Long-Term Antibacterial Film Nanocomposite Incorporated with Patchouli Essential Oil Prepared by Supercritical CO2 Cyclic Impregnation for Wound Dressing
Abstract
:1. Introduction
2. Results
2.1. Structure and Morphology of PEO-MSNs and PEO-FNC
2.2. Drug Load of PEO-MSNs and the Effects of SCCI Parameters
2.3. Physical and Mechanical Properties
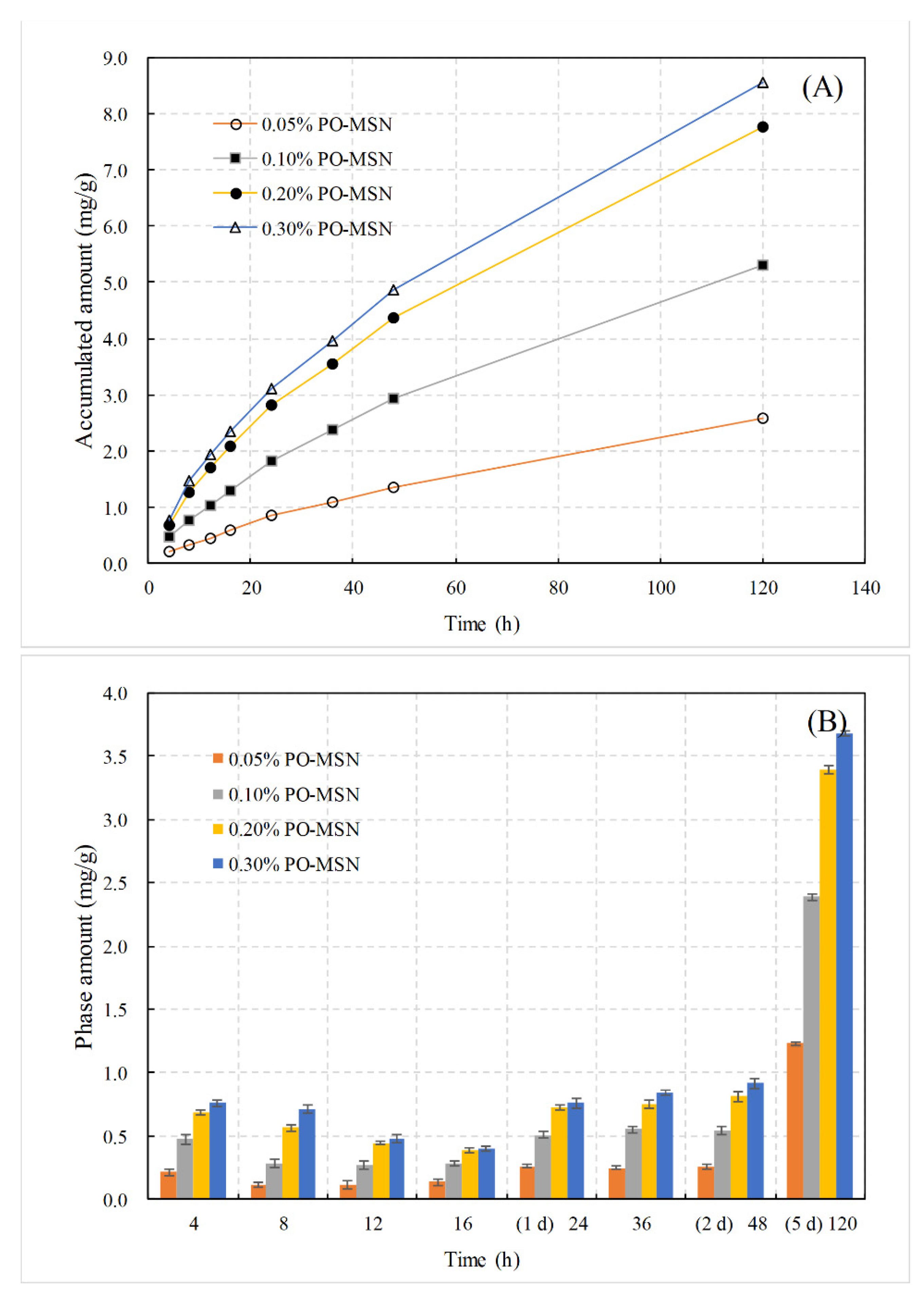
2.4. Release Behavior of PEO from PEO-FNC
2.5. Cytotoxicity Exhibition of PEO-FNC
2.6. Antibacterial Properties
3. Materials and Methods
3.1. Materials
3.2. Loading PEO into MSNs via Supercritical CO2 Cyclic Impregnation (SCCI)
3.3. Film Nanocomposite Fabrication
3.4. Characterization
3.4.1. Structure and Morphology of MSNs and PEO-FNC
3.4.2. Mechanical Properties of Film Nanocomposite
3.5. Contents and Release Behavior of PEO
3.6. Cytotoxicity Test
3.7. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vowden, K.; Vowden, P. Wound dressings: Principles and practice. Surgery 2017, 35, 489–494. [Google Scholar]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. USA 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surfaces B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Qi, H.; Shi, C.; Wei, G.; Xiao, L.; Huang, Z.; Liu, S.; Yu, H.; Teng, C.; et al. Nanocomposite sponges of sodium alginate/graphene oxide/polyvinyl alcohol as potential wound dressing: In vitro and in vivo evaluation. Compos. Part B Eng. 2019, 167, 396–405. [Google Scholar] [CrossRef]
- Ye, S.; Jiang, L.; Wu, J.; Su, C.; Huang, C.; Liu, X.; Shao, W. Flexible Amoxicillin-Grafted Bacterial Cellulose Sponges for Wound Dressing: In Vitro and in Vivo Evaluation. ACS Appl. Mater. Interfaces 2018, 10, 5862–5870. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohyd. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Colobatiu, L.; Gavan, A.; Mocan, A.; Bogdan, C.; Mirel, S.; Tomuta, I. Development of bioactive compounds-loaded chitosan films by using a QbD approach—A novel and potential wound dressing material. React. Funct. Polym. 2019, 138, 46–54. [Google Scholar] [CrossRef]
- Shi, R.; Niu, Y.; Gong, M.; Ye, J.; Tian, W.; Zhang, L. Antimicrobial gelatin-based elastomer nanocomposite membrane loaded with ciprofloxacin and polymyxin B sulfate in halloysite nanotubes for wound dressing. Mater. Sci. Eng. C 2018, 87, 128–138. [Google Scholar] [CrossRef]
- Dabiri, G.; Damstetter, E.; Phillips, T. Choosing a Wound Dressing Based on Common Wound Characteristics. Adv. Wound Care 2016, 5, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Pilehvar-Soltanahmadi, Y.; Dadashpour, M.; Mohajeri, A.; Fattahi, A.; Sheervalilou, R.; Zarghami, N. An overview on application of natural substances incorporated with electrospun nanofibrous scaffolds to development of innovative wound dressings. Mini-Rev. Med. Chem. 2018, 18, 414–427. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohyd. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, H.; Yang, S.; Xi, Z.; Tang, T.; Yin, R.; Zhang, W. Electrospun PLGA membrane incorporated with andrographolide-loaded mesoporous silica nanoparticles for sustained antibacterial wound dressing. Nanomedicine 2018, 13, 2881–2899. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, X.; Tang, H.; Zheng, M.; Huang, F. A novel candidate for wound dressing: Transparent porous maghemite/cellulose nanocomposite membranes with controlled release of doxorubicin from a simple approach. Mater. Sci. Eng. C 2017, 79, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Chakradhar, S. What’s old is new: Reconfiguring known antibiotics to fight drug resistance. Nat. Med. 2016, 22, 1197–1199. [Google Scholar] [CrossRef]
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st Century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584–592. [Google Scholar] [CrossRef]
- Cordell, G.A.; Colvard, M.D. Natural products and traditional medicine: Turning on a paradigm. J. Nat. Prod. 2012, 75, 514–525. [Google Scholar] [CrossRef]
- Orchard, A.; van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evid. Based Complement. Alt. 2017, 2017, 4517971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef]
- Velmurugan, P.; Ganeshan, V.; Nishter, N.F.; Jonnalagadda, R.R. Encapsulation of orange and lavender essential oils in chitosan nanospherical particles and its application in leather for aroma enrichment. Surfaces Interfaces 2017, 9, 124–132. [Google Scholar] [CrossRef]
- Varona, S.; Martín, A.; Cocero, M.J. Liposomal incorporation of lavandin essential oil by a thin-film hydration method and by particles from gas-saturated solutions. Ind. Eng. Chem. Res. 2011, 50, 2088–2097. [Google Scholar] [CrossRef]
- de Dicastillo, C.L.; Villegas, C.; Garrido, L.; Roa, K.; Torres, A.; Galotto, M.J.; Rojas, A.; Romero, J. Modifying an active compound’s release kinetic using a supercritical impregnation process to incorporate an active agent into PLA electrospun mats. Polymers 2018, 10, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas, A.; Torres, A.; Añazco, A.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Effect of pressure and time on scCO2-assisted incorporation of thymol into LDPE-based nanocomposites for active food packaging. J. CO2 Util. 2018, 26, 434–444. [Google Scholar] [CrossRef]
- Champeau, M.; Thomassin, J.M.; Tassaing, T.; Jérôme, C. Drug loading of polymer implants by supercritical CO2 assisted impregnation: A review. J. Control Release 2015, 209, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, G.; Pantić, M.; Novak, Z.; Knez, Ž. Supercritical impregnation of drugs and supercritical fluid deposition of metals into aerogels. J. Mater. Sci. 2015, 50, 1–12. [Google Scholar] [CrossRef]
- Jia, J.; Liu, X.; Wu, K.; Zhou, X.; Ge, F. Loading zedoary oil into pH-sensitive chitosan grafted mesoporous silica nanoparticles via gate-penetration by supercritical CO2 (GPS). J. CO2 Util. 2019, 33, 12–20. [Google Scholar] [CrossRef]
- Liu, X.; Jia, J.; Duan, S.; Zhou, X.; Xiang, A.; Lian, Z.; Ge, F. Zein/MCM-41 nanocomposite film incorporated with cinnamon essential oil loaded by modified supercritical CO2 impregnation for long-term antibacterial packaging. Pharmaceutics 2020, 12, 169. [Google Scholar] [CrossRef] [Green Version]
- Nouri, A.; Yaraki, M.T.; Ghorbanpour, M.; Agarwal, S.; Gupta, V.K. Enhanced Antibacterial effect of chitosan film using Montmorillonite/CuO nanocomposite. Int. J. Biol. Macromol. 2018, 109, 1219–1231. [Google Scholar] [CrossRef]
- Niedermayer, S.; Weiss, V.; Herrmann, A.; Schmidt, A.; Datz, S.; Müller, K.; Wagner, E.; Bein, T.; Bräuchle, C. Multifunctional polymer-capped mesoporous silica nanoparticles for pH-responsive targeted drug delivery. Nanoscale 2015, 7, 7953–7964. [Google Scholar] [CrossRef] [Green Version]
- Shankar, S.; Wang, L.; Rhim, J. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Mater. Sci. Eng. C 2018, 93, 289–298. [Google Scholar] [CrossRef]
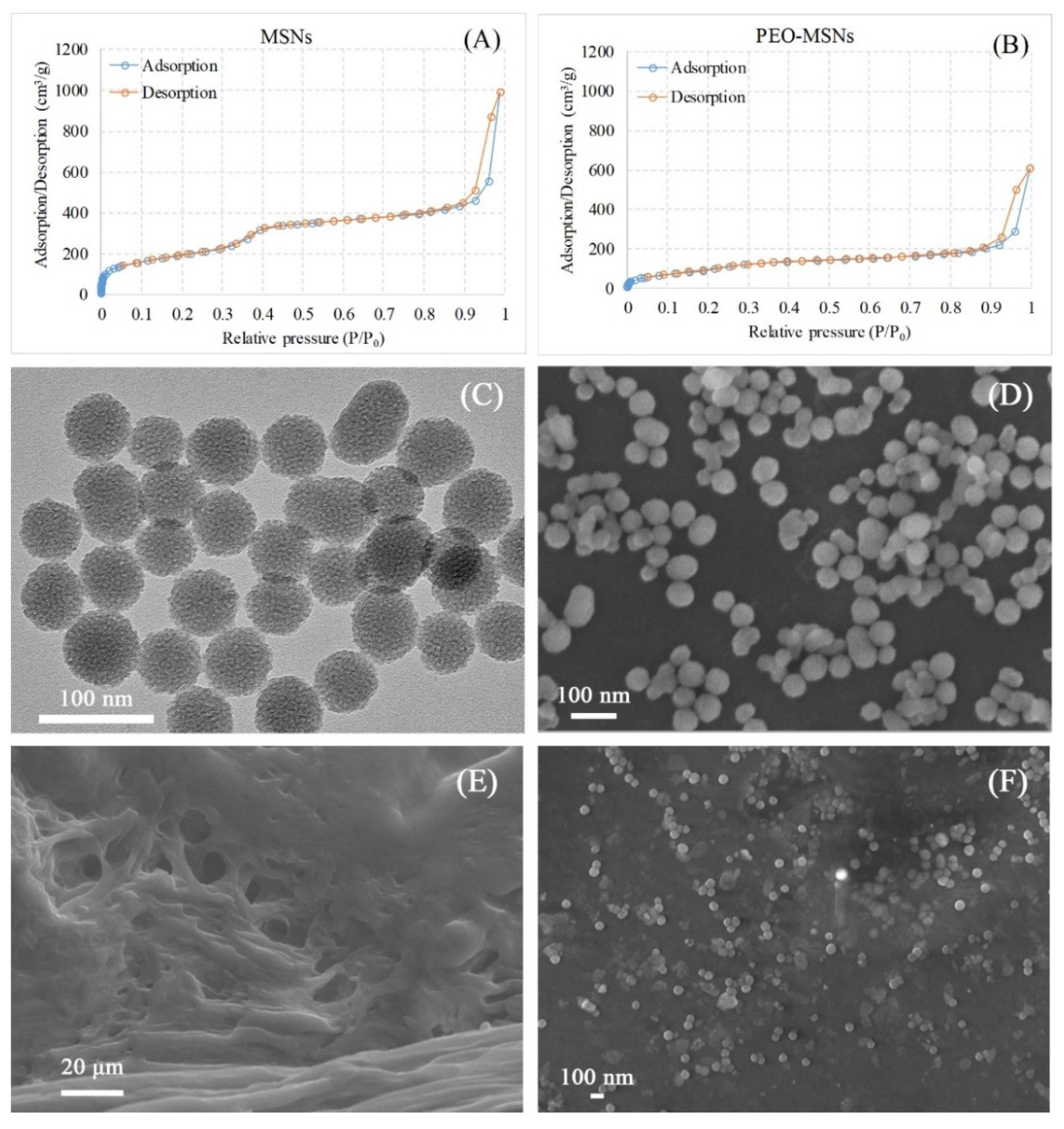
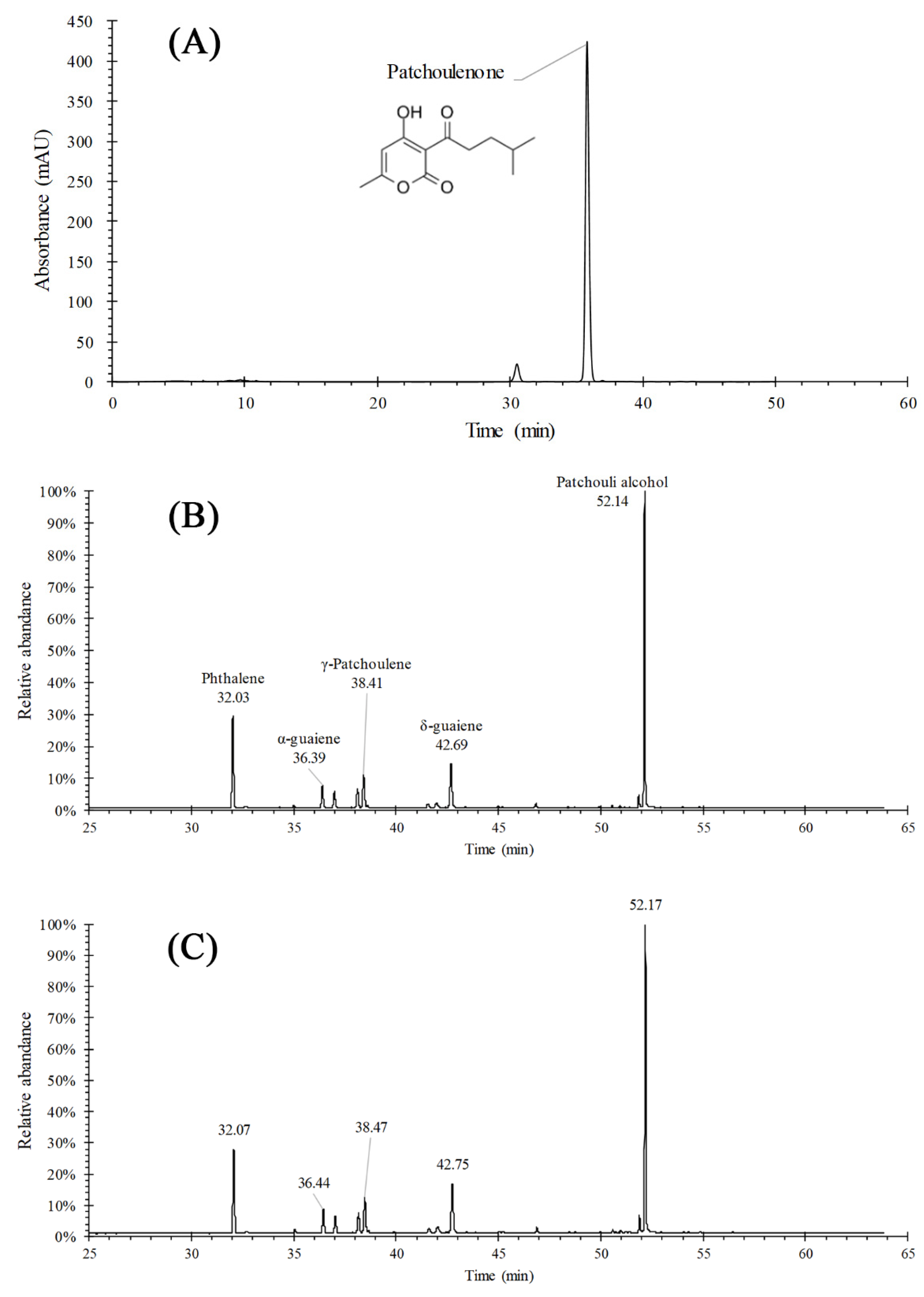
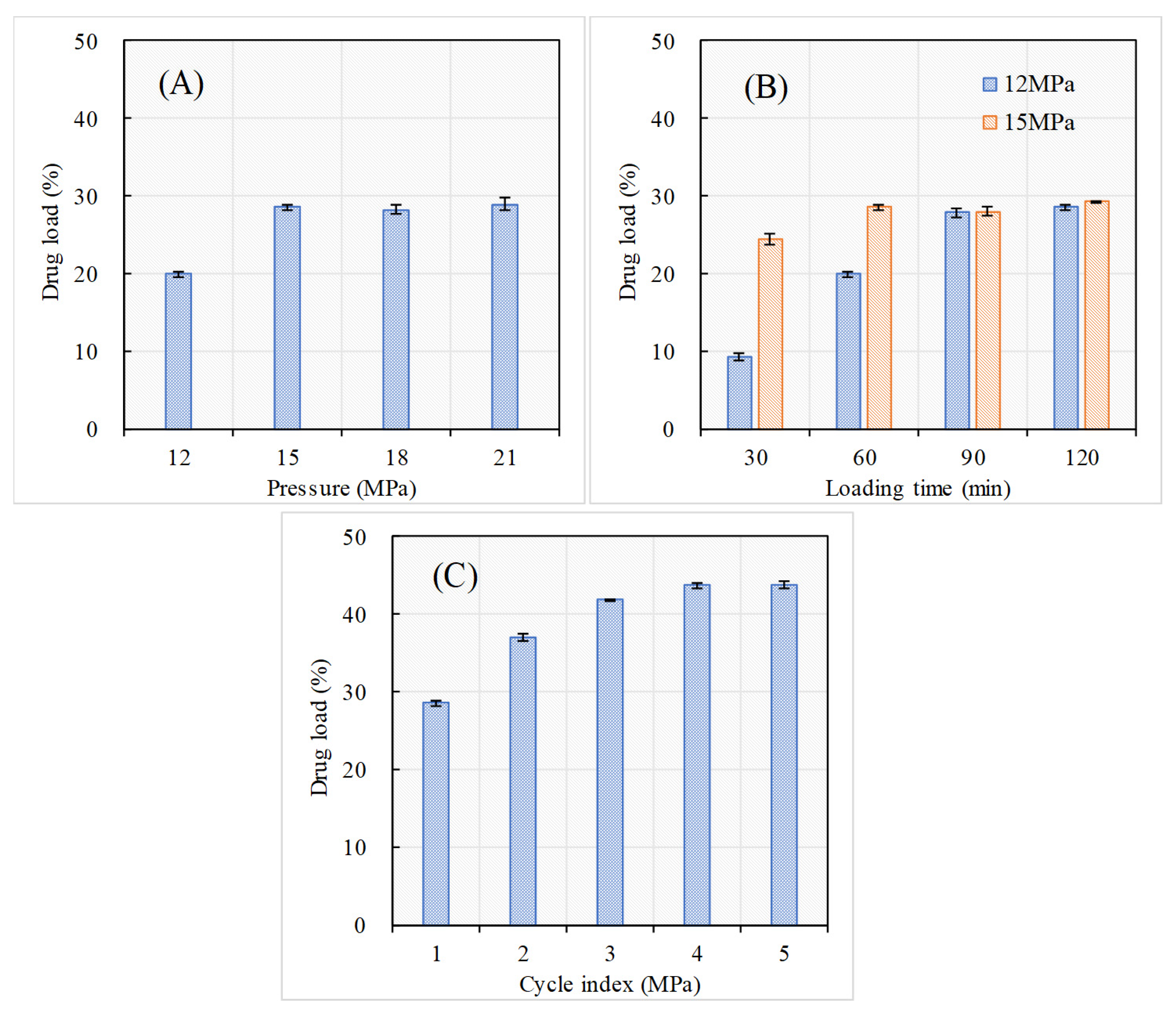
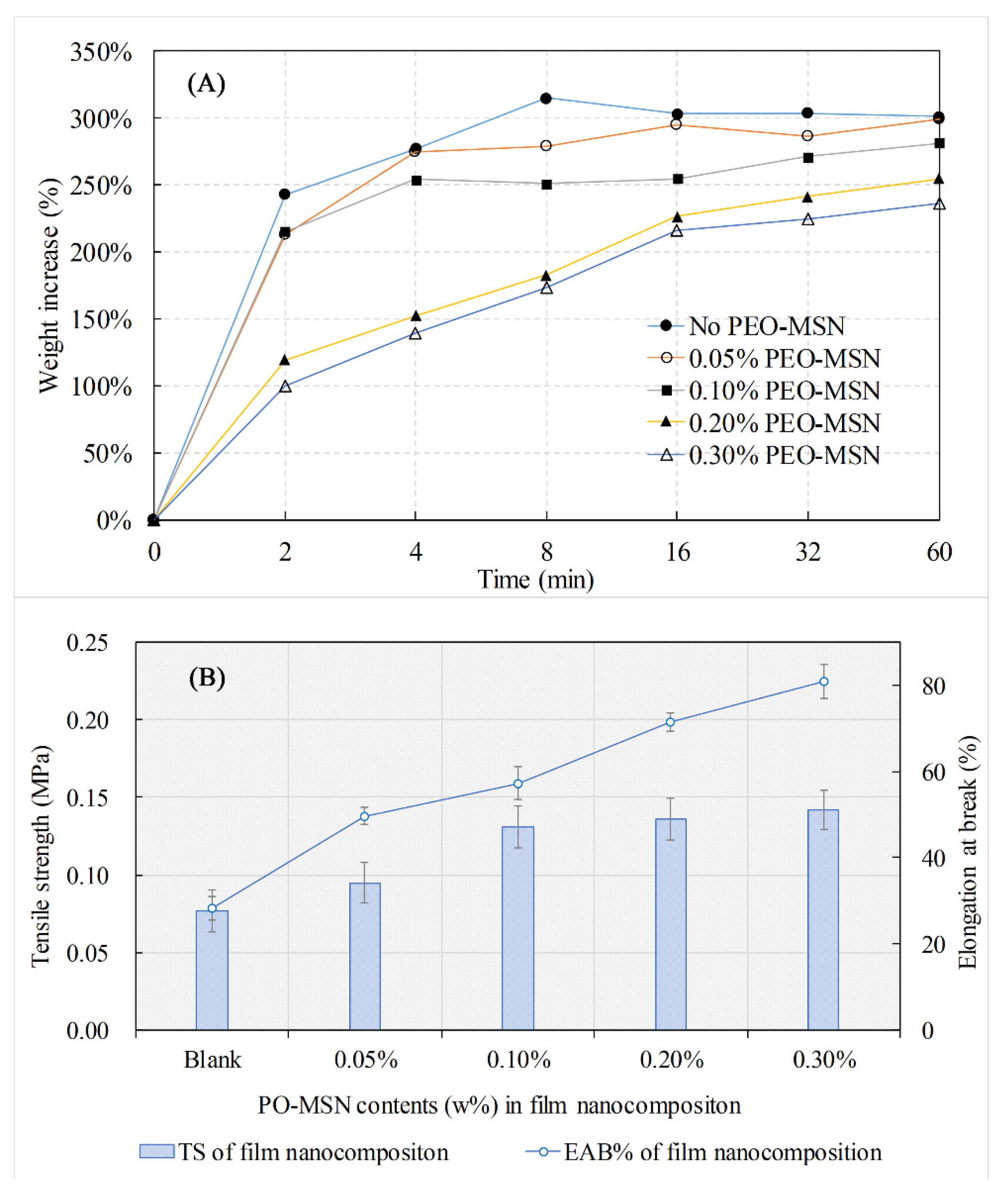

| Name | MSN | PEO-MSN |
|---|---|---|
| BET surface area | 610.67 m2/g | 259.18 m2/g |
| BJH adsorption surface area | 868.68 m2/g | 396.72 m2/g |
| BJH desorption surface area | 873.01 m2/g | 448.66 m2/g |
| BJH adsorption pore volume | 1.58 cm3/g | 0.45 cm3/g |
| BJH desorption pore volume | 1.61 cm3/g | 0.80 cm3/g |
| BJH adsorption pore width | 7.37 nm | 4.47 nm |
| BJH desorption pore width | 7.29 nm | 7.04 nm |
| Concentration (μg/mL) | 1.6 | 3.1 | 6.3 | 12.5 | 25.0 | 50.0 |
|---|---|---|---|---|---|---|
| Inhibition rate | 0.4% | −2.4% | 2.6% | 24.7% | 49.6% | 81.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, J.; Duan, S.; Zhou, X.; Sun, L.; Qin, C.; Li, M.; Ge, F. Long-Term Antibacterial Film Nanocomposite Incorporated with Patchouli Essential Oil Prepared by Supercritical CO2 Cyclic Impregnation for Wound Dressing. Molecules 2021, 26, 5005. https://doi.org/10.3390/molecules26165005
Jia J, Duan S, Zhou X, Sun L, Qin C, Li M, Ge F. Long-Term Antibacterial Film Nanocomposite Incorporated with Patchouli Essential Oil Prepared by Supercritical CO2 Cyclic Impregnation for Wound Dressing. Molecules. 2021; 26(16):5005. https://doi.org/10.3390/molecules26165005
Chicago/Turabian StyleJia, Jingfu, Shulei Duan, Xue Zhou, Lifang Sun, Chengyuan Qin, Ming Li, and Fahuan Ge. 2021. "Long-Term Antibacterial Film Nanocomposite Incorporated with Patchouli Essential Oil Prepared by Supercritical CO2 Cyclic Impregnation for Wound Dressing" Molecules 26, no. 16: 5005. https://doi.org/10.3390/molecules26165005





