Postharvest Drying Techniques Regulate Secondary Metabolites and Anti-Neuroinflammatory Activities of Ganoderma lucidum
Abstract
:1. Introduction
2. Results
2.1. Primary Metabolites as Affected by Different Drying Methods
2.2. Proteomic Analysis
2.3. Cytotoxicity Assay
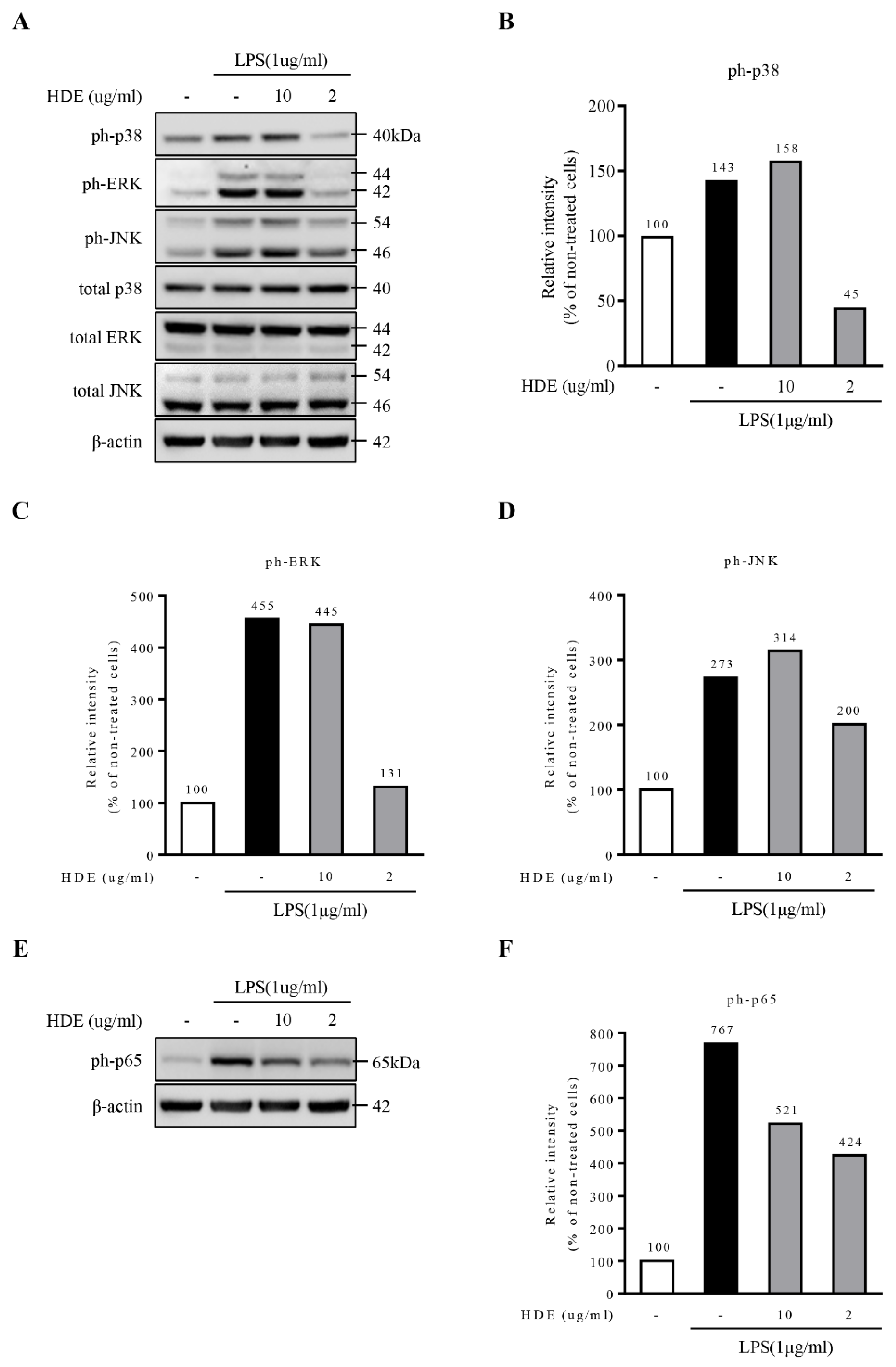
2.4. Heat-Dried GL Extract Inhibits the Activation of NF-κB Signaling in LPS-Treated BV2 Cells
3. Discussion
4. Materials and Methods
4.1. General Procedure
4.2. Gas Chromatography Coupled with Time-of-Flight Mass Spectrometric (GC-TOF-MS) Analysis
4.3. Protein Extraction
4.4. Peptide Analysis
4.5. Cell Viability Assays
4.6. LPS Treatment to BV2 Cells for Inflammation Induction
4.7. Western-Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Desa, M.; Nurlaila, W.; Mohammad, M.; Fudholi, A. Review of drying technology of Figure. Trends Food Sci. Technol. 2019, 88, 93–103. [Google Scholar] [CrossRef]
- Adali, M.B.; Barresi, A.A.; Boccardo, G.; Pisano, R. Spray Freeze-Drying as a Solution to Continuous Manufacturing of Pharmaceutical Products in Bulk. Processes 2020, 8, 709. [Google Scholar] [CrossRef]
- Prosapio, V.; Norton, I. Influence of osmotic dehydration pre-treatment on oven drying and freeze drying performance. LWT 2017, 80, 401–408. [Google Scholar] [CrossRef]
- Marques, L.G.; Prado, M.M.; Freire, J.T. Rehydration characteristics of freeze-dried tropical fruits. LWT 2009, 42, 1232–1237. [Google Scholar] [CrossRef]
- Barimah, J.; Yanney, P.; Laryea, D.; Quarcoo, C. Effect of Drying Methods on Phytochemicals, Antioxidant Activity and Total Phenolic Content of Dandelion Leaves. Am. J. Food Nutr. 2017, 5, 136–141. [Google Scholar]
- Holmer, R.; Linwattana, G.; Nath, P.; Keatinge, J. High Value Vegetables in Southeast Asia: Production, Supply and Demand; Proceedings-SEAVEG 2012. In Proceedings of the Regional Symposium on High Value Vegetables in Southeast Asia: Production, Supply and Demand (SEAVEG2012), Chiang Mai, Thailand, 24–26 January 2012. [Google Scholar]
- Bhatta, S.; Janezic, T.S.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Martysiak-Żurowska, D.; Rożek, P.; Puta, M. The effect of freeze-drying and storage on lysozyme activity, lactoferrin content, superoxide dismutase activity, total antioxidant capacity and fatty acid profile of freeze-dried human milk. Dry. Technol. 2020, 1–11. [Google Scholar] [CrossRef]
- Tetteh, O.N.A.; Ulrichs, C.; Huyskens-Keil, S.; Mewis, I.; Amaglo, N.K.; Oduro, I.N.; Adarkwah, C.; Obeng-Ofori, D.; Förster, N. Effects of harvest techniques and drying methods on the stability of glucosinolates in Moringa oleifera leaves during post-harvest. Sci. Hortic. 2019, 246, 998–1004. [Google Scholar] [CrossRef]
- Salvatierra-Rojas, A.; Nagle, M.; Gummert, M.; de Bruin, T.; Müller, J. Development of an inflatable solar dryer for improved postharvest handling of paddy rice in humid climates. Int. J. Agric. Biol. Eng. 2017, 10, 269–282. [Google Scholar]
- Stathers, T.; Holcroft, D.; Kitinoja, L.; Mvumi, B.M.; English, A.; Omotilewa, O.; Kocher, M.; Ault, J.; Torero, M. A scoping review of interventions for crop postharvest loss reduction in sub-Saharan Africa and South Asia. Nat. Sustain. 2020, 3, 821–835. [Google Scholar] [CrossRef]
- Chauhan, R.S.; Nautiyal, M.C.; Figueredo, G.; Rana, V.S. Effect of Post Harvest Drying Methods on the Essential Oil Composition of Nardostachys jatamansi DC. J. Essent. Oil Bear. Plants 2017, 20, 1090–1096. [Google Scholar] [CrossRef]
- Kwon, O.C.; Park, Y.J.; Kim, H.I.; Kong, W.S.; Cho, J.H.; Lee, C.S. Taxonomic position and species identity of the cultivated Yeongji ‘Ganoderma lucidum’in Korea. Mycobiology 2016, 44, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Ann. Rev. 2007, 13, 265–301. [Google Scholar] [CrossRef]
- Sanodiya, B.S.; Thakur, G.S.; Baghel, R.K.; Prasad, G.B.K.S.; Bisen, P.S. Ganoderma lucidum: A Potent Pharmacological Macrofungus. Curr. Pharm. Biotechnol. 2009, 10, 717–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 913. [Google Scholar] [CrossRef] [Green Version]
- Bishop, K.S.; Kao, C.H.; Xu, Y.; Glucina, M.P.; Paterson, R.; Ferguson, L.R. From 2000years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry 2015, 114, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.-B.; Lin, S.-Q.; Liu, J.-H.; Lin, Z.-B. Polysaccharide Extract Isolated from Ganoderma lucidum Protects Rat Cerebral Cortical Neurons from Hypoxia/Reoxygenation Injury. J. Pharmacol. Sci. 2004, 95, 294–298. [Google Scholar] [CrossRef] [Green Version]
- Cheung, W.M.; Hui, W.S.; Chu, P.W.; Chiu, S.W.; Ip, N.Y. Ganoderma extract activates MAP kinases and induces the neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000, 486, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Tang, Q.; Zhou, C.; Jia, W.; Da Silva, L.; Nguyen, L.D.; Reutter, W.; Fan, H. GLIS, a bioactive proteoglycan fraction from Ganoderma lucidum, displays anti-tumour activity by increasing both humoral and cellular immune response. Life Sci. 2010, 87, 628–637. [Google Scholar] [CrossRef]
- Zhang, K.; Pu, Y.-Y.; Sun, D.-W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Kim, S.U.; de Vellis, J. Microglia in health and disease. J. Neurosci. Res. 2005, 81, 302–313. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Perry, V.H. Microglial Physiology: Unique Stimuli, Specialized Responses. Annu. Rev. Immunol. 2009, 27, 119–145. [Google Scholar] [CrossRef]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Soliman, K.F. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells. J. Neuroimmunol. 2018, 320, 87–97. [Google Scholar] [CrossRef]
- Sato, S.; St-Pierre, C.; Bhaumik, P.; Nieminen, J. Galectins in innate immunity: Dual functions of host soluble β-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol. Rev. 2009, 230, 172–187. [Google Scholar] [CrossRef]
- Cloutier, A.; Ear, T.; Blais-Charron, E.; Dubois, C.M.; McDonald, P.P. Differential involvement of NF-κB and MAP kinase pathways in the generation of inflammatory cytokines by human neutrophils. J. Leukoc. Biol. 2006, 81, 567–577. [Google Scholar] [CrossRef] [Green Version]
- Morrison, D.K. MAP Kinase Pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Perry, V.H.; Teeling, J. Microglia and macrophages of the central nervous system: The contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin. Immunopathol. 2013, 35, 601–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- So, T.; Nagashima, H.; Ishii, N. TNF Receptor-Associated Factor (TRAF) Signaling Network in CD4+ T-Lymphocytes. Tohoku J. Exp. Med. 2015, 236, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.Y.; Eung-Ryoung Lee, J.-Y.K.; Cho, S.-G. Protein Phosphorylation as a Regulatory Mechanism of Various Cellular Function. Cancer Prev. Res. 2006, 11, 1–8. [Google Scholar]
- Harry, G.J.; Kraft, A.D. Neuroinflammation and microglia: Considerations and approaches for neurotoxicity assessment. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1265–1277. [Google Scholar] [CrossRef]
- Ryu, D.H.; Cho, J.Y.; Bin Sadiq, N.; Kim, J.-C.; Lee, B.; Hamayun, M.; Lee, T.S.; Kim, H.S.; Park, S.H.; Nho, C.W.; et al. Optimization of antioxidant, anti-diabetic, and anti-inflammatory activities and ganoderic acid content of differentially dried Ganoderma lucidum using response surface methodology. Food Chem. 2021, 335, 127645. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-M.; Jang, K.-J.; Han, M.S.; Jeong, J.-W.; Kim, G.Y.; Lee, J.-H.; Choi, Y.H. Ganoderma lucidum ethanol extract inhibits the inflammatory response by suppressing the NF-κB and toll-like receptor pathways in lipopolysaccharide-stimulated BV2 microglial cells. Exp. Ther. Med. 2013, 5, 957–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilliard, A.; Mendonca, P.; Soliman, K.F. Involvement of NFκB and MAPK signaling pathways in the preventive effects of Ganoderma lucidum on the inflammation of BV-2 microglial cells induced by LPS. J. Neuroimmunol. 2020, 345, 577269. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Li, Y.; Pei, G. Polysaccharides from Ganoderma lucidum attenuate microglia-mediated neuroinflammation and modulate microglial phagocytosis and behavioural response. J. Neuroinflammation 2017, 14, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satria, D.; Tamrakar, S.; Suhara, H.; Kaneko, S.; Shimizu, K. Mass Spectrometry-Based Untargeted Metabolomics and α-Glucosidase Inhibitory Activity of Lingzhi (Ganoderma lingzhi) During the Developmental Stages. Molecules 2019, 24, 2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, C.; Bi, J.; Li, X.; Lyu, J.; Zhou, M.; Wu, X. Antioxidant profile of thinned young and ripe fruits of Chinese peach and nectarine varieties. Int. J. Food Prop. 2020, 23, 1272–1286. [Google Scholar] [CrossRef]
- Famiani, F.; Bonghi, C.; Chen, Z.-H.; Drincovich, M.F.; Farinelli, D.; Lara, M.V.; Proietti, S.; Rosati, A.; Vizzotto, G.; Walker, R.P. Stone Fruits: Growth and Nitrogen and Organic Acid Metabolism in the Fruits and Seeds—A Review. Front. Plant Sci. 2020, 11, 1427. [Google Scholar] [CrossRef]
- Park, J.; Min, J.-S.; Kim, B.; Chae, U.-B.; Yun, J.W.; Choi, M.-S.; Kong, I.-K.; Chang, K.-T.; Lee, D.-S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef]
- Xing, B.; Xin, T.; Hunter, R.; Bing, G. Pioglitazone inhibition of lipopolysaccharide-induced nitric oxide synthase is associated with altered activity of p38 MAP kinase and PI3K/Akt. J. Neuroinflammation 2008, 5, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luyben, K.C.A.M.; Liou, J.K.; Bruin, S. Enzyme degradation during drying. Biotechnol. Bioeng. 1982, 24, 533–552. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Krokida, M.K.; Maroulis, Z.B.; Saravacos, G.D. The effect of the method of drying on the colour of dehydrated products. Int. J. Food Sci. Technol. 2001, 36, 53–59. [Google Scholar] [CrossRef]
- Knorr, D. Impact of non-thermal processing on plant metabolites. J. Food Eng. 2003, 56, 131–134. [Google Scholar] [CrossRef]
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Dek, M.S.P.; Hairuddin, M.R. Effect of Freeze-Drying on the Antioxidant Compounds and Antioxidant Activity of Selected Tropical Fruits. Int. J. Mol. Sci. 2011, 12, 4678–4692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Han, Y.-K.; Yun, S.-H.; Lee, Y.-W. Roles of the Glyoxylate and Methylcitrate Cycles in Sexual Development and Virulence in the Cereal Pathogen Gibberella zeae. Eukaryot. Cell 2009, 8, 1155–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenz, M.C.; Fink, G.R. Life and death in a macrophage: Role of the glyoxylate cycle in virulence. Eukaryot. Cell 2002, 1, 657–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quin, M.; Flynn, C.M.; Schmidt-Dannert, C. Traversing the fungal terpenome. Nat. Prod. Rep. 2014, 31, 1449–1473. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.J.; Hattori, T.; Shimada, M. A metabolic role of the glyoxylate and tricarboxylic acid cycles for development of the copper-tolerant brown-rot fungus Fomitopsis palustris. FEMS Microbiol. Lett. 2002, 217, 9–14. [Google Scholar] [CrossRef]
- Garrett, R.H.; Grisham, C.M. Principles of Biochemistry: With a Human Focus; Brooks/Cole Publishing Company: Pacific Grove, CA, USA, 2001. [Google Scholar]
- Minárik, P.; Tomásková, N.; Kollárová, M.; Antalík, M. Malate dehydrogenases--structure and function. Gen. Physiol. Biophys. 2002, 21, 257–266. [Google Scholar]
- Akihisa, T.; Nakamura, Y.; Tagata, M.; Tokuda, H.; Yasukawa, K.; Uchiyama, E.; Suzuki, T.; Kimura, Y. Anti-Inflammatory and Anti-Tumor-Promoting Effects of Triterpene Acids and Sterols from the FungusGanoderma lucidum. Chem. Biodivers. 2007, 4, 224–231. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, J.; Liu, J.; Huang, Y.; Zhong, J.-J.; Tang, W. Enhancement of IL-2 and IFN-γ expression and NK cells activity involved in the anti-tumor effect of ganoderic acid Me in vivo. Int. Immunopharmacol. 2007, 7, 864–870. [Google Scholar] [CrossRef]
- Ma, H.-T.; Hsieh, J.-F.; Chen, S.-T. Anti-diabetic effects of Ganoderma lucidum. Phytochemistry 2015, 114, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L. Ganoderic acid produced from submerged culture of Ganoderma lucidum induces cell cycle arrest and cytotoxicity in human hepatoma cell line BEL7402. Biotechnol. Lett. 2005, 27, 835–838. [Google Scholar] [CrossRef]
- Chen, N.H.; Liu, J.W.; Zhong, J.J. Ganoderic acid Me inhibits tumor invasion through down-regulating matrix metalloproteinases 2/9 gene expression. J. Pharmacol. Sci. 2008, 108, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-H.; Zhong, J.-J. p53 is important for the anti-invasion of ganoderic acid T in human carcinoma cells. Phytomedicine 2011, 18, 719–725. [Google Scholar] [CrossRef]
- Liu, J.; Shimizu, K.; Konishi, F.; Noda, K.; Kumamoto, S.; Kurashiki, K.; Kondo, R. Anti-androgenic activities of the triterpenoids fraction of Ganoderma lucidum. Food Chem. 2007, 100, 1691–1696. [Google Scholar] [CrossRef]
- Chang, T.-S.; Chiang, C.-M.; Kao, Y.-H.; Wu, J.-Y.; Wu, Y.-W.; Wang, T.-Y. A New Triterpenoid Glucoside from a Novel Acidic Glycosylation of Ganoderic Acid A via Recombinant Glycosyltransferase of Bacillus subtilis. Molecules 2019, 24, 3457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.; Modi, H. Statistical optimization of culture conditions for enhanced mycelial biomass production using Ganoderma lucidum. J. Appl. Biol. Biotechnol. 2018, 6, 41–45. [Google Scholar]
- Salmon, M.; Thimmappa, R.B.; Minto, R.; Melton, R.; Hughes, R.K.; O’Maille, P.E.; Hemmings, A.; Osbourn, A. A conserved amino acid residue critical for product and substrate specificity in plant triterpene synthases. Proc. Natl. Acad. Sci. USA 2016, 113, E4407–E4414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.J.; Choi, J.; Kim, K.W.; Kil Ahn, S.; Ha, S.-H.; Choi, Y.; Park, N.I.; Kim, J.K. Metabolite Profiling of Peppers of Various Colors Reveals Relationships Between Tocopherol, Carotenoid, and Phytosterol Content. J. Food Sci. 2017, 82, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Song, D.-G.; Kim, Y.S.; Jung, B.C.; Rhee, K.-J.; Pan, C.-H. Parkin Induces Upregulation of 40S Ribosomal Protein SA and Posttranslational Modification of Cytokeratins 8 and 18 in Human Cervical Cancer Cells. Appl. Biochem. Biotechnol. 2013, 171, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Haange, S.-B.; Jehmlich, N.; Hoffmann, M.; Weber, K.; Lehmann, J.; Von Bergen, M.; Slanina, U. Disease Development Is Accompanied by Changes in Bacterial Protein Abundance and Functions in a Refined Model of Dextran Sulfate Sodium (DSS)-Induced Colitis. J. Proteome Res. 2019, 18, 1774–1786. [Google Scholar] [CrossRef] [PubMed]
- Mairuae, N.; Cheepsunthorn, P.; Buranrat, B. Anti-inflammatory and anti-oxidative effects of Centella asiatica extract in lipopolysaccharide-stimulated BV2 microglial cells. Pharmacogn. Mag. 2019, 15, 140. [Google Scholar] [CrossRef]




| No | RT (s) | Identified Compound | Formula | S/N | Temp (°C) | Peak Area | p-Value |
|---|---|---|---|---|---|---|---|
| Organic acid | |||||||
| 1 | 336.475 | L-(+)-Lactic acid | C3H6O3 | 259.64 ± 46.50 | −80 | 623,113 ± 220,546 | 0.005 |
| 60 | 1,353,365 ± 61,640 | ||||||
| 2 | 388.033 | 3-Furoic acid | C5H4O3 | 120.63 ± 49.69 | −80 | 268,829 ± 14,818 | 0.000 |
| 60 | 99,835 ± 4186 | ||||||
| 3 | 495.075 | Succinic acid | C4H6O4 | 843.21 ± 31.93 | −80 | 6,780,625 ± 1,330,300 | 0.000 |
| 60 | ND | ||||||
| 4 | 590.617 | Malic acid | C4H6O5 | 5741.99 ± 23.93 | −80 | 3,368,389 ± 350,517 | 0.001 |
| 60 | 4,294,037 ± 67,108 | ||||||
| 5 | 731.117 | Propanoic acid | C3H6O4 | 52.45 ± 2.66 | −80 | ND | 0.000 |
| 60 | 19,674± 1922 | ||||||
| 6 | 745.183 | Citric acid | C6H8O7 | 10,707.06 ± 39.46 | −80 | 7,468,684 ± 1,296,440 | 0.009 |
| 60 | 3,794,228 ±237,974 | ||||||
| 7 | 824.033 | D-Gluconic acid | C6H12O7 | 1322.40 ± 100.12 | −80 | 54,672 ± 16,882 | 0.000 |
| 60 | 1,060,360 ± 119,163 | ||||||
| Amino acid/Amine | |||||||
| 8 | 355.775 | L-Valine | C5H11NO2 | 300.84 ± 94.39 | −80 | 292,186 ± 64,672 | 0.002 |
| 60 | 2,214,288 ± 462,985 | ||||||
| 9 | 361.750 | N-Butylamine | C4H11N | 500.24 ± 85.16 | −80 | 36,110 ± 538 | 0.002 |
| 60 | 798,463 ± 103,414 | ||||||
| 10 | 365.325 | L-Alanine | C3H7NO2 | 2235.52 ± 70.06 | −80 | 2,218,555 ± 620,914 | 0.000 |
| 60 | 9,117,948 ± 656,401 | ||||||
| 11 | 439.383 | D-Valine | C5H11NO2 | 5420.60 ± 103.17 | −80 | 475,411 ± 136,233 | 0.000 |
| 60 | 12,989,286 ± 224,714 | ||||||
| 12 | 470.525 | Ethanolamine | C2H7NO | 568.73 ± 56.24 | −80 | 281,663 ± 94,049 | 0.000 |
| 60 | 822,952 ± 83,137 | ||||||
| 13 | 472.700 | L-Norleucine | C6H13NO2 | 2223.83 ± 104.51 | −80 | 167,868 ± 61,864 | 0.002 |
| 60 | 6,097,243 ± 864,121 | ||||||
| 14 | 486.208 | L-Leucine | C6H13NO2 | 2240.74 ± 102.79 | −80 | 225,995 ± 70,362 | 0.001 |
| 60 | 6,090,595 ± 690,905 | ||||||
| 15 | 490.150 | L-Proline | C5H9NO2 | 1541.72 ± 107.91 | −80 | 66,414 ± 33,100 | 0.000 |
| 60 | 4,183,781 ± 920,171 | ||||||
| 16 | 523.325 | L-Serine | C3H7NO3 | 1096.97 ± 90.24 | −80 | 346,714 ± 128,627 | 0.001 |
| 60 | 3,385,954 ± 645,120 | ||||||
| 17 | 538.683 | L-Threonine | C4H9NO3 | 821.96 ± 9134 | −80 | 163,522 ± 45,729 | 0.001 |
| 60 | 1,784,039 ± 211,063 | ||||||
| 18 | 607.075 | L-Aspartic acid | C4H7NO4 | 2225.12 ± 62.37 | −80 | 622,475 ± 115,745 | 0.002 |
| 60 | 2,060,365 ± 6360 | ||||||
| 19 | 653.967 | L-Glutamic acid | C5H9NO4 | 1138.54 ± 49.91 | −80 | 356,869 ± 131,194 | 0.005 |
| 60 | 855,723 ± 81,835 | ||||||
| 20 | 662.317 | Phenylalanine | C9H11NO2 | 467.51 ± 86.40 | −80 | 144,403 ± 35,274 | 0.000 |
| 60 | 1,228,814 ± 130,812 | ||||||
| 21 | 679.717 | L-Asparagine | C4H8N2O3 | 173.74 ± 81.35 | −80 | 112,420 ± 56,004 | 0.000 |
| 60 | 727,603 ± 65,606 | ||||||
| 22 | 785.425 | L-Lysine | C6H14N2O2 | 726.90 ± 84.47 | −80 | 18,913 ± 41,286 | 0.000 |
| 60 | 1,631,107 ± 166,827 | ||||||
| Monosaccharides | |||||||
| 23 | 709.058 | L-Rhamnose | C6H12O5 | 452.46 ± 101.89 | −80 | 164,194 ± 29,950 | 0.000 |
| 60 | 3,186,455 ± 211,877 | ||||||
| 24 | 751.675 | D-(-)-Erythorose | C4H8O4 | 261.62 ± 69.45 | −80 | 7,495,006 ± 2,173,719 | 0.002 |
| 60 | 2,337,360 ± 444,077 | ||||||
| 25 | 770.233 | D-(-)-Fructose | C6H12O6 | 196.91 ± 28.92 | −80 | 137,329 ± 25,989 | 0.003 |
| 60 | 1,301,930 ± 50,758 | ||||||
| 26 | 781.567 | D-(+)-Mannose | C6H12O6 | 12,225.38 ± 84.45 | −80 | 1,339,283 ± 309,865 | 0.000 |
| 60 | 9,066,007 ± 634,819 | ||||||
| 27 | 794.742 | D-Mannitol | C6H14O6 | 18,746.15 ± 103.89 | −80 | 502,455 ± 193,329 | 0.000 |
| 60 | 16,399,566 ± 2,209,653 | ||||||
| 28 | 857.492 | Inositol | C6H12O6 | 5254.67 ± 30.80 | −80 | 2,211,218 ± 527,434 | 0.026 |
| 60 | 3,361,783 ± 225,679 | ||||||
| 29 | 887.150 | D-Sorbitol | C6H14O6 | 71.30 ± 7.61 | −80 | ND | 0.000 |
| 60 | 43,967 ± 4705 | ||||||
| 30 | 938.000 | D-(-)-Ribofuranose | C5H10O5 | 54.96 ± 9.83 | −80 | ND | 0.000 |
| 60 | 134,705 ± 13,507 | ||||||
| 31 | 1070.190 | D-(+)-Trehalose | C12H22O11 | 42,467.71 ± 32.92 | −80 | 19,711,971 ± 726,018 | 0.000 |
| 60 | 11,425,742 ± 1,430,771 | ||||||
| Alcohols and its derivatives | |||||||
| 32 | 748.625 | 3-Deoxyhexitol | C6H14O5 | 48.303 ± 40.76 | −80 | 16,294 ± 6668 | 0.024 |
| 60 | 44,286 ± 894 | ||||||
| 33 | 794.742 | D Mannitol | C6H14O6 | 18,746.15 ± 103.88 | −80 | 502,454 ± 17,291 | 0.007 |
| 60 | 16,399,565 ± 19,763 | ||||||
| 34 | 454.675 | Diethylene glycol | C4H10O3 | 48.874 ± 61.899 | −80 | 408,235 ± 5816 | 0.05 |
| 60 | 116,301 ± 6540 | ||||||
| 35 | 887.150 | D-Sorbitol | C6H14O6 | 71.296 ± 7.608 | −80 | ND | |
| 60 | 43,967 ± 420 | ||||||
| 36 | 224.233 | Ethylene glycol | C2H6O2 | 2745.569 ± 8.64 | −80 | 19,748,319 ± 53,976 | 0.02 |
| 60 | 17,779,139 ± 9579 | ||||||
| 37 | 472.950 | Glycerol | C3H8O3 | 1073.492 ± 80.425 | −80 | 426,256 ± 10,359 | 0.001 |
| 60 | 2,784,540 ± 4976 | ||||||
| 38 | 475.608 | Silanol | SiH4O | 28,812.306 ± 56.396 | −80 | 8,028,737 ± 31,479 | 0.005 |
| 60 | 27,759,383 ± 2333 | ||||||
| 39 | 597.350 | L(-) Arabitol | C5H12O5 | 16.866 ± 26.507 | −80 | 29,802 ± 1857 | 0.03 |
| 60 | 48,490 ± 446 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadiq, N.-b.; Ryu, D.-H.; Cho, J.-Y.; Lee, A.-H.; Song, D.-G.; Dorjsembe, B.; Kim, J.-C.; Jung, J.-H.; Nho, C.-W.; Hamayun, M.; et al. Postharvest Drying Techniques Regulate Secondary Metabolites and Anti-Neuroinflammatory Activities of Ganoderma lucidum. Molecules 2021, 26, 4484. https://doi.org/10.3390/molecules26154484
Sadiq N-b, Ryu D-H, Cho J-Y, Lee A-H, Song D-G, Dorjsembe B, Kim J-C, Jung J-H, Nho C-W, Hamayun M, et al. Postharvest Drying Techniques Regulate Secondary Metabolites and Anti-Neuroinflammatory Activities of Ganoderma lucidum. Molecules. 2021; 26(15):4484. https://doi.org/10.3390/molecules26154484
Chicago/Turabian StyleSadiq, Nooruddin-bin, Da-Hye Ryu, Jwa-Yeong Cho, A-Hyeon Lee, Dae-Geun Song, Banzragch Dorjsembe, Jin-Chul Kim, Je-Hyeong Jung, Chu-Won Nho, Muhammad Hamayun, and et al. 2021. "Postharvest Drying Techniques Regulate Secondary Metabolites and Anti-Neuroinflammatory Activities of Ganoderma lucidum" Molecules 26, no. 15: 4484. https://doi.org/10.3390/molecules26154484






