The Fumarprotocetraric Acid Inhibits Tau Covalently, Avoiding Cytotoxicity of Aggregates in Cells
Abstract
:1. Introduction
2. Results
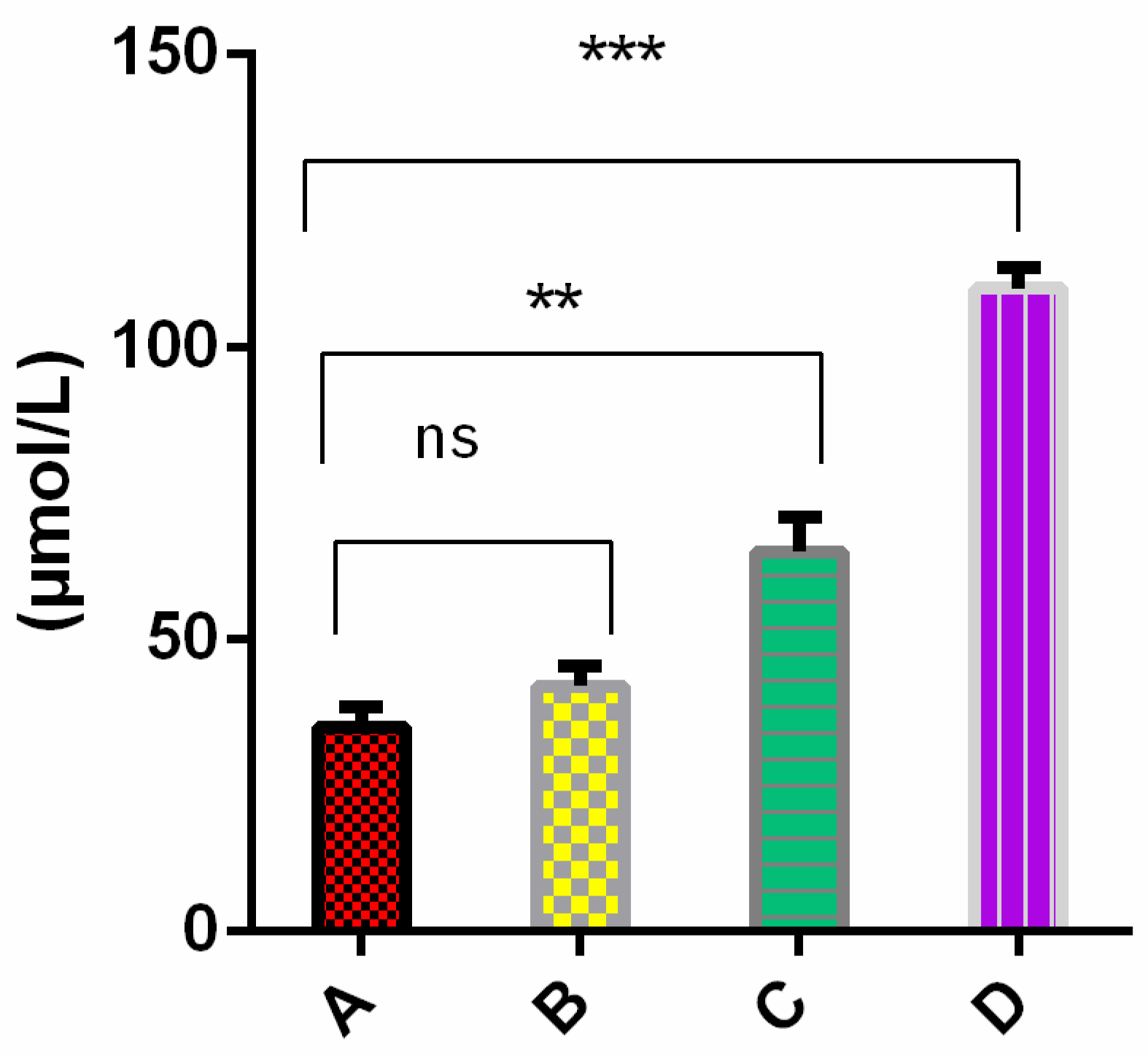
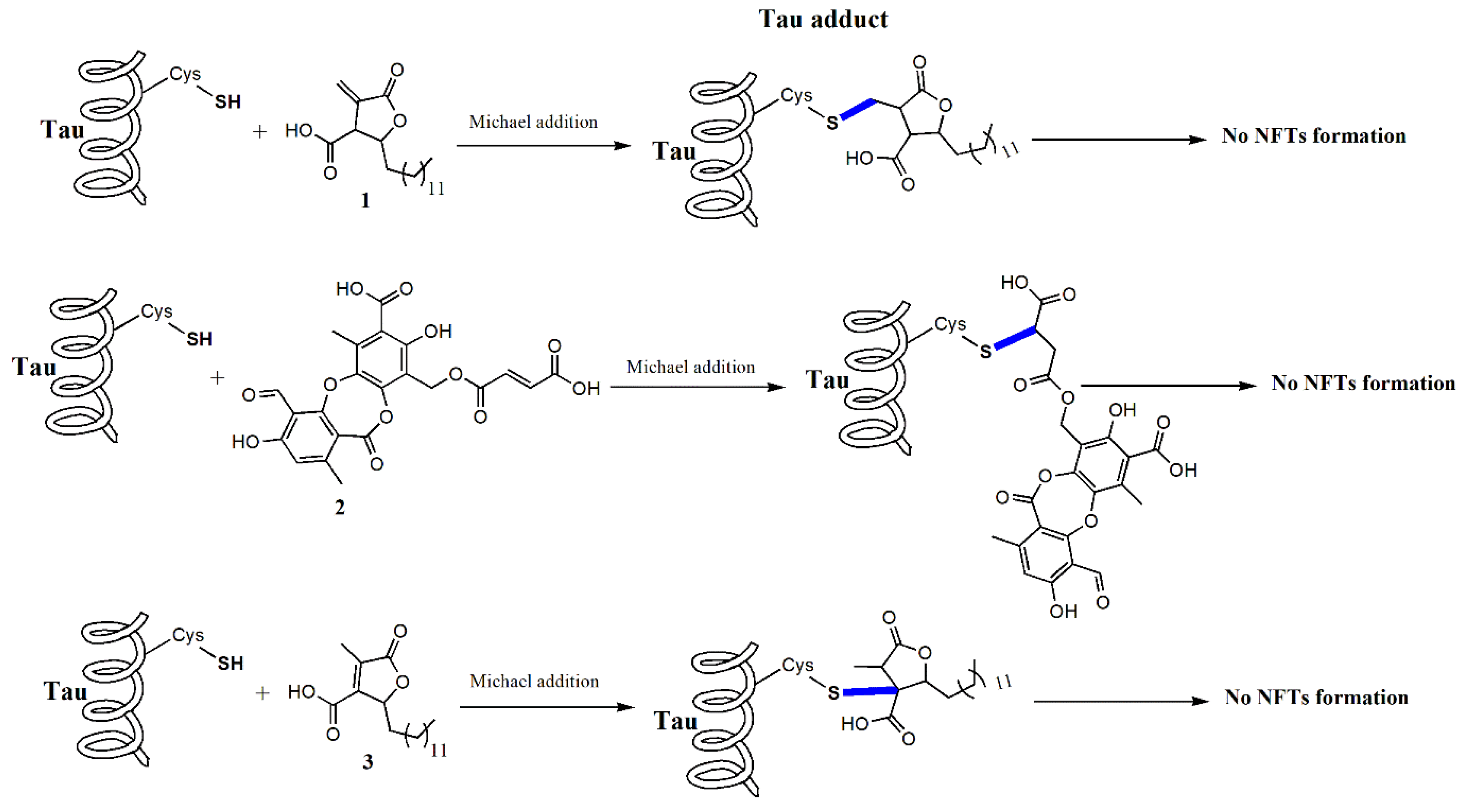
2.1. Lichens Compounds Inhibit the Progression of Tau Aggregation through the Interaction with Cysteines
2.2. The Compound 2 Diminished β Sheet Content and Remodeled Aggregates Morphology
2.3. Cells Incubated with Treated Oligomers by Compound 2 Prevent Morphological Changes
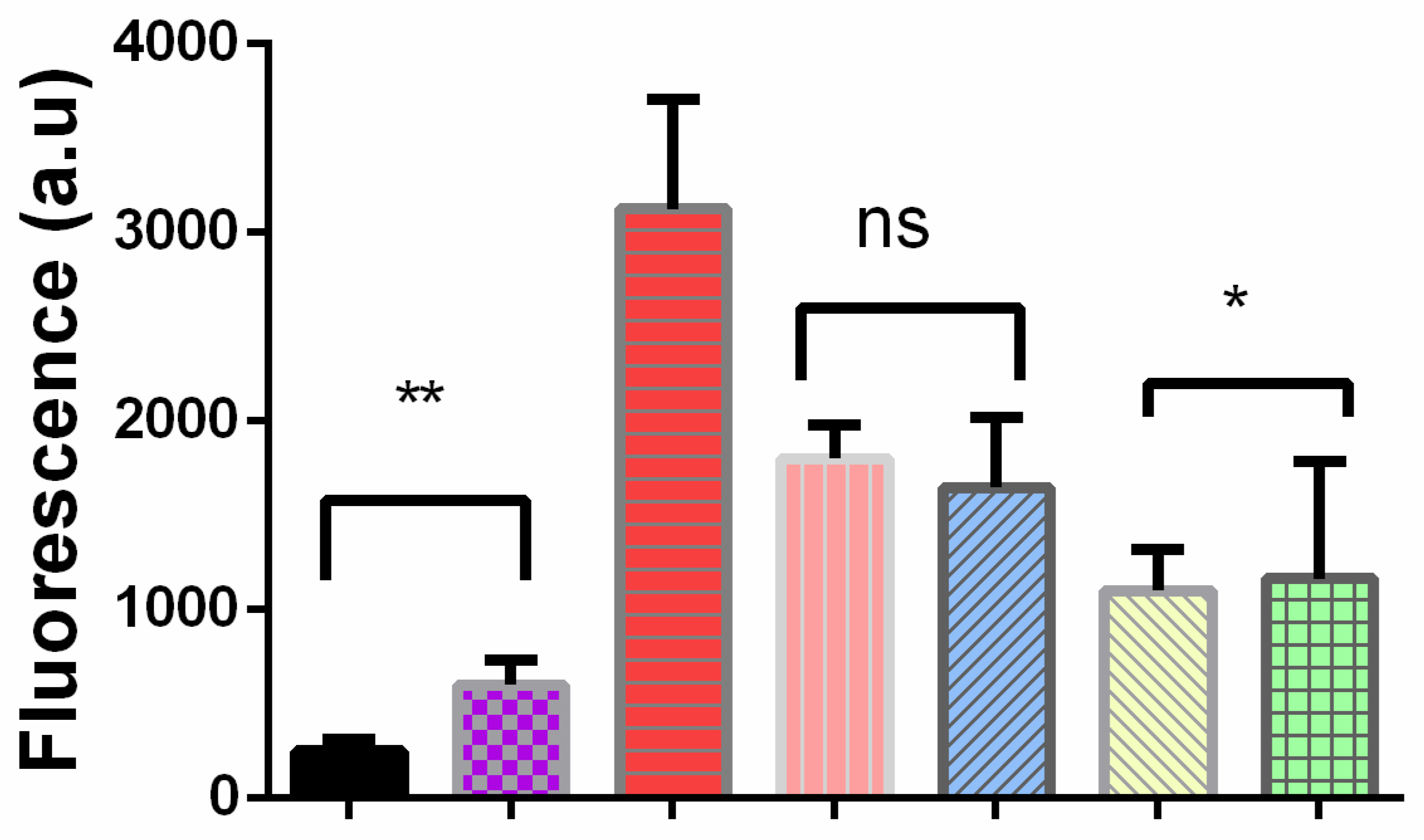
2.4. Cells Cultured in the Presence of External Oligomers Provoke Membrane Damage
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. Protein Purification
4.4. Thioflavin T Assay
4.5. Atomic Force Images
4.6. Maleimide Labeling
4.7. Total Internal Reflections Fluorescence Microscopy (TIRFM)
4.8. Cells Culture, Immunofluorescence, and LDH Assay
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- López, O.L.; DeKosky, S.T. Clinical Symptoms in Alzheimer’s Disease. Handb. Clin. Neurol. 2008, 89, 207–216. [Google Scholar] [PubMed]
- Mebane-Sims, I. Alzheimer’s Association 2018 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2018, 14, 367–429. [Google Scholar]
- Gong, C.-X.; Iqbal, K. Hyperphosphorylation of Microtubule-Associated Protein Tau: A Promising Therapeutic Target for Alzheimer Disease. Curr. Med. Chem. 2008, 15, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Leugers, C.J. Tau and Tauopathies. Prog. Mol. Biol. Transl. Sci. 2012, 107, 263–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, K.; Rank, K.B.; Evans, D.B.; Sharma, S.K. Role of Cysteine-291 and Cysteine-322 in the Polymerization of Human Tau into Alzheimer-like Filaments. Biochem. Biophys. Res. Commun. 2001, 285, 20–26. [Google Scholar] [CrossRef]
- Ramachandran, G.; Milan-Garces, E.A.; Udgaonkar, J.B.; Puranik, M. Resonance Raman Spectroscopic Measurements Delineate the Structural Changes That Occur during Tau Fibril Formation. Biochemistry 2014, 53, 6550–6565. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Sahara, N.; Saito, Y.; Murayama, M.; Yoshiike, Y.; Kim, H.; Miyasaka, T.; Murayama, S.; Ikai, A.; Takashima, A. Granular Tau Oligomers as Intermediates of Tau Filaments. Biochemistry 2007, 46, 3856–3861. [Google Scholar] [CrossRef]
- Gotz, J.; Ittner, A.; Ittner, L.M. Tau-Targeted Treatment Strategies in Alzheimer’s Disease. Br. J. Pharmacol. 2012, 165, 1246–1259. [Google Scholar] [CrossRef] [Green Version]
- Kametani, F.; Hasegawa, M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hochgrafe, K.; Sydow, A.; Mandelkow, E.M. Regulatable Transgenic Mouse Models of Alzheimer Disease: Onset, Reversibility and Spreading of Tau Pathology. FEBS J. 2013, 280, 4371–4381. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Goedert, M. Tau Pathology and Neurodegeneration. Lancet Neurol. 2013, 12, 609–622. [Google Scholar] [CrossRef]
- Medina, L.; González-Lizárraga, F.; Dominguez-Meijide, A.; Ploper, D.; Parrales, V.; Sequeira, S.; Cima-Omori, M.-S.; Zweckstetter, M.; Del Bel, E.; Michel, P.P.; et al. Doxycycline Interferes With Tau Aggregation and Reduces Its Neuronal Toxicity. Front. Aging Neurosci. 2021, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- George, R.C.; Lew, J.; Graves, D.J. Interaction of Cinnamaldehyde and Epicatechin with Tau: Implications of Beneficial Effects in Modulating Alzheimer’s Disease Pathogenesis. J. Alzheimer’s Dis. 2013, 36, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Areche, C.; Zapata, F.; González, M.; Díaz, E.; Montecinos, R.; Hernández, M.; Melo, F.; Cornejo, A. Anthraquinone Derivative Reduces Tau Oligomer Progression by Inhibiting Cysteine-Cysteine Interaction. ChemistryOpen 2019, 8, 554–559. [Google Scholar] [CrossRef] [Green Version]
- VandeVrede, L.; Boxer, A.L.; Polydoro, M. Targeting Tau: Clinical Trials and Novel Therapeutic Approaches. Neurosci. Lett. 2020, 731, 134919. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.; James, M.J.; Lee, V.M.-Y.; Smith, A.B.; Trojanowski, J.Q.; Ballatore, C.; Brunden, K.R. Aminothienopyridazines and Methylene Blue Affect Tau Fibrillization via Cysteine Oxidation. J. Biol. Chem. 2013, 288, 11024–11037. [Google Scholar] [CrossRef] [Green Version]
- Wischik, C.M.; Harrington, C.R.; Storey, J.M. Tau-Aggregation Inhibitor Therapy for Alzheimer’s Disease. Biochem. Pharmacol. 2014, 88, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, S.; Feldman, H.H.; Schneider, L.S.; Wilcock, G.K.; Frisoni, G.B.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Wischik, D.J. Efficacy and Safety of Tau-Aggregation Inhibitor Therapy in Patients with Mild or Moderate Alzheimer’s Disease: A Randomised, Controlled, Double-Blind, Parallel-Arm, Phase 3 Trial. Lancet 2016, 388, 2873–2884. [Google Scholar] [CrossRef] [Green Version]
- Bomprezzi, R. Dimethyl Fumarate in the Treatment of Relapsing–Remitting Multiple Sclerosis: An Overview. Ther. Adv. Neurol. Disord. 2015, 8, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Linker, R.A.; Gold, R. Dimethyl Fumarate for Treatment of Multiple Sclerosis: Mechanism of Action, Effectiveness, and Side Effects. Curr. Neurol. Neurosci. Rep. 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Chan, F.K.M.; Moriwaki, K.; De Rosa, M.J. Detection of Necrosis by Release of Lactate Dehydrogenase (LDH) Activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [PubMed] [Green Version]
- Gudjónsdóttir, G.A.; Ingólfsdóttir, K. Quantitative Determination of Protolichesterinic- and Fumarprotocetraric Acids in Cetraria Islandica by High-Performance Liquid Chromatography. J. Chromatogr. A 1997, 757, 303–306. [Google Scholar] [CrossRef]
- Huneck, S.; Yoshimura, I. (Eds.) Identification of Lichen Substances. In Identification of Lichen Substances; Springer: Berlin/Heidelberg, Germany, 1996; pp. 11–123. ISBN 978-3-642-85243-5. [Google Scholar]
- Cornejo, A.; Jimenez, J.M.; Caballero, L.; Melo, F.; Maccioni, R.B. Fulvic Acid Inhibits Aggregation and Promotes Disassembly of Tau Fibrils Associated with Alzheimer’s Disease. J. Alzheimers Dis. 2011, 27, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, S.W.; Cornejo, A.; van Eersel, J.; Stevens, C.H.; Vaca, I.; Cueto, M.; Kassiou, M.; Gladbach, A.; Macmillan, A.; Lewis, L.; et al. The Polyphenol Altenusin Inhibits in Vitro Fibrillization of Tau and Reduces Induced Tau Pathology in Primary Neurons. ACS Chem. Neurosci. 2017, 8, 743–751. [Google Scholar] [CrossRef]
- Viklickỳ, V.; Dráber, P.; Hašek, J.; Bártek, J. Production and Characterization of a Monoclonal Antitubulin Antibody. Cell Biol. Int. Rep. 1982, 6, 725–731. [Google Scholar] [CrossRef]
- Draber, P.; Rubino, S.; Draberova, E.; Viklickỳ, V.; Cappuccinelli, P. A Broad Spectrum Monoclonal Antibody to Alpha-Tubulin Does Not Recognize All Protozoan Tubulins. Protoplasma 1985, 128, 201–207. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, C.; Cartagena, C.; Caballero, L.; Melo, F.; Areche, C.; Cornejo, A. The Fumarprotocetraric Acid Inhibits Tau Covalently, Avoiding Cytotoxicity of Aggregates in Cells. Molecules 2021, 26, 3760. https://doi.org/10.3390/molecules26123760
González C, Cartagena C, Caballero L, Melo F, Areche C, Cornejo A. The Fumarprotocetraric Acid Inhibits Tau Covalently, Avoiding Cytotoxicity of Aggregates in Cells. Molecules. 2021; 26(12):3760. https://doi.org/10.3390/molecules26123760
Chicago/Turabian StyleGonzález, Camila, Constanza Cartagena, Leonardo Caballero, Francisco Melo, Carlos Areche, and Alberto Cornejo. 2021. "The Fumarprotocetraric Acid Inhibits Tau Covalently, Avoiding Cytotoxicity of Aggregates in Cells" Molecules 26, no. 12: 3760. https://doi.org/10.3390/molecules26123760





