −0.5 | 413, 341, 311, 283 | Musanga | |
| 241, 310sh, 327 | P23 | 5.87 | trans-Caftaric acid (2-O-caffeoyl-L-tartaric acid) | C14H14O9 | 311,0403 | 311,0401 | 0.6 | 179, 149 | Ocimum | |
| 232, 272sh, 338 | P24 | 8.70 | Apigenin 8- C-rhamnoside-6-hexoside | C27H30O14 | 577,1557 | 577,1553 | 0.7 | 503, 473, 383, 353 | Musanga | |
| 262, 277 | P25 | 9.90 | Feruloyl tartaric acid isomer 1 | C14H14O9 | 325,0560 | 325,0555 | 1.5 | 193, 178, 149, 134 | trace | Musanga |
| 261, 276 | P26 | 10.07 | Feruloyl tartaric acid isomer 2 | C14H14O9 | 325,0560 | 325,0562 | −0.6 | 193, 149, 134 | trace | Musanga |
| 261, 275 | P27 | 10.20 | Feruloyl tartaric acid isomer 3 | C14H14O9 | 325,0560 | 325,0559 | 0.3 | 193, 149 | Ocimum | Musanga |
| 229, 302sh, 325 | P28 | 22.25 | 3,5-di-O-Caffeoylquinic acid | C25H24O12 | 515,1195 | 515,1198 | −0.6 | 353, 191, 179, 173, 175 | Ocimum | |
| 241, 297sh, 327 | P29 | 26.15 | trans-Caffeic acid | C9H8O8 | 179,0344 | 179,034 | 2.2 | 135, 107 | trace | trace |
| 241, 305sh, 327 | P30 | 29.02 | Chicoric acid (2,3-dicaffeoyl-L-tartaric acid) | C22H18O12 | 473,0720 | 473,0717 | 0.6 | 311, 293, 179, 149, 135 | Ocimum | Musanga |
| 242, 300sh, 330 | P31 | 35.85 | Rosmarinic acid | C18H16O8 | 359,0767 | 359,0763 | 1.1 | 161, 197, 179 | Ocimum | |
| 299, 325 | P32 | 37.10 | 1,3,5-tri-O-Caffeoylquinic acid | C34H30O15 | 677,1506 | 677,1509 | −0.4 | 615, 515, 453, 35, 191, 179, 161 | trace | trace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awwad, A.; Poucheret, P.; Idres, Y.A.; Tshibangu, D.S.T.; Servent, A.; Ferrare, K.; Lazennec, F.; Bidel, L.P.R.; Cazals, G.; Tousch, D. In Vitro Tests for a Rapid Evaluation of Antidiabetic Potential of Plant Species Containing Caffeic Acid Derivatives: A Validation by Two Well-Known Antidiabetic Plants, Ocimum gratissimum L. Leaf and Musanga cecropioides R. Br. ex Tedlie (Mu) Stem Bark. Molecules 2021, 26, 5566. https://doi.org/10.3390/molecules26185566
Awwad A, Poucheret P, Idres YA, Tshibangu DST, Servent A, Ferrare K, Lazennec F, Bidel LPR, Cazals G, Tousch D. In Vitro Tests for a Rapid Evaluation of Antidiabetic Potential of Plant Species Containing Caffeic Acid Derivatives: A Validation by Two Well-Known Antidiabetic Plants, Ocimum gratissimum L. Leaf and Musanga cecropioides R. Br. ex Tedlie (Mu) Stem Bark. Molecules. 2021; 26(18):5566. https://doi.org/10.3390/molecules26185566
Chicago/Turabian StyleAwwad, Abdulmomem, Patrick Poucheret, Yanis A. Idres, Damien S. T. Tshibangu, Adrien Servent, Karine Ferrare, Françoise Lazennec, Luc P. R. Bidel, Guillaume Cazals, and Didier Tousch. 2021. "In Vitro Tests for a Rapid Evaluation of Antidiabetic Potential of Plant Species Containing Caffeic Acid Derivatives: A Validation by Two Well-Known Antidiabetic Plants, Ocimum gratissimum L. Leaf and Musanga cecropioides R. Br. ex Tedlie (Mu) Stem Bark" Molecules 26, no. 18: 5566. https://doi.org/10.3390/molecules26185566







