Effects of Insecticides and Microbiological Contaminants on Apis mellifera Health
Abstract
:1. Introduction
2. The Significance of Bees to Humans as an Economically Important Species
3. Products of Bee Origin—Effect on Human Health, Its Various Applications, and a Source of Chemical Contaminants
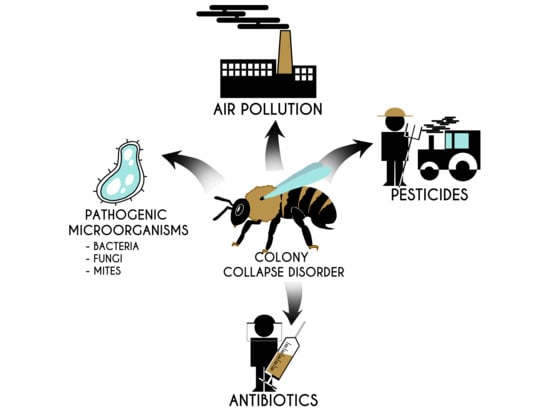
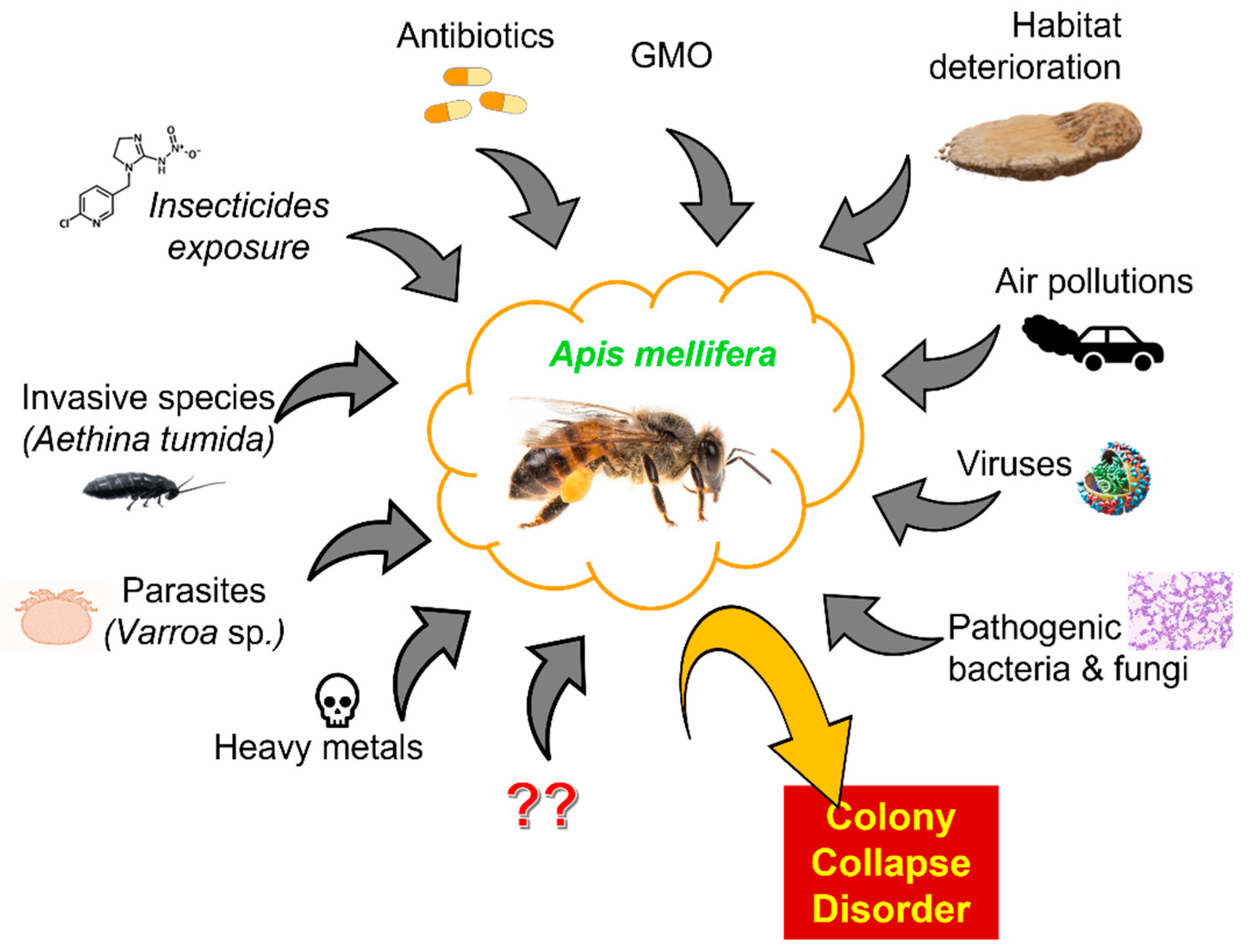
4. Colony Collapse Disorder and Factors Presumably Causing It
5. Pesticides in a Bee Environment and Their Consequences
5.1. Impact of Pesticides on Living Organisms and the Environment
5.2. Insecticides Present in the Honey Bee Environment and Their Effects
5.2.1. Neonicotinoids
5.2.2. Coumaphos
5.2.3. Chlorpyrifos
5.2.4. Spinosad
5.2.5. Fipronil
5.2.6. Possible Effects of Chosen Pesticides on A. mellifera Health
6. Bee Legal Protection against Pesticides
- Protecting plants or plant products against all harmful organisms or preventing the action of such organisms, unless the main purpose of these products is considered to be for reasons of hygiene rather than for the protection of plants or plant products;
- Influencing the life processes of plants, such as substances influencing their growth, other than as a nutrient;
- Preserving plant products, in so far as such substances or products are not subject to special community provisions on preservatives;
- Destroying undesired plants or parts of plants, except algae, unless the products are applied on soil or water to protect plants;
- Checking or preventing undesired growth of plants, except algae, unless the products are applied on soil or water to protect plants.
- One of the criteria for the approval of active substances (as well as safeners and synergists) is to demonstrate that this substance does not cause significant exposure to honey bees, or cause unacceptable acute or chronic effects on the survival and development of honey bee colonies, including effects on bee larvae honey and honey bees behavior;
- As a mandatory element of the documentation of the active substance, the applicant is required to submit reviewed, publicly available scientific publications on the active substance and its relevant metabolites, devoted to side effects on health, the environment and non-target species of this substance (including honey bees), in accordance with an indication of EFSA, published in the last 10 years before the date of submission of the dossier.
7. Microorganisms as One of the Factors Triggering CCD
7.1. Bacteria
7.1.1. Paenibacillus larvae
7.1.2. Melissococcus plutonius
7.1.3. Serratia marcescens
7.2. Fungi
7.2.1. Ascosphaera apis
7.2.2. Aspergillus sp.
7.2.3. Nosema sp.
7.3. Varroa destructor
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staveley, J.P.; Law, S.A.; Fairbrother, A.; Menzie, C.A. A Causal Analysis of Observed Declines in Managed Honey Bees (Apis mellifera). Hum. Ecol. Risk Assess. 2014, 20, 566–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, R.; Melathopoulos, A.P.; White, R.; Pernal, S.F.; Guarna, M.M.; Foster, L.J. Ecological adaptation of diverse honey bee (Apis mellifera) populations. PLoS ONE 2010, 5, e11096. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Wallberg, A.; Webster, M.T. From where did the Western honeybee (Apis mellifera) originate? Ecol. Evol. 2012, 2, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Szczuka, D.; Górczyńska, A.; Motyl, I.; Kręgiel, D. Characterization of Apis mellifera Gastrointestinal Microbiota and Lactic Acid Bacteria for Honeybee Protection—A Review. Cells 2021, 10, 701. [Google Scholar] [CrossRef]
- Nowak, A.; Nowak, I.; Leska, A. American foulbrood as an infectious disease of honeybees—Selected legal and environmental aspects. Studia Prawno-Ekon. 2020, 115, 87–107. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Giannini, T.C.; Cordeiro, G.D.; Freitas, B.M.; Saraiva, A.M.; Imperatriz-Fonseca, V.L. The Dependence of Crops for Pollinators and the Economic Value of Pollination in Brazil. J. Econ. Entomol. 2015, 108, 849–857. [Google Scholar] [CrossRef]
- Tulchinsky, T. Micronutrient Deficiency Conditions: Global Health Issues. Public Health Rev. 2010, 32, 243–255. [Google Scholar] [CrossRef] [Green Version]
- Bradbear, N. Bees and Their Role in Forest Livelihoods; FAO: Rome, Italy, 2019. [Google Scholar]
- Hung, K.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, S.R.; Tarpy, D.R.; Burrack, H.J. Bee species diversity enhances productivity and stability in a perennial crop. PLoS ONE 2014, 9, e97307. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, G.; Baldock, K.C.R.; Rendell, L.; Willmer, P.G. Pollinator importance networks illustrate the crucial value of bees in a highly speciose plant community. Sci. Rep. 2017, 7, 8389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klatt, B.K.; Holzschuh, A.; Westphal, C.; Clough, Y.; Smit, I.; Pawelzik, E.; Tscharntke, T. Bee pollination improves crop quality, shelf life and commercial value. Proc. Biol. Sci. 2013, 281, 20132440. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, D.; Greenwood, S. Pollinator biodiversity and crop pollination in temperate ecosystems, implications for national pollinator conservation strategies: Mini review. Sci. Total Environ. 2020, 744, 140880. [Google Scholar] [CrossRef] [PubMed]
- Bergman, A.; Yanai, J.; Weiss, J.; Bell, D.; David, M.P. Acceleration of wound healing by topical application of honey. An animal model. Am. J. Surg. 1983, 145, 374–376. [Google Scholar] [CrossRef]
- Atwa, A.D.; AbuShahba, R.Y.; Mostafa, M.; Hashem, M.I. Effect of honey in preventing gingivitis and dental caries in patients undergoing orthodontic treatment. Saudi Dent. J. 2014, 26, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, I.; Moniruzzaman, M.; Boukraâ, L.; Benhanifia, M.; Islam, A.; Islam, N.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Algerian honey. Molecules 2012, 17, 11199–11215. [Google Scholar] [CrossRef] [Green Version]
- Saeed, S.; Fariborz, S.; Taghavi, M. Antiproliferative and cytotoxic properties of honey in human prostate cancer cell line (PC-3): Possible mechanism of cell growth inhibition and apoptosis induction. Afr. J. Pharm. Pharm. 2014, 8, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Estevinho, L.; Pereira, A.; Moreira, L.; Dias, L.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef]
- Gheldof, N.; Wang, X.; Engeseth, N. Buckwheat Honey Increases Serum Antioxidant Capacity in Humans. J. Agric. Food Chem. 2003, 51, 1500–1505. [Google Scholar] [CrossRef]
- White, J.W., Jr.; Subers, M.H.; Schepartz, A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta 1963, 73, 57–70. [Google Scholar] [CrossRef]
- Kwakman, P.; Velde, A.; Boer, L.; Speijer, D.; Christina Vandenbroucke-Grauls, M.; Zaat, S. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almaraz-Abarca, N.; Campos, M.D.G.; Ávila-Reyes, J.A.; Naranjo-Jiménez, N.; Herrera-Corral, J.; González-Valdez, L.S. Variability of antioxidant activity among honeybee-collected pollen of different botanical origin. Interciencia 2004, 29, 574–578. [Google Scholar]
- Li, Q.; Wang, K.; Marcucci, M.C.; Sawaya, A.C.H.F.; Hu, L.; Xue, X.-F.; Wu, L.-M.; Hu, F.-L. Nutrient-rich bee pollen: A treasure trove of active natural metabolites. J. Funct. Foods 2018, 49, 472–484. [Google Scholar] [CrossRef]
- Negri, G.; Barreto, L.; Sper, F.; Carvalho, C.; Campos, M. Phytochemical Analysis and Botanical Origin of Apis mellifera Bee Pollen From The Municipality of Canavieiras, Bahia State, Brazil. Braz. J. Food Technol. 2020, 21. [Google Scholar] [CrossRef] [Green Version]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mărgăoan, R.; Mărghitaş, L.A.; Dezmirean, D.S.; Dulf, F.V.; Bunea, A.; Socaci, S.; Bobiş, O. Predominant and Secondary Pollen Botanical Origins Influence the Carotenoid and Fatty Acid Profile in Fresh Honeybee-Collected Pollen. J. Agric. Food Chem. 2014, 62, 6306–6316. [Google Scholar] [CrossRef] [PubMed]
- Salles, J.; Cardinault, N.; Patrac, V.; Berry, A.; Giraudet, C.; Collin, M.-L.; Chanet, A.; Tagliaferri, C.; Denis, P.; Pouyet, C.; et al. Bee Pollen Improves Muscle Protein and Energy Metabolism in Malnourished Old Rats through Interfering with the Mtor Signaling Pathway and Mitochondrial Activity. Nutrients 2014, 6, 5500–5516. [Google Scholar] [CrossRef] [Green Version]
- Olczyk, P.; Koprowski, R.; Kaźmierczak, J.; Mencner, L.; Wojtyczka, R.D.; Stojko, J.; Olczyk, K.; Komosinska-Vassev, K. Bee Pollen as a Promising Agent in the Burn Wounds Treatment. Evid. Based Complement. Altern. Med. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hausen, B.M.; Wollenweber, E.; Senff, H.; Post, B. Propolis allergy. (II). The sensitizing properties of 1,1-dimethylallyl caffeic acid ester. Contact Dermat. 1987, 17, 171–177. [Google Scholar] [CrossRef]
- Batista, L.; Campesatto, E.; Assis, M.; Barbosa, A.; Grillo, L.; Dornelas, C. Estudo comparativo do uso tópico de própolis verde e vermelha na reparação de feridas em ratos. Rev. Do Colégio Bras. De Cir. 2012, 39, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.S.; Lee, J.H.; Cho, M.H.; Lee, J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 2015, 31, 1–11. [Google Scholar] [CrossRef]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.P.; Boersma, B.J.; Crawford, J.H.; Hogg, N.; Kirk, M.; Kalyanaraman, B.; Parks, D.A.; Barnes, S.; Darley-Usmar, V. Antioxidant mechanisms of isoflavones in lipid systems: Paradoxical effects of peroxyl radical scavenging. Free Radic. Biol. Med. 2001, 31, 1570–1581. [Google Scholar] [CrossRef]
- Buttstedt, A.; Moritz, R.; Erler, S. More than royal food—Major royal jelly protein genes in sexuals and workers of the honeybee Apis mellifera. Front. Zool. 2013, 10, 72. [Google Scholar] [CrossRef] [Green Version]
- Nagai, T.; Inoue, R. Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Chem. 2004, 84, 181–186. [Google Scholar] [CrossRef]
- Izuta, H.; Chikaraishi, Y.; Shimazawa, M.; Mishima, S.; Hara, H. 10-Hydroxy-2-decenoic acid, a major fatty acid from royal jelly, inhibits VEGF-induced angiogenesis in human umbilical vein endothelial cells. Evid. Based Complement. Altern. Med. 2009, 6, 489–494. [Google Scholar] [CrossRef] [PubMed]
- El-Hanoun, A.; Elkomy, A.; Fares, W.; Shahien, E. Impact of royal jelly to improve reproductive performance of male rabbits under hot summer conditions. World Rabbit Sci. 2014, 22, 241. [Google Scholar] [CrossRef]
- Najafi, G.; Nejati, V.; Shalizar Jalali, A.; Zahmatkesh, E. Protective Role of Royal Jelly in Oxymetholone-induced Oxidative Injury in Mouse Testis. IJT 2014, 8, 1073–1080. [Google Scholar]
- Velicer, C. Antibiotic Use in Relation to the Risk of Breast Cancer. JAMA 2004, 291, 827. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, S.; Henry, M.; Stout, J.; White, B. Neonicotinoid residues in honey from urban and rural environments. Environ. Sci. Pollut. Res. 2021, 28, 28179–28190. [Google Scholar] [CrossRef]
- Singh, C.; Sivaram, V. Detection of Carbamates in Honeybees and Bee-products of Karnataka State. Int. J. Adv. Res. 2014, 2, 757–762. [Google Scholar] [CrossRef]
- Ruiz-Toledo, J.; Vandame, R.; Castro-Chan, R.A.; Penilla-Navarro, R.P.; Gómez, J.; Sánchez, D. Organochlorine Pesticides in Honey and Pollen Samples from Managed Colonies of the honey bee Apis mellifera Linnaeus and the Stingless Bee Scaptotrigona mexicana Guérin from Southern, Mexico. Insects 2018, 9, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Naggar, Y.; Codling, G.; Vogt, A.; Naiem, A.; Mona, M.; Seif, A.; Giesy, J.P. Organophosphorus insecticides in honey, pollen and bees (Apis mellifera L.) and their potential hazard to bee colonies in Egypt. Ecotoxicol. Environ. Saf. 2015, 114, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. Contaminants of bee products. Apidologie 2005, 37, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Burden, C.; Morgan, M.; Hladun, K.; Amdam, G.; Trumble, J.; Smith, B. Acute sublethal exposure to toxic heavy metals alters honey bee (Apis mellifera) feeding behavior. Sci. Rep. 2019, 9, 4253. [Google Scholar] [CrossRef]
- Herrera, A.; Perez-Arquillue, C.; Conchello, P.; Bayarri, S.; Lazaro, R.; Yague, C.; Arino, A. Determination of pesticides and PCBs in honey by solid-phase extraction cleanup followed by gas chromatography with electron-capture and nitrogen phosphorus detection. Anal. Bioanal. Chem. 2005, 381, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Lambert, O.; Veyrand, B.; Durand, S.; Marchand, P.; Le Bizec, B.; Piroux, M.; Puyo, S.; Thorin, C.; Delbac, F.; Pouliquen, H. Polycyclic aromatic hydrocarbons: Bees, honey and pollen as sentinels for environmental chemical contaminants. Chemosphere 2012, 86, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Ciemniak, A.; Witczak, A.; Mocek, K. Assessment of honey contamination with polycyclic aromatic hydrocarbons. J. Environ. Sci. Health Part B 2013, 48, 993–998. [Google Scholar] [CrossRef]
- Dobrinas, S.; Birghila, S.; Coatu, V. Assessment of polycyclic aromatic hydrocarbons in honey and propolis produced from various flowering trees and plants in Romania. J. Food Compos. Anal. 2008, 21, 71–77. [Google Scholar] [CrossRef]
- Perugini, M.; Di Serafino, G.; Giacomelli, A.; Medrzycki, P.; Sabatini, A.G.; Persano Oddo, L.; Marinelli, E.; Amorena, M. Monitoring of Polycyclic Aromatic Hydrocarbons in Bees (Apis mellifera) and Honey in Urban Areas and Wildlife Reserves. J. Agric. Food Chem. 2009, 57, 7440–7444. [Google Scholar] [CrossRef]
- Iwegbue, C.; Tesi, G.; Obi, G.; Obi-Iyeke, G.; Igbuku, U.; Martincigh, B. Concentrations, health risks and sources of polycyclic aromatic hydrocarbons in Nigerian honey. Toxicol. Environ. Health Sci. 2016, 8, 28–42. [Google Scholar] [CrossRef]
- Al-Alam, J.; Fajloun, Z.; Chbani, A.; Millet, M. Determination of 16 PAHs and 22 PCBs in honey samples originated from different region of Lebanon and used as environmental biomonitors sentinel. J. Environ. Sci. Health Part A 2018, 54, 9–15. [Google Scholar] [CrossRef]
- Kazazic, M.; Djapo-Lavic, M.; Mehic, E.; Jesenkovic-Habul, L. Monitoring of honey contamination with polycyclic aromatic hydrocarbons in Herzegovina region. Chem. Ecol. 2020, 36, 726–732. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Hayes, J., Jr.; Underwood, R.M.; Pettis, J. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar] [CrossRef]
- Aizen, M.A.; Harder, L.D. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef] [Green Version]
- National Agricultural Statistics Service (NASS); Agricultural Statistics Board, United States Department of Agriculture (USDA). Honey Bee Colonies. 2020. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/rn301137d/nc5819380/t148g6070/hcny0820.pdf (accessed on 14 July 2021).
- Ferrier, P.M.; Rucker, R.R.; Thurman, W.N.; Burgett, M. Economic Effects and Responses to Changes in Honey Bee Health. USDA Econ. Res. Serv. 2018. [Google Scholar] [CrossRef]
- Johnson, R.; Evans, J.; Robinson, G.; Berenbaum, M. Changes in transcript abundance relating to colony collapse disorder in honey bees (Apis mellifera). Proc. Natl. Acad. Sci. USA 2009, 106, 14790–14795. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.; Tarpy, D.; vanEngelsdorp, D.; Chauzat, M.P.; Cox-Foster, D.L.; Delaplane, K.S.; Neumann, P.; Pettis, J.S.; Rogers, R.E.; Shutler, D. Colony Collapse Disorder in context. BioEssays 2010, 32, 845–846. [Google Scholar] [CrossRef] [Green Version]
- Potts, S.; Roberts, S.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honey bees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Allsopp, M.H.; de Lange, W.J.; Veldtman, R. Valuing insect pollination services with cost of replacement. PLoS ONE 2008, 3, e3128. [Google Scholar] [CrossRef] [PubMed]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Van Engelsdorp, D.; Traynor, K.S.; Andree, M.; Lichtenberg, E.M.; Chen, Y.; Saegerman, C.; Cox-Foster, D.L. Colony Collapse Disorder (CCD) and bee age impact honey bee pathophysiology. PLoS ONE 2017, 12, e0179535. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.A.; Telfer, M.G.; Roy, D.B.; Preston, C.D.; Greenwood, J.J.; Asher, J.; Fox, R.; Clarke, R.T.; Lawton, J.H. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 2004, 303, 1879–1881. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Niu, C.Y.; Lei, C.L.; Cui, J.J.; Desneux, N. Quantification of toxins in a Cry1Ac + CpTI cotton cultivar and its potential effects on the honey bee Apis mellifera L. Ecotoxicology 2010, 19, 1452–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Highfield, A.C.; El Nagar, A.; Mackinder, L.C.; Noël, L.M.; Hall, M.J.; Martin, S.J.; Schroeder, D.C. Deformed wing virus implicated in overwintering honeybee colony losses. Appl. Environ. Microbiol. 2009, 75, 7212–7220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meikle, W.G.; Diaz, R. Factors affecting pupation success of the small hive beetle, Aethina tumida. J. Insect Sci. 2012, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, M.; Floris, I.; Buffa, F.; Salaris, E.; Satta, A. Agonistic interactions between the honeybee (Apis mellifera ligustica) and the European wasp (Vespula germanica) reveal context-dependent defense strategies. PLoS ONE 2017, 12, e0180278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFrederick, Q.; Kathilankal, J.; Fuentes, J. Air pollution modifies floral scent trails. Atmos. Environ. 2008, 42, 2336–2348. [Google Scholar] [CrossRef]
- van der Sluijs, J.; Simon-Delso, N.; Goulson, D.; Maxim, L.; Bonmatin, J.; Belzunces, L. Neonicotinoids, bee disorders and the sustainability of pollinator services. Curr. Opin. Environ. Sustain. 2013, 5, 293–305. [Google Scholar] [CrossRef]
- Schönfelder, M.L.; Bogner, F.X. Individual perception of bees: Between perceived danger and willingness to protect. PLoS ONE 2017, 12, e0180168. [Google Scholar] [CrossRef] [Green Version]
- Hristov, P.; Shumkova, R.; Palova, N.; Neov, B. Factors Associated with Honey Bee Colony Losses: A Mini-Review. Vet. Sci. 2020, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, C.; Merlo, F.D. Pesticides: Human health Effects. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2011; pp. 438–453. [Google Scholar]
- Osmad, K.A. Pesticides and Human Health. Pesticides in the Modern World—Effects of Pesticides Exposure; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Pathera, A.K.; Saini, P.; Kumar, M. Harmful effects of pesticides on human health. Ann. Agric. Bio Res. 2012, 17, 165–168. [Google Scholar]
- Woodcock, B.A.; Isaac, N.J.; Bullock, J.M.; Roy, D.B.; Garthwaite, D.G.; Crove, A.; Pywell, R.F. Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat. Commun. 2016, 7, 12459. [Google Scholar] [CrossRef] [Green Version]
- Grab, H.; Branstetter, M.G.; Amon, N.; Urban-Mead, K.R.; Park, M.G.; Gibbs, J.; Blitzer, E.J.; Poveda, K.; Loeb, G.; Danforth, B.N. Agriculturally dominated landscapes reduce bee phylogenetic diversity and pollination services. Science 2019, 363, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Mullin, C.A.; Frazier, M.; Frazier, J.L.; Ashcraft, S.; Simonds, R.; vanEngelsdrop, D.; Pettis, J.S. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE 2010, 5, e9754. [Google Scholar] [CrossRef] [Green Version]
- Feazel-Orr, H.K.; Catalfamo, K.M.; Brewster, C.C.; Fell, R.D.; Anderson, T.D.; Traver, B.E. Effects of Pesticide Treatments on Nutrient Levels in Worker Honey Bees (Apis mellifera). Insects 2016, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Claudianos, C.; Ranson, H.; Johnson, R.M.; Biswas, S.; Schuler, M.A.; Berenbaum, M.R.; Feyereisen, R.; Oakeshott, J.G. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 2006, 15, 615–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahl, O.; Ulm, K. Influence of pollen feeding and physiological condition on pesticide sensitivity of the honey bee Apis mellifera carnica. Oecologia 1983, 59, 106–128. [Google Scholar] [CrossRef]
- Wu, J.Y.; Anelli, C.M.; Sheppard, W.S. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS ONE 2011, 6, e14720. [Google Scholar] [CrossRef] [Green Version]
- Fisher, A., 2nd; Rangel, J. Exposure to pesticides during development negatively affects honey bee (Apis mellifera) drone sperm viability. PLoS ONE 2018, 13, e0208630. [Google Scholar] [CrossRef]
- Pettis, J.S.; Lichtenberg, E.M.; Andree, M.; Stitzinger, J.; Rose, R.; Vanengelsdorp, D. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE 2013, 8, e70182. [Google Scholar] [CrossRef]
- Pettis, J.S.; vanEngelsdorp, D.; Johnson, J.; Dively, G. Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 2012, 99, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Boncristiani, H.; Underwood, R.; Schwarz, R.; Evans, J.; Pettis, J.; vanEngelsdorp, D. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 2012, 58, 613–620. [Google Scholar] [CrossRef]
- Park, M.G.; Blitzer, E.J.; Gibbs, J.; Losey, J.E.; Danforth, B.N. Negative effects of pesticides on wild bee communities can be buffered by landscape context. Proc. Biol. Sci. 2015, 282, 20150299. [Google Scholar] [CrossRef] [Green Version]
- McArt, S.; Fersch, A.; Milano, N.; Truitt, L.; Böröczky, K. High pesticide risk to honey bees despite low focal crop pollen collection during pollination of a mass blooming crop. Sci. Rep. 2017, 7, 46554. [Google Scholar] [CrossRef] [PubMed]
- Tasman, K.; Hidalgo, S.; Zhu, B.; Rands, S.; Hodge, J. Neonicotinoids disrupt memory, circadian behaviour and sleep. Sci. Rep. 2021, 11, 2061. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Galligan, J.J.; Hollingworth, R.M. Agonist actions of neonicotinoids on nicotinic acetylcholine receptors expressed by cockroach neurons. Neurotoxicology 2007, 28, 829–842. [Google Scholar] [CrossRef]
- Lundin, O.; Rundlöf, M.; Smith, H.G.; Fries, I.; Bommarco, R. Neonicotinoid Insecticides and Their Impacts on Bees: A Systematic Review of Research Approaches and Identification of Knowledge Gaps. PLoS ONE 2015, 10, e0136928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon-Delso, N.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Chagnon, M.; Downs, C.; Furlan, L.; Gibbons, D.W.; Giorio, C.; Girolami, V.; et al. Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. Int. 2015, 22, 5–34. [Google Scholar] [CrossRef]
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Agudo, M.; González-Cabrera, J.; Picó, Y.; Calatayud-Vernich, P.; Urbaneja, A.; Dicke, M.; Tena, A. Neonicotinoids in excretion product of phloem-feeding insects kill beneficial insects. Proc. Natl. Acad. Sci. USA 2019, 116, 16817–16822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsvetkov, N.; Samson-Robert, O.; Sood, K.; Patel, H.S.; Malena, D.A.; Gajiwala, P.H.; Maciukiewicz, P.; Fournier, V.; Zayed, A. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science 2017, 356, 1395–1397. [Google Scholar] [CrossRef] [Green Version]
- Laycock, I.; Lenthall, K.M.; Barratt, A.T.; Cresswell, J.E. Effects of imidacloprid, a neonicotinoid pesticide, on reproduction in worker bumble bees (Bombus terrestris). Ecotoxicology 2012, 21, 1937–1945. [Google Scholar] [CrossRef]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. Biol. Sci. 2017, 284, 20171711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forfert, N.; Troxler, A.; Retschnig, G.; Gauthier, L.; Straub, L.; Moritz, R.F.A.; Neumann, P.; Williams, G.R. Neonicotinoid pesticides can reduce honeybee colony genetic diversity. PLoS ONE 2017, 12, e0186109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 2012, 7, e29268. [Google Scholar] [CrossRef]
- Stafford, K.; Coles, G. Drug Resistance in Ectoparasites of Medical and Veterinary Importance. In Antimicrobial Drug Resistance. Infectious Disease; Mayers, D.L., Ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Williamson, S.M.; Moffat, C.; Gomersall, M.A.; Saranzewa, N.; Connolly, C.N.; Wright, G.A. Exposure to acetylcholinesterase inhibitors alters the physiology and motor function of honeybees. Front. Physiol. 2013, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.M.; Pollock, H.S.; Berenbaum, M.R. Synergistic interactions between in-hive miticides in Apis mellifera. J. Econ. Entomol. 2009, 102, 474–479. [Google Scholar] [CrossRef]
- Berry, J.A.; Hood, W.M.; Pietravalle, S.; Delaplane, K.S. Field-level sublethal effects of approved bee hive chemicals on Honey Bees (Apis mellifera L). PLoS ONE 2013, 8, e76536. [Google Scholar] [CrossRef] [Green Version]
- Gregorc, A.; Alburaki, M.; Rinderer, N.; Sampson, B.; Knight, P.R.; Karim, S.; Adamczyk, J. Effects of coumaphos and imidacloprid on honey bee (Hymenoptera: Apidae) lifespan and antioxidant gene regulations in laboratory experiments. Sci. Rep. 2018, 8, 15003. [Google Scholar] [CrossRef]
- Premrov Bajuk, B.; Babnik, K.; Snoj, T.; Milcinski, L.; Pislak Ocepek, M.; Skof, M.; Jencic, V.; Filazi, A.; Stajnbaher, D.; Kobal, S. Coumaphos residues in honey, bee brood, and beeswax after Varroa treatment. Apidologie 2017, 48, 588–598. [Google Scholar] [CrossRef]
- Testai, E.; Buratti, F.; Di Consiglio, E. Chlorpyrifos. In Hayes’ Handbook of Pesticide Toxicology; Academic Press: Cambridge, MA, USA, 2010; pp. 1505–1526. [Google Scholar] [CrossRef]
- Eastmond, D.; Balakrishnan, S. Genotoxicity of Pesticides. In Hayes’ Handbook of Pesticide Toxicology; Academic Press: Cambridge, MA, USA, 2010; pp. 357–380. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Huang, J.; Ma, C.; Xiao, L.; Huang, Q.; Zhao, Y.; Nie, H.; Su, S. Effects of Sublethal Concentrations of Chlorpyrifos on Olfactory Learning and Memory Performances in Two Bee Species, Apis mellifera and Apis cerana. Sociobiology 2017, 64, 174. [Google Scholar] [CrossRef] [Green Version]
- DeGrandi-Hoffman, G.; Chen, Y.; Simonds, R. The Effects of Pesticides on Queen Rearing and Virus Titers in Honey Bees (Apis mellifera L.). Insects 2013, 4, 71–89. [Google Scholar] [CrossRef] [Green Version]
- Fulton, M.; Key, P.; DeLorenzo, M. Insecticide Toxicity in Fish. Fish. Physiol. 2013, 309–368. [Google Scholar] [CrossRef]
- Sporleder, M.; Lacey, L. Biopesticides. Insect Pests Potato 2013, 463–497. [Google Scholar] [CrossRef]
- Mayes, M.A.; Thompson, G.D.; Husband, B.; Miles, M.M. Spinosad toxicity to pollinators and associated risk. Rev. Environ. Contam. Toxicol. 2003, 179, 37–71. [Google Scholar] [CrossRef]
- Qu, H.; Ma, R.X.; Liu, D.H.; Wang, P.; Huang, L.D.; Qiu, X.X.; Zhou, Z.Q. Enantioselective toxicity and degradation of the chiral insecticide fipronil in Scenedesmus obliguus suspension system. Environ. Toxicol. Chem. 2014, 33, 2516–2521. [Google Scholar] [CrossRef] [PubMed]
- Bonmatin, J.M.; Marchand, P.A.; Cotte, J.F.; Aajoud, A.; Casabianca, H.; Goutailler, G.; Courtiade, M. Bees and systemic insecticides (imidacloprid, fipronil) in pollen: Subnano-quantification by HPLC/MS/MS and GC/MS. Environ. Fate Ecol. Eff. Pestic. 2007, 837–845. [Google Scholar]
- Nicodemo, D.; Maioli, M.; Medeiros, H.; Guelfi, M.; Balieira, K.; De Jong, D.; Mingatto, F. Fipronil and imidacloprid reduce honeybee mitochondrial activity. Environ. Toxicol. Chem. 2014, 33, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Zaluski, R.; Kadri, S.M.; Alonso, D.P.; Martins Ribolla, P.E.; de Oliveira Orsi, R. Fipronil promotes motor and behavioral changes in honey bees (Apis mellifera) and affects the development of colonies exposed to sublethal doses. Environ. Toxicol. Chem. 2015, 34, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Kairo, G.; Provost, B.; Tchamitchian, S.; Abdelkader, F.; Bonnet, M.; Cousin, M.; Senechal, J.; Benet, P.; Kretzschmar, A.; Belzunces, L.P.; et al. Drone exposure to the systemic insecticide Fipronil indirectly impairs queen reproductive potential. Sci. Rep. 2016, 6, 31904. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.H.; Ogbourne, S. Eco-toxicological effects of the avermectin family with a focus on abamectin and ivermectin. Chemosphere 2016, 154, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Aljedani, D.M. Effects of abamectin and deltamethrin to the foragers honeybee workers of Apis mellifera jemenatica (Hymenoptera: Apidae) under laboratory conditions. Saudi J. Biol. Sci. 2017, 24, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Pradhan, S.; Goswami, A. Preparation and characterisation of acephate nano-encapsulated complex. Nanosci. Methods 2012, 1, 9–15. [Google Scholar] [CrossRef]
- Yao, J.; Zhu, Y.C.; Adamczyk, J.; Luttrell, R. Influences of acephate and mixtures with other commonly used pesticides on honey bee (Apis mellifera) survival and detoxification enzyme activities. Comp. Biochem. Physiol. C Toxicol. Pharm. 2018, 209, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Hillier, N.; Frost, E.; Shutler, D. Fate of Dermally Applied Miticides Fluvalinate and Amitraz within Honey Bee (Hymenoptera: Apidae) Bodies. J. Econ. Entomol. 2013, 106, 558–565. [Google Scholar] [CrossRef]
- Medici, S.; Castro, A.; Sarlo, E.; Marioli, J.; Eguaras, M. The concentration effect of selected acaricides present in beeswax foundation on the survival of Apis mellifera colonies. J. Apic. Res. 2012, 51, 164–168. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, P.; Demaeght, P.; Dermauw, W.; Khalighi, M.; Stevens, C.V.; Vanholme, B.; Tirry, L.; Lummen, P.; Van Leeuwen, T. On the mode of action of bifenazate: New evidence for a mitochondrial target site. Pestic. Biochem. Physiol. 2012, 104, 88–95. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X. Toxicity and risk of spinetoram and bifenazate to bumblebee Bombus terrestris (Hymenoptera: Apidae). Acta Entomol. Sin. 2019, 62, 334–342. [Google Scholar] [CrossRef]
- Hougard, J.; Duchon, S.; Zaim, M.; Guillet, P. Bifenthrin: A Useful Pyrethroid Insecticide for Treatment of Mosquito Nets. J. Med. Entomol. 2002, 39, 526–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, P.; Wang, Q.; Sun, J.; Liu, F.; Wang, X.; Wu, Y.; Zhou, T. Effects of sublethal concentrations of bifenthrin and deltamethrin on fecundity, growth, and development of the honeybee Apis mellifera ligustica. Environ. Toxicol. Chem. 2010, 29, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Ganguly, P.; Barik, S.R.; Samanta, A. Dissipation kinetics and risk assessment of chlorfenapyr on tomato and cabbage. Environ. Monit. Assess. 2018, 190, 71. [Google Scholar] [CrossRef] [PubMed]
- N’Guessan, R.; Boko, P.; Odjo, A.; Akogbéto, M.; Yates, A.; Rowland, M. Chlorfenapyr: A pyrrole insecticide for the control of pyrethroid or DDT resistant Anopheles gambiae (Diptera: Culicidae) mosquitoes. Acta Trop. 2007, 102, 69–78. [Google Scholar] [CrossRef]
- Costa, E.; Araujo, E.; Maia, A.; Silva, F.; Bezerra, C.; Silva, J. Toxicity of insecticides used in the Brazilian melon crop to the honey bee Apis mellifera under laboratory conditions. Apidologie 2013, 45, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Smodis Skerl, M.I.; Kmecl, V.; Gregorc, A. Exposure to pesticides at sublethal level and their distribution within a honey bee (Apis mellifera) colony. Bull. Environ. Contam. Toxicol. 2010, 85, 125–128. [Google Scholar] [CrossRef]
- Zikic, B.; Aleksic, N.; Ristanic, M.; Glavinic, U.; Vejnovic, B.; Krnjaic, I.; Stanimirovic, Z. Anti-Varroa Efficiency of Coumaphos and Its Influence on Oxidative Stress and Survival of Honey Bees. Acta Vet. Brno 2020, 70, 355–373. [Google Scholar] [CrossRef]
- Bendahou, N.; Bounias, M.; Fleche, C. Toxicity of cypermethrin and fenitrothion on the hemolymph carbohydrates, head acetylcholinesterase, and thoracic muscle Na+, K+-ATPase of emerging honeybees (Apis mellifera mellifera. L). Ecotoxicol. Environ. Saf. 1999, 44, 139–146. [Google Scholar] [CrossRef]
- Fent, K.; Haltiner, T.; Kunz, P.; Christen, V. Insecticides cause transcriptional alterations of endocrine related genes in the brain of honey bee foragers. Chemosphere 2020, 260, 127542. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.; Hashem, K. Deltamethrin-induced oxidative stress and biochemical changes in tissues and blood of catfish (Clarias gariepinus): Antioxidant defense and role of alpha-tocopherol. BMC Vet. Res. 2012, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Li, Z.; Huang, Q.; Zhang, X.W.; Ke, L.; Yan, W.Y.; Zhang, L.Z.; Zeng, Z.J. Deltamethrin Impairs Honeybees (Apis mellifera) Dancing Communication. Arch. Environ. Contam. Toxicol. 2019, 78, 117–123. [Google Scholar] [CrossRef]
- Boussabbeh, M.; Ben Salem, I.; Hamdi, M.; Ben Fradj, S.; Abid-Essefi, S.; Bacha, H. Diazinon, an organophosphate pesticide, induces oxidative stress and genotoxicity in cells deriving from large intestine. Environ. Sci. Pollut. Res. Int. 2016, 23, 2882–2889. [Google Scholar] [CrossRef]
- Weick, J.; Thorn, R.S. Effects of acute sublethal exposure to coumaphos or diazinon on acquisition and discrimination of odor stimuli in the honey bee (Hymenoptera: Apidae). J. Econ. Entomol. 2002, 95, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Joho, Y.; Vogel, M.; Fent, K. Transcriptional and physiological effects of the pyrethroid deltamethrin and the organophosphate dimethoate in the brain of honey bees (Apis mellifera). Environ. Pollut. 2019, 244, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Holder, P.; Jones, A.; Tyler, C.; Cresswell, J. Fipronil pesticide as a suspect in historical mass mortalities of honey bees. Proc. Natl. Acad. Sci. USA 2018, 115, 13033–13038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berheim, E.; Jenks, J.; Lundgren, J.; Michel, E.; Grove, D.; Jensen, W. Effects of Neonicotinoid Insecticides on Physiology and Reproductive Characteristics of Captive Female and Fawn White-tailed Deer. Sci. Rep. 2019, 9, 4534. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.; Huang, Z.; Milbrath, M. Neonicotinoid Pesticides Are More Toxic to Honey Bees at Lower Temperatures: Implications for Overwintering Bees. Front. Ecol. Evol. 2020, 8, 316. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Interaction of field realistic doses of clothianidin and Varroa destructor parasitism on adult honey bee (Apis mellifera L.) health and neural gene expression, and antagonistic effects on differentially expressed genes. PLoS ONE 2020, 15, e0229030. [Google Scholar] [CrossRef] [Green Version]
- Vergara-Amado, J.; Manzi, C.; Franco, L.; Contecha, S.C.; Marquez, S.J.; Solano-Iguaran, J.J.; Haro, R.E.; Silva, A.X. Effects of residual doses of neonicotinoid (imidacloprid) on metabolic rate of queen honey bees Apis mellifera (Hymenoptera: Apidae). Apidologie 2020, 51, 1091–1099. [Google Scholar] [CrossRef]
- Miles, M.; Mayes, M.; Dutton, R. The effects of spinosad, a naturally derived insect control agent, to the honeybee (Apis melifera). Mededelingen 2002, 67, 611–616. [Google Scholar] [PubMed]
- Miles, M.J.; Alix, A.; Bourgouin, C.; Schmitzer, S. Effects of spinosad on honey bees (Apis mellifera): Findings from over ten years of testing and commercial use. Julius-Kühn-Archiv 2012, 437, 107. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Muller, A.; Sumser, H.; Horren, T.; et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [Green Version]
- Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions the European Green Deal, European Commission: Brussels, 11 December 2019 COM (2019) 640 Final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 4 August 2021).
- Regulation (EC) No 1107/2009 150 of the European Parliament and of the Council of 21 October 2009, Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R1107 (accessed on 4 August 2021).
- Directive 2009/128/EC 151 of the European Parliament and of the Council of 21 October 2009. Available online: https://www.europarl.europa.eu/RegData/etudes/STUD/2018/627113/EPRS_STU(2018)627113_EN.pdf (accessed on 4 August 2021).
- Commission Implementing Regulation (EU) No 485/2013 of 24 May 2013 Amending Implementing Regulation (EU) No 540/2011, as Regards the Conditions of Approval of the Active Substances Clothianidin, Thiamethoxam and Imidacloprid, and Prohibiting the Use and Sale of Seeds Treated with Plant Protection Products Containing Those Active Substances. Available online: https://eur-lex.europa.eu/eli/reg_impl/2013/485/oj (accessed on 4 August 2021).
- Rundlöf, M.; Andersson, G.; Bommarco, R.; Fries, I.; Hederström, V.; Herbertsson, L.; Jonsson, O.; Klatt, B.K.; Pedersen, T.R.; Yourstone, J.; et al. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 2015, 521, 77–80. [Google Scholar] [CrossRef]
- Wood, T.; Goulson, D. The environmental risks of neonicotinoid pesticides: A review of the evidence post 2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, B.; Bullock, J.; Shore, R.; Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commission Implementing Regulation (EU) 2018/783 of 29 May 2018 Amending Implementing Regulation (EU) No 540/2011 as Regards the Conditions of Approval of the Active Substance Imidacloprid. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018R0783 (accessed on 4 August 2021).
- Commission Implementing Regulation (EU) 2018/784 of 29 May 2018 Amending Implementing Regulation (EU) No 540/2011 as Regards the Conditions of Approval of the Active Substance Clothianidin. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018R0784 (accessed on 4 August 2021).
- Commission Implementing Regulation (EU) 2018/785 of 29 May 2018 Amending Implementing Regulation (EU) No 540/2011 as Regards the Conditions of Approval of the Active Substance Thiamethoxam. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32018R0785 (accessed on 4 August 2021).
- Maini, S.; Medrzycki, P.; Porrini, C. The puzzle of honey bee loses: The review. Bull. Insectol. 2010, 63, 153–160. [Google Scholar]
- Goulson, D. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Godfray, H.; Blacquière, T.; Field, L.; Hails, R.; Petrokofsky, G.; Potts, S.; Raine, N.; Vanbergen, A.; McLean, A. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140558. [Google Scholar] [CrossRef] [Green Version]
- EFSA, European Food Safety Authority. Scientific Opinion on the science behind the development of a risk assessment of Plant Protection Products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 2012, 10, 2668. [Google Scholar] [CrossRef]
- Sgolastra, F.; Medrzycki, P.; Bortolotti, L.; Maini, S.; Porrini, C.; Simon-Delso, N.; Bosch, J. Bees and pesticide regulation: Lessons from the neonicotinoid experience. Biol. Conserv. 2020, 241, 108356. [Google Scholar] [CrossRef]
- Beekman, M.; Ratnieks, F. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Protecting Pollinators from Pesticides—Bees, Beekeeping & Protecting Pollinators|Honey Bee Program. Bees.caes.uga.edu. Available online: https://bees.caes.uga.edu/bees-beekeeping-pollination/pollination/pollination-protecting-pollinators-from-pesticides.html (accessed on 10 May 2021).
- World Organisation for Animal Health (OIE). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organisation for Animal Health: Paris, France, 2013. [Google Scholar]
- Schuch, D.; Madden, R.; Sattler, A. An improved method for the detection and presumptive identification of Paenibacillus larvae subsp.larvae spores in honey. J. Apic. Res. 2001, 40, 59–64. [Google Scholar] [CrossRef]
- Genersch, E.; Forsgren, E.; Pentikäinen, J.; Ashiralieva, A.; Rauch, S.; Kilwinski, J.; Fries, I. Reclassification of Paenibacillus larvae subsp. pulvifaciens and Paenibacillus larvae subsp. larvae as Paenibacillus larvae without subspecies differentiation. J. Syst. Evol. Microbiol. 2006, 56, 501–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemurot, M.; Brunain, M.; Akol, A.M.; Descamps, T.; de Graaf, D.C. First detection of Paenibacillus larvae the causative agent of American Foulbrood in a Ugandan honeybee colony. Springerplus 2016, 5, 1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauch, S.; Ashiralieva, A.; Hedtke, K.; Genersch, E. Negative correlation between individual-insect-level virulence and colony-level virulence of Paenibacillus larvae, the etiological agent of American foulbrood of honeybees. Appl. Environ. Microbiol. 2009, 75, 3344–3347. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gonzalez, E.; Poppinga, L.; Fünfhaus, A.; Hertlein, G.; Hedtke, K.; Jakubowska, A.; Genersch, E. Paenibacillus larvae chitin-degrading protein PlCBP49 is a key virulence factor in American Foulbrood of honey bees. PLoS Pathog. 2014, 10, e1004284. [Google Scholar] [CrossRef] [PubMed]
- Khezri, M.; Moharrami, M.; Modirrousta, H.; Torkaman, M.; Rokhzad, B.; Khanbabaie, H. Prevalence of American foulbrood in asymptomatic apiaries of Kurdistan, Iran. Vet. World 2018, 11, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Piccini, C.; D’Alessandro, B.; Antúnez, K.; Zunino, P. Detection of Paenibacillus larvae subspecies larvae spores in naturally infected bee larvae and artificially contaminated honey by PCR. World J. Microbiol. Biotechnol. 2002, 18, 761–765. [Google Scholar] [CrossRef]
- Beims, H.; Wittmann, J.; Bunk, B.; Spröer, C.; Rohde, C.; Günther, G.; Rohde, M.; von der Ohe, W.; Steinert, M. Paenibacillus larvae-Directed Bacteriophage HB10c2 and Its Application in American Foulbrood-Affected Honey Bee Larvae. Appl. Environ. Microbiol. 2015, 81, 5411–5419. [Google Scholar] [CrossRef] [Green Version]
- Beims, H.; Bunk, B.; Erler, S.; Mohr, K.; Sproer, C.; Pradella, S.; Gunther, G.; Rohde, M.; von der Ohe, W.; Steinert, M. Discovery of Paenibacillus larvae ERIC V: Phenotypic and genomic comparison to genotypes ERIC I-IV reveal different inventories of virulence factors which correlate with epidemiological prevalences of American Foulbrood. Int. J. Med. Microbiol. 2020, 310, 151394. [Google Scholar] [CrossRef]
- Ansari, M.J.; Al-Ghamdi, A.; Nuru, A.; Ahmed, A.M.; Ayaad, T.H.; Al-Qarni, A.; Alattal, Y.; Al-Waili, N. Survey and molecular detection of Melissococcus plutonius, the causative agent of European Foulbrood in honeybees in Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Djukic, M.; Erler, S.; Leimbach, A.; Grossar, D.; Charrière, J.D.; Gauthier, L.; Hartken, D.; Dietrich, S.; Nacke, H.; Daniel, R.; et al. Comparative Genomics and Description of Putative Virulence Factors of Melissococcus plutonius, the Causative Agent of European Foulbrood Disease in Honey Bees. Genes 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otten, C. A General overview on AFB and EFB Pathogen, Way of infection, Multiplication, Clinical symptoms and Outbreak. Apiacta 2003, 38, 106–113. [Google Scholar]
- Lewkowski, O.; Erler, S. Virulence of Melissococcus plutonius and secondary invaders associated with European foulbrood disease of the honey bee. Microbiologyopen 2019, 8, e00649. [Google Scholar] [CrossRef] [PubMed]
- Erban, T.; Ledvinka, O.; Kamler, M.; Hortova, B.; Nesvorna, M.; Tyl, J.; Titera, D.; Markovic, M.; Hubert, J. Bacterial community associated with worker honeybees (Apis mellifera) affected by European foulbrood. PeerJ 2017, 5, e3816. [Google Scholar] [CrossRef] [Green Version]
- Rusenova, N.; Parvanov, P. EUROPEAN FOULBROOD DISEASE –AETIOLOGY, DIAGNOSTICS AND CONTROL. Trakia J. Sci. 2005, 3, 10–16. [Google Scholar]
- Lye, D.C.; Earnest, A.; Ling, M.L.; Lee, T.E.; Yong, H.C.; Fisher, D.A.; Krishnan, P.; Hsu, L.Y. The impact of multidrug resistance in healthcare-associated and nosocomial Gram-negative bacteraemia on mortality and length of stay: Cohort study. Clin. Microbiol. Infect. 2012, 18, 502–508. [Google Scholar] [CrossRef] [Green Version]
- Burritt, N.L.; Foss, N.J.; Neeno-Eckwall, E.C.; Church, J.O.; Hilger, A.M.; Hildebrand, J.A.; Warshauer, D.M.; Perna, N.T.; Burritt, J.B. Sepsis and Hemocyte Loss in Honey Bees (Apis mellifera) Infected with Serratia marcescens Strain Sicaria. PLoS ONE 2016, 11, e0167752. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Blom, J.; Walker, E.D. Genomic, Physiologic, and Symbiotic Characterization of Serratia marcescens Strains Isolated from the Mosquito Anopheles stephensi. Front. Microbiol. 2017, 8, 1483. [Google Scholar] [CrossRef]
- Celejewski-Marciniak, P.; Tyski, S. Bacilli of the genus Serratia: Species characteristics, pathogenicity and antibiotic resistance of Serratia marcescens. Postepy Mikrobiol. 2011, 50, 291–302. [Google Scholar]
- Raymann, K.; Coon, K.L.; Shaffer, Z.; Salisbury, S.; Moran, N.A. Pathogenicity of Serratia marcescens Strains in Honey Bees [published correction appears in mBio. mBio 2018, 9, e01649-18. [Google Scholar] [CrossRef] [Green Version]
- Jensen, A.; Aronstein, K.; Flores, J.; Vojvodic, S.; Palacio, M.; Spivak, M. Standard methods for fungal brood disease research. J. Apic. Res. 2013, 52, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; You, X.L.; Wang, L.L.; Yan, Z.T.; Zhou, Z.Y. Spore morphology and ultrastructure of an Ascosphaera apis strain from the honeybees (Apis mellifera) in southwest China. Mycologia 2018, 110, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Reynaldi, F.J.; Lucia, M.; Genchi Garcia, M.L. Ascosphaera apis, the entomopathogenic fungus affecting larvae of native bees (Xylocopa augusti): First report in South America. Rev. Iberoam. Micol. 2015, 32, 261–264. [Google Scholar] [CrossRef]
- Maxfield-Taylor, S.A.; Mujic, A.B.; Rao, S. First detection of the larval chalkbrood disease pathogen Ascosphaera apis (Ascomycota: Eurotiomycetes: Ascosphaerales) in adult bumble bees. PLoS ONE 2015, 10, e0124868. [Google Scholar] [CrossRef]
- Mráz, P.; Hýbl, M.; Kopecký, M.; Bohatá, A.; Konopická, J.; Hoštičková, I.; Konvalina, P.; Šipoš, J.; Rost, M.; Čurn, V. The Effect of Artificial Media and Temperature on the Growth and Development of the Honey Bee Brood Pathogen Ascosphaera apis. Biology 2021, 10, 431. [Google Scholar] [CrossRef]
- Balajee, S.A.; Houbraken, J.; Verweij, P.E.; Hong, S.B.; Yaghuchi, T.; Varga, J.; Samson, R.A. Aspergillus species identification in the clinical setting. Stud. Mycol. 2007, 59, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Bhabhra, R.; Askew, D.S. Thermotolerance and virulence of Aspergillus fumigatus: Role of the fungal nucleolus. Med. Mycol. 2005, 43 (Suppl. 1), S87–S93. [Google Scholar] [CrossRef] [PubMed]
- Diba, K.; Kordbacheh, P.; Mirhendi, S.H.; Rezaie, S.; Mahmoudi, M. Identification of Aspergillus species using morphological characteristic and the effect of temperature on the protease activity. Int. J. Biochem. Biotechnol. 2013, 2, 2167–2404. [Google Scholar]
- Foley, K.; Fazio, G.; Jensen, A.B.; Hughes, W.O. The distribution of Aspergillus spp. opportunistic parasites in hives and their pathogenicity to honey bees. Vet. Microbiol. 2014, 169, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Underwood, R.; VanEngelsdorp, D. Colony Collapse Disorder: Have we seen this before? Bee Cult. 2007, 35, 13–18. [Google Scholar]
- Cilia, G.; Sagona, S.; Giusti, M.; Jarmela dos Santos, P.; Nanetti, A.; Felicioli, A. Nosema ceranae infection in honeybee samples from Tuscanian Archipelago (Central Italy) investigated by two qPCR methods. Saudi J. Biol. Sci. 2019, 26, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; García-Palencia, P.; Martín-Hernández, R.; Meana, A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef] [PubMed]
- García-Palencia, P.; Martín-Hernández, R.; González-Porto, A.; Marin, P.; Meana, A.; Higes, M. Natural infection by Nosema ceranae causes similar lesions as in experimentally infected caged-worker honey bees (Apis mellifera). J. Apic. Res. 2010, 49, 278–283. [Google Scholar] [CrossRef]
- Kasprzak, S.; Topolska, G. [Nosema ceranae (Eukaryota: Fungi: Microsporea)—A new parasite of western honey bee Apis mellifera L.]. Wiadomości Parazytol. 2007, 53, 281–284. [Google Scholar]
- Gage, S.; Kramer, C.; Calle, S.; Carroll, M.; Heien, M.; DeGrandi-Hoffman, G. Nosema ceranae parasitism impacts olfactory learning and memory and neurochemistry in honey bees (Apis mellifera). J. Exp. Biol. 2017, 221, jeb161489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.P.; Evans, J.D.; Murphy, C.; Gutell, R.; Zuker, M.; Gundensen-Rindal, D.; Pettis, J.S. Morphological, molecular, and phylogenetic characterization of Nosema ceranae, a microsporidian parasite isolated from the European honey bee, Apis mellifera. J. Eukaryot. Microbiol. 2009, 56, 142–147. [Google Scholar] [CrossRef] [Green Version]
- Naug, D.; Gibbs, A. Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie 2009, 40, 595–599. [Google Scholar] [CrossRef] [Green Version]
- Rubanov, A.; Russell, K.; Rothman, J.; Nieh, J.; McFrederick, Q. Intensity of Nosema ceranae infection is associated with specific honey bee gut bacteria and weakly associated with gut microbiome structure. Sci. Rep. 2019, 9, 3820. [Google Scholar] [CrossRef]
- Faita, M.; Cardozo, M.; Amandio, D.; Orth, A.; Nodari, R. Glyphosate-based herbicides and Nosema sp. microsporidia reduce honey bee (Apis mellifera L.) survivability under laboratory conditions. J. Apic. Res. 2020, 59, 332–342. [Google Scholar] [CrossRef]
- Naeef Ayoub, Z. Virulence of Varroa destructor in Colonies of Honey Bee Apis mellifera. In Beekeeping-New Challenges; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J. Varroa Mite, Varroa destructor Anderson and Trueman (Acari: Varroidae). Encycl. Entomol. 2008, 4041–4048. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Eccles, L.; Calvete, Y.; Mcgowan, J.; Kelly, P.; Correa-Benítez, A. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, E.H.; Jones, B.; Bowman, A. Salivary secretions from the honeybee mite, Varroa destructor: Effects on insect haemocytes and preliminary biochemical characterization. Parasitology 2011, 138, 602–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinkevich, F.D.; Danka, R.G.; Healy, K.B. Influence of Varroa Mite (Varroa destructor) Management Practices on Insecticide Sensitivity in the Honey Bee (Apis mellifera). Insects 2017, 8, 9. [Google Scholar] [CrossRef] [PubMed]
| Pesticide | Application | Possible Effect on Bees | References |
|---|---|---|---|
| Abamectin | Control of insect pests and mites destroying horticultural and agricultural crops. | Negatively affects the viability and cytotoxic midgut cells which can lead to digestive diseases. | [119,120] |
| Acephate | Control a wide range of chewing and sucking insect pests threating agricultural crops. | Suppresses esterase activity and reduces body weight. | [121,122] |
| Amitraz | Control of ticks, mites, and lice on domestic animals. | Increases mortality, leads to behavioral changes in adult honey bees. | [123,124] |
| Bifenazate | Control of spider mites. | Affects the physiology and behavior. | [125,126] |
| Bifenthrin | Control of insect pests, treatment of mosquito nets, suppression of malaria transmission. | Increases mortality, affects central and peripheral nervous systems. | [127,128] |
| Chlorfenapyr | Control of insect pests threating agricultural crops and animals. | Increases mortality, causes paralysis, affects nervous system. | [129,130,131] |
| Coumaphos | Control of insect pests threating agricultural crops and animals. | Reduces foraging activity, affects colony mortality, and affects the size of hypopharyngeal glands, significantly affects oxidative status. | [105,132,133] |
| Cypermethrin | Control of insect pests threating agricultural crops. | Leads to a significant hypoglucosemia and hypotrehalosemia, causes minor expressional changes of genes. | [134,135] |
| Deltamethrin | Control of disease vectors and eradicating unwanted insects. | Interferes with the nervous system such as memory-related characteristics and dance behavior. | [136,137] |
| Diazinon | Control of household insects and insect pests threating agricultural crops. | Reduces activity of acetylcholineasterase (an enzyme essential to the transmission of nerve impulses), affects odor learning. | [138,139] |
| Dimethoate | Control of insect pests threating agricultural crops. | Leads to inhibition of the acetylcholinesterase, affects physiological traits. | [140] |
| Fenitrothion | Control of insect pests threating agricultural crops. | Leads to a significant hypoglucosemia and hypotrehalosemia, inhibits acetylcholinesterase activity. | [134] |
| Fipronil | Control of insect pests threating agricultural crops (mostly sunflowers). | Increases mortality, affects foraging intensity and homing success. | [141] |
| Neonicotinoids | Control of insect pests threating agricultural crops and deterring pests on domesticated animals. | Increases mortality, affects central nervous system, increases the levels of deformed wing virus, affects a large number of up and down-regulated differentially expressed genes, and reduces standard metabolic rate in queens. | [142,143,144,145] |
| Spinosad | Control of insect pests affecting agricultural crops. | Absence of significant impact on honey bee colonies | [146,147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leska, A.; Nowak, A.; Nowak, I.; Górczyńska, A. Effects of Insecticides and Microbiological Contaminants on Apis mellifera Health. Molecules 2021, 26, 5080. https://doi.org/10.3390/molecules26165080
Leska A, Nowak A, Nowak I, Górczyńska A. Effects of Insecticides and Microbiological Contaminants on Apis mellifera Health. Molecules. 2021; 26(16):5080. https://doi.org/10.3390/molecules26165080
Chicago/Turabian StyleLeska, Aleksandra, Adriana Nowak, Ireneusz Nowak, and Anna Górczyńska. 2021. "Effects of Insecticides and Microbiological Contaminants on Apis mellifera Health" Molecules 26, no. 16: 5080. https://doi.org/10.3390/molecules26165080