Fe3O4/Graphene Composite Anode Material for Fast-Charging Li-Ion Batteries
Abstract
:1. Introduction
2. Results
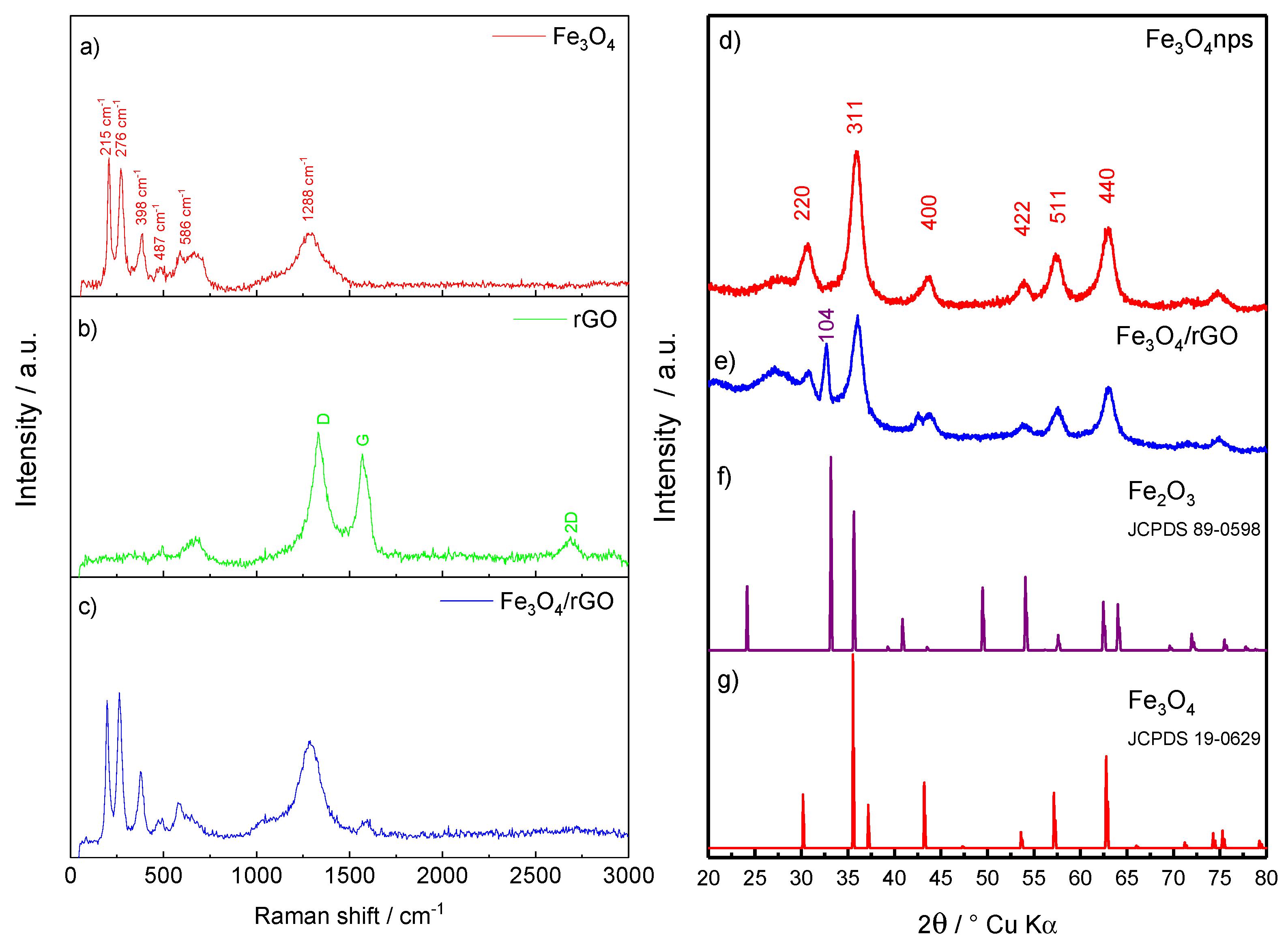
2.1. Structural and Morphological Characterization
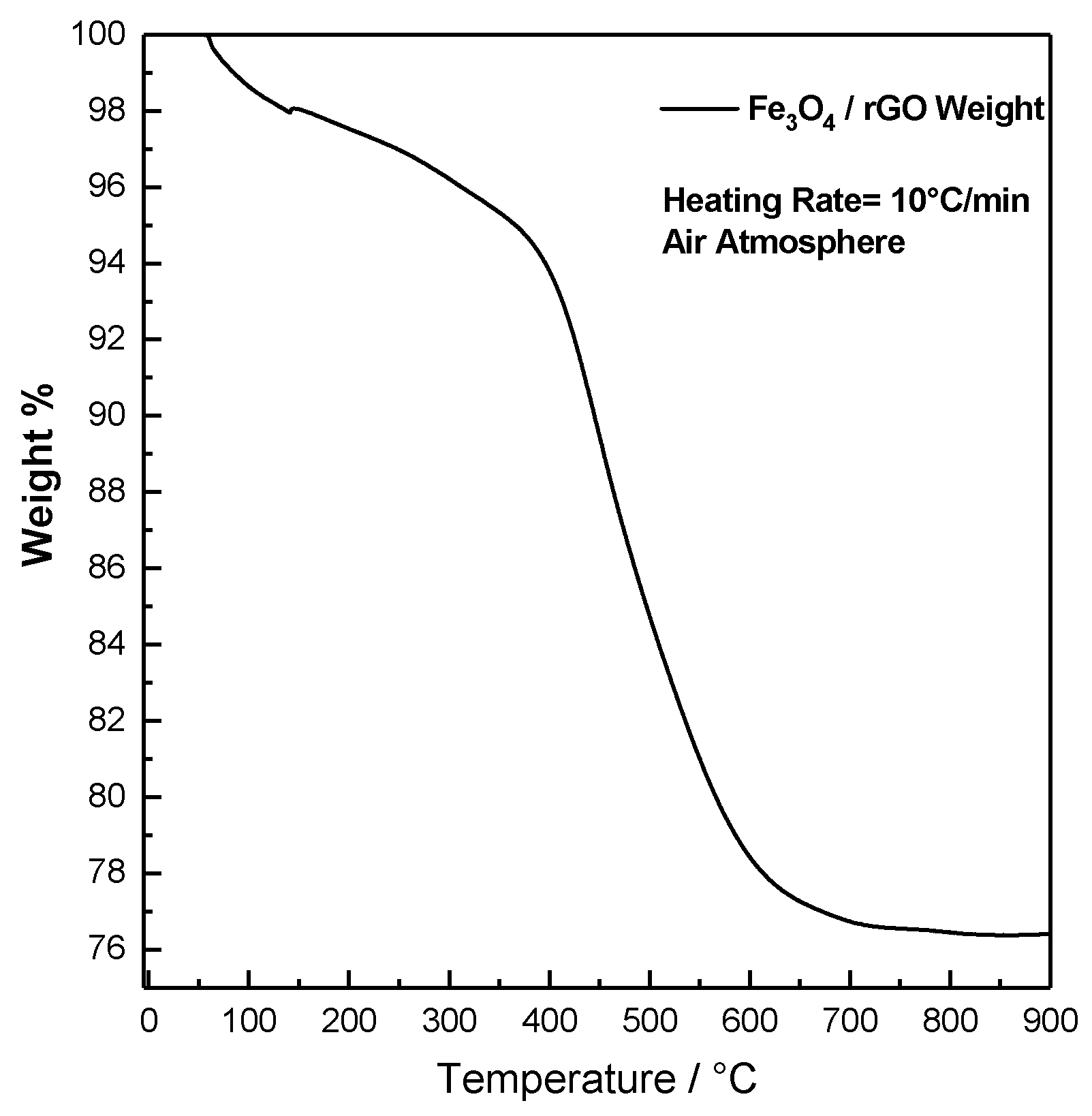
2.2. Thermal Characterization
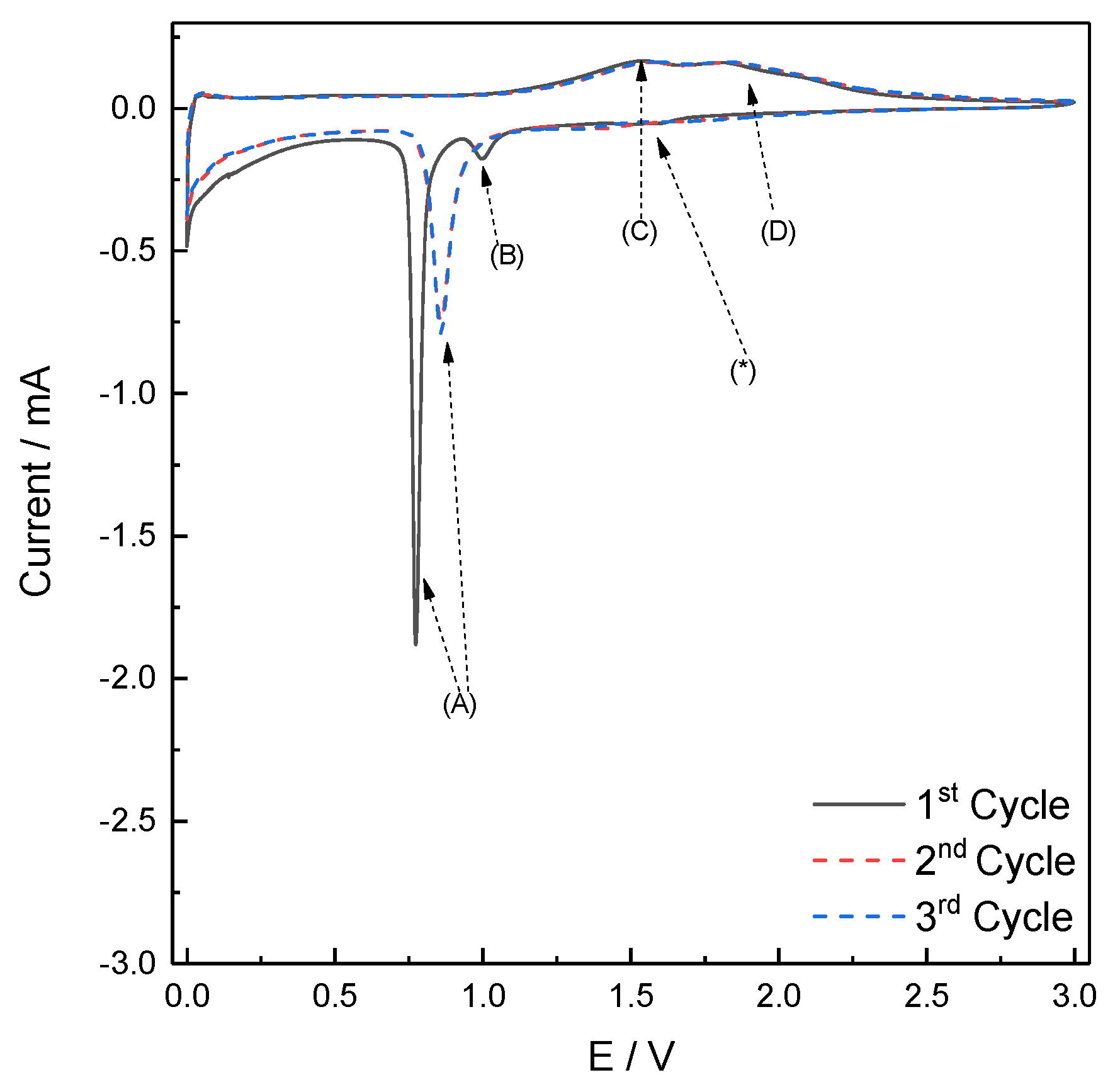
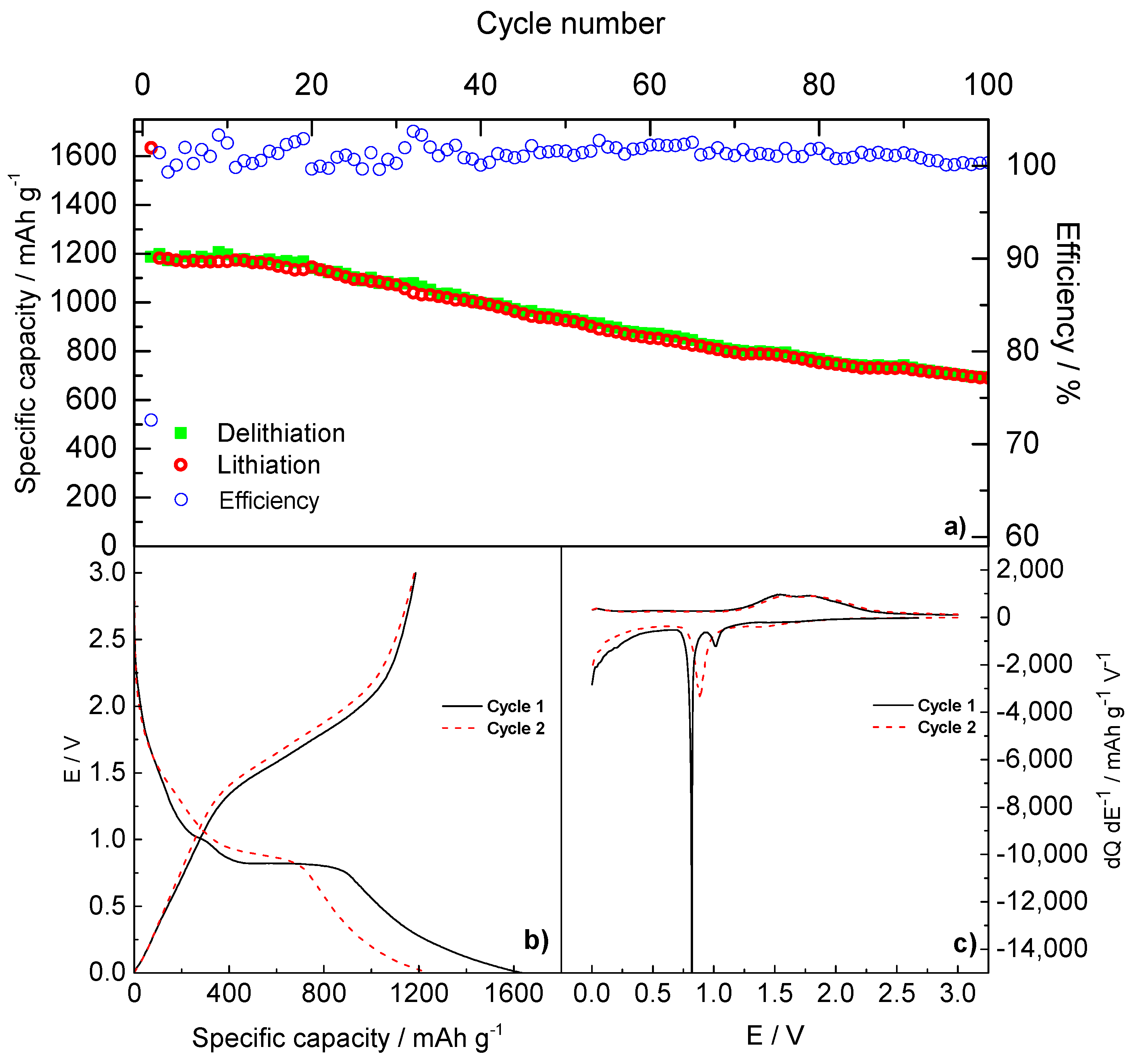
2.3. Electrochemical Characterization
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Electrode Processing
3.4. Structural and Electrochemical Characterizaiton
3.5. Cell Assembly and Electrochemical Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steele, B.C.H. Fast Ion Transport in Solids: Solid-State Batteries and Devices; North-Holland/American Elsevier: Amsterdam, The Netherlands; London, UK; New York, NY, USA, 1973; ISBN 978-072-040-223-0. [Google Scholar]
- Kristin Persson, L.D.; Sethuraman, V.A.; Hardwick, L.J.; Hinuma, Y.; Meng, Y.S.; Ven, A.; Srinivasan, V.; Kostecki, R.; Ceder, G. Lithium Diffusion in Graphitic Carbon. J. Phys. Chem. 2010, 1, 1176–1180. [Google Scholar] [CrossRef] [Green Version]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond intercalation-based Li-ion batteries: The state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. 2010, 22, 170–192. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; He, K.; Stach, E.A. Comparison of Co3O4 and CoO Nanoparticles as Anodes for Lithium-ion Batteries. Microsc. Microanal. 2015, 21, 477–478. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Chung, M.K.; Ka, B.H.; Ku, J.H.; Park, S.; Ryu, J.; Oh, S.M. The Role of Metallic Fe and Carbon Matrix in Fe2O3/Fe/Carbon Nanocomposite for Lithium-Ion Batteries. J. Electrochem. Soc. 2010, 157, 412–417. [Google Scholar] [CrossRef]
- Maroni, F.; Gabrielli, S.; Palmieri, A.; Marcantoni, E.; Croce, F.; Nobili, F. High cycling stability of anodes for lithium-ion batteries based on Fe3O4 nanoparticles and poly(acrylic acid) binder. J. Power Sources 2016, 332, 79–87. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Hu, A.; Chen, X.; Tang, Y.; Tang, Q.; Yang, L.; Zhang, S. Self-assembly of Fe3O4 nanorods on graphene for lithium ion batteries with high rate capacity and cycle stability. Electrochem. Commun. 2013, 28, 139–142. [Google Scholar] [CrossRef]
- Chen, Y.; Xia, H.; Lu, L.; Xue, J. Synthesis of porous hollow Fe3O4 beads and their applications in lithium ion batteries. J. Mater. Chem. 2012, 22, 5006–5012. [Google Scholar] [CrossRef]
- Wu, H.; Du, N.; Wang, J.; Zhang, H. Three-dimensionally porous Fe3O4 as high-performance anode materials for lithiumeion batteries. Deren Yang. J. Power Sources 2014, 246, 198–203. [Google Scholar] [CrossRef]
- Kang, E.; Jung, Y.S.; Cavanagh, A.S.; Kim, G.H.; George, S.M.; Dillon, A.C.; Kim, J.K.; Lee, J. Fe3O4 nanoparticles confined in mesocellular carbon foam for high performance anode materials for lithium-ion batteries. Adv. Funct. Mater. 2011, 21, 2430–2438. [Google Scholar] [CrossRef]
- Carbonari, G.; Maroni, F.; Gabrielli, S.; Staffolani, A.; Tossici, R.; Palmieri, A.; Nobili, F. Synthesis and characterization of vanillin-templated Fe2O3 nanoparticles as a sustainable anode material for Li-ion batteries. ChemElectroChem 2019, 6. [Google Scholar] [CrossRef]
- Yoon, T.; Kim, J.; Kim, J.; Lee, J.K. Electrostatic Self-Assembly of Fe3O4 Nanoparticles on Graphene Oxides for High Capacity Lithium-Ion Battery Anodes. Energy 2013, 6, 4830–4840. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Marinho, B.; Ghislandi, M.; Tkalya, E.; Koning, C.E.; de With, G. Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder. Powder Technol. 2012, 221, 351–358. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Y.; Ahn, H.; Wang, G. Microwave hydrothermal synthesis of high performance tin-graphene nanocomposites for lithium ion batteries. J. Power Sources 2012, 216, 22–27. [Google Scholar] [CrossRef]
- Su, J.; Cao, M.; Ren, L.; Hu, C. Fe3O4–Graphene Nanocomposites with Improved Lithium Storage and Magnetism Properties. J. Phys. Chem. 2011, 115, 14469–14477. [Google Scholar] [CrossRef]
- Yang, S.; Cui, G.; Pang, S.; Cao, Q.; Kolb, U.; Feng, X.; Maier, J.; Müllen, K. Fabrication of Cobalt and Cobalt Oxide/Graphene Composites: Towards High-Performance Anode Materials for Lithium Ion Batteries. ChemSusChem 2010, 3, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cui, L.-F.; Yang, Y.; Casalongue, H.S.; Robinson, J.T.; Liang, Y.; Cui, Y.; Dai, H. Mn3O4-Graphene Hybrid as a High-Capacity Anode Material for Lithium Ion Batteries. J. Am. Chem. Soc. 2010, 132, 13978–13980. [Google Scholar] [CrossRef] [Green Version]
- Bresser, D.; Buchholz, D.; Moretti, A.; Varzi, A.; Passerini, S. Alternative binders for sustainable electrochemical energy storage—The transition to aqueous electrode processing and bio-derived polymers. Energy Environ. Sci. 2018, 11, 3096–3127. [Google Scholar] [CrossRef] [Green Version]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward Efficient Binders for Li-Ion Battery Si-Based Anodes: Polyacrylic Acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef]
- Valvo, B.D.M.; Liivat, D.A.; Eriksson, D.H.; Edström, K. Iron-Based Electrodes Meet Water-Based Preparation, Fluorine-Free Electrolyte and Binder: A Chance for More Sustainable Lithium-Ion Batteries? ChemSusChem 2017, 11, 2431–2448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Bt Ahmad Khairudin, N.B.; Bt Mohamad, S.E.; Pl Hara, H.; Bt Mad Nordin, M.F.; Lee, K.X. An eco-friendly means of biosynthesis of superparamagnetic magnetite nanoparticles via marine polymer. IEEE Trans. Nanotechnol. 2017, 16, 1047–1052. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Nano magnetite decorated multiwalled carbon nanotubes: A robust nanomaterial for enhanced carbon dioxide adsorption. Energy Environ. Sci. 2011, 4, 889–895. [Google Scholar] [CrossRef]
- Yuvakkumar, R. Hong. Green Synthesis of Spinel Magnetite Iron Oxide Nanoparticles. Adv. Mater. Res. 2014, 1051, 39–42. [Google Scholar] [CrossRef]
- Childres, I.; Jauregui, L.A.; Park, W.; Cao, H.; Chen, Y.P. Raman spectroscopy of graphene and related materials. New Dev. Phot. Mater. Res. 2013, 1, 1–20. [Google Scholar]
- Scherrer, P.S. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachr. Ges. Wiss. Gött. Math. Physikalische Kl. 1918, 2, 98–100. [Google Scholar]
- Kim, H.; Seo, D.-H.; Kim, S.-W.; Kim, J.; Kang, K. Highly reversible Co3O4/graphene hybrid anode for lithium rechargeable batteries. Carbon 2011, 49, 326–332. [Google Scholar] [CrossRef]
- Thackeray, M.M.; de Picciotto, L.A.; de Kock, A.; Johnson, P.J.; Nicholas, V.A.; Adendorff, K.T. Spinel Electrodes for Lithium Batteries. J. Am. Ceram. Soc. 1987, 82, 1–8. [Google Scholar] [CrossRef]
- Combined, X.R.D.; Larcher, D.; Bonnin, D.; Cortes, R.; Rivals, I.; Personnaz, L.; Tarascon, J.-M. Combined XRD, EXAFS, and Mossbauer Studies of the Reduction by Lithium of a-Fe2O3 with Various Particle Sizes. J. Electrochem. Soc. 2003, 150, 1643–1647. [Google Scholar] [CrossRef]
- Laruelle, S.; Grugeon, S.; Poizot, P.; Dollé, M.; Dupont, L.; Tarascon, J.-M. On the Origin of the Extra Electrochemical Capacity Displayed by MO/Li Cells at Low Potential. J. Electrochem. Soc. 2002, 149, A627. [Google Scholar] [CrossRef]
- Gireaud, L.; Grugeon, S.; Pilard, S.; Guenot, P.; Tarascon, J.-M.; Laruelle, S. Mass Spectrometry Investigations on Electrolyte Degradation Products for the Development of Nanocomposite Electrodes in Lithium Ion Batteries. Anal. Chem. 2006, 78, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, R.; Jaraba, M.; Lavela, P.; Tirado, J.L.; Jumas, J.C.; Olivier-Fourcade, J. Changes in oxidation state and magnetic order of iron atoms during the electrochemical reaction of lithium with NiFe2O4. Electrochem. Commun. 2003, 5, 16–21. [Google Scholar] [CrossRef]
- Yamakawa, N.; Jiang, M.; Key, B.; Grey, C.P. Identifying the Local Structures Formed during Lithiation of the Conversion Material, Iron Fluoride, in a Li Ion Battery: A Solid-State NMR, X-ray Diffraction, and Pair Distribution Function Analysis Study. J. Am. Chem. Soc. 2009, 131, 10525–10536. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, Z.-J.; Yang, L.; Cheng, S.; Liu, M. A high-performance anode for lithium ion batteries: Fe3O4 microspheres encapsulated in hollow graphene shells. J. Mater. Chem. A 2015, 3, 11847–11856. [Google Scholar] [CrossRef]
- Han, S.; Wang, X.; Huang, Y.; Tang, Y.; Ai, Y.; Jiang, J.; Wu, D. Carbon encapsulated Fe3O4/graphene framework with oriented macropores for lithium ion battery anode with enhanced cycling stability. RSC Adv. 2015, 5, 98399–98403. [Google Scholar] [CrossRef]
- Zhu, W.; Kierzek, K.; Wang, S.; Li, S.; Holze, R.; Chen, X. Improved performance in lithium ion battery of CNT- Fe3O4@graphene induced by three-dimensional structured construction. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 126014. [Google Scholar] [CrossRef]
- Liu, Y.; Siddique, A.H.; Huang, H.; Fang, Q.; Deng, W.; Zhou, X.; Lu, H.; Liu, Z. In situ preparation of Fe3O4 in a carbon hybrid of graphene nanoscrolls and carbon nanotubes as high performance anode material for lithium-ion batteries. Nanotechnology 2017, 28, 465401. [Google Scholar] [CrossRef] [Green Version]
- Maroni, F.; Raccichini, R.; Birrozzi, A.; Carbonari, G.; Tossici, R.; Croce, F.; Marassi, R.; Nobili, F. Graphene/silicon nanocomposite anode with enhanced electrochemical stability for lithium-ion battery applications. J. Power Sources 2014, 269, 873–882. [Google Scholar] [CrossRef]
- Nobili, F.; Mancini, M.; Stallworth, P.E.; Croce, F.; Greenbaum, S.G.; Marassi, R. Tin-coated graphite electrodes as composite anodes for Li-ion batteries. Effects of tin coatings thickness toward intercalation behavior. J. Power Sources 2012, 198, 243–250. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Nonlinear Least Squares Fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Barsoukov, E.; Ross Macdonald, J. Impedance Spectroscopy. Theory, Experiment, and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2005; ISBN 0-471-64749-7. [Google Scholar]
- Mascolo, M.C.; Pei, Y.; Ring, T.A. Room Temperature Co-Precipitation Synthesis of Magnetite Nanoparticles in a Large pH Window with Different Bases. Materials 2013, 6, 5549–5567. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Cycle Number | C-Rate | Capacity/mAh g−1 | Efficiency/% | |
|---|---|---|---|---|
| A | 3 | C/10 | 1253 | 88.6 |
| B | 8 | C/5 | 1069 | 93.6 |
| C | 13 | C/2 | 925 | 96.4 |
| D | 18 | 1C | 834 | 97.7 |
| E | 23 | 2C | 742 | 98.0 |
| F | 28 | 5C | 610 | 99.1 |
| G | 33 | 10C | 484 | 99.2 |
| H | 50 | 1C | 874 | 99.6 |
| Low C-Rate | High C-Rate | ||||
|---|---|---|---|---|---|
| Electrode | Current/A g−1 | Capacity/mAh g−1 (Cycle Number) | Current/A g−1 | Capacity/mAh g−1 (Cycle Number) | Ref. |
| Fe3O4@rGO | 1 | 1260 (250) | 10 | 357 (65) | [37] |
| C-Fe3O4 | 0.2 | 1065 (200) | 8 | 470 (50) | [38] |
| CNT-Fe3O4@graphene | 0.2 C * | ≈600 (5) | 10 C * | 177 (25) | [39] |
| Fe3O4@rGNSs-CNTs | 0.1 | 1232.9 (100) | 5 | 500 (30) | [40] |
| Fe3O4/rGO | 0.88 | 688 (100) | 8.81 | 484 (33) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staffolani, A.; Darjazi, H.; Carbonari, G.; Maroni, F.; Gabrielli, S.; Nobili, F. Fe3O4/Graphene Composite Anode Material for Fast-Charging Li-Ion Batteries. Molecules 2021, 26, 4316. https://doi.org/10.3390/molecules26144316
Staffolani A, Darjazi H, Carbonari G, Maroni F, Gabrielli S, Nobili F. Fe3O4/Graphene Composite Anode Material for Fast-Charging Li-Ion Batteries. Molecules. 2021; 26(14):4316. https://doi.org/10.3390/molecules26144316
Chicago/Turabian StyleStaffolani, Antunes, Hamideh Darjazi, Gilberto Carbonari, Fabio Maroni, Serena Gabrielli, and Francesco Nobili. 2021. "Fe3O4/Graphene Composite Anode Material for Fast-Charging Li-Ion Batteries" Molecules 26, no. 14: 4316. https://doi.org/10.3390/molecules26144316
APA StyleStaffolani, A., Darjazi, H., Carbonari, G., Maroni, F., Gabrielli, S., & Nobili, F. (2021). Fe3O4/Graphene Composite Anode Material for Fast-Charging Li-Ion Batteries. Molecules, 26(14), 4316. https://doi.org/10.3390/molecules26144316






