Frequency-Dependent Sonochemical Processing of Silicon Surfaces in Tetrahydrofuran Studied by Surface Photovoltage Transients
Abstract
:1. Introduction
2. Materials and Methods
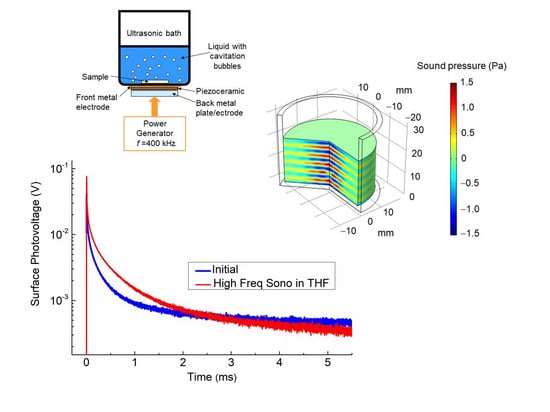
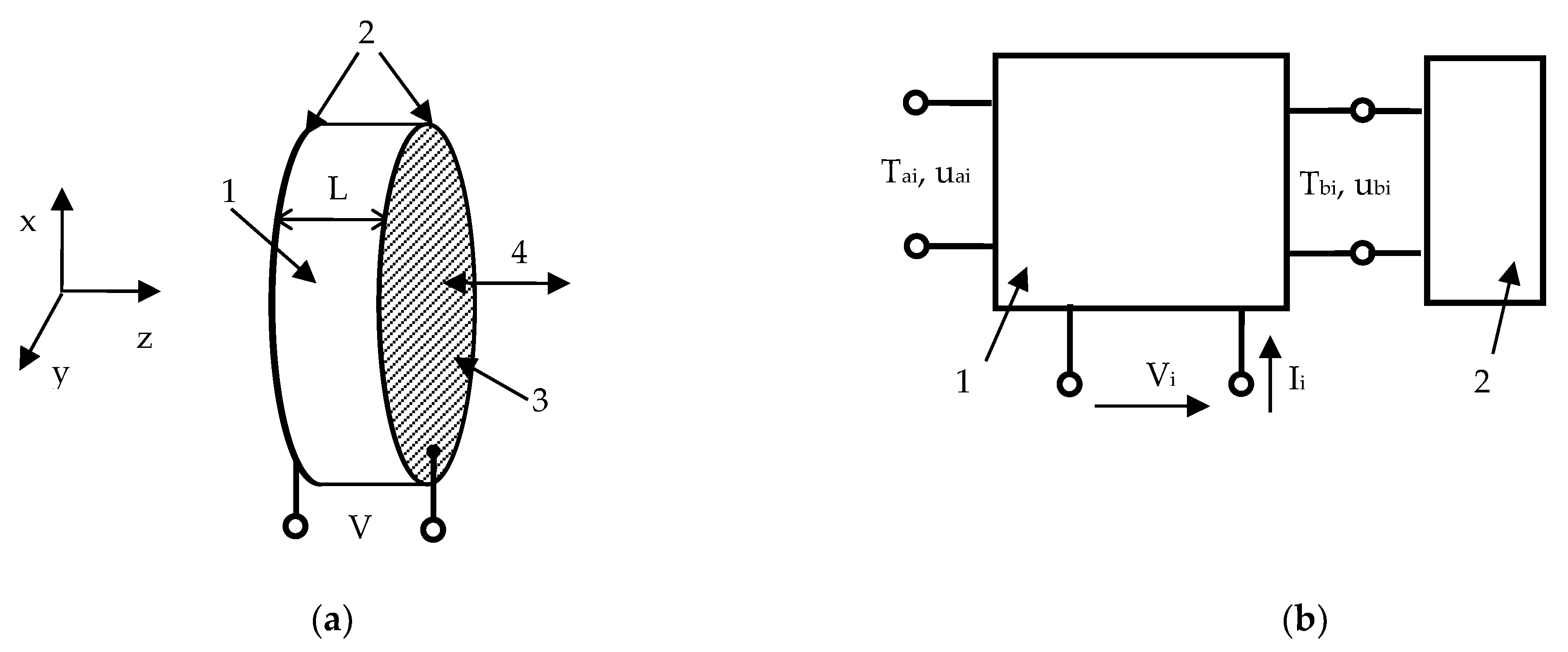
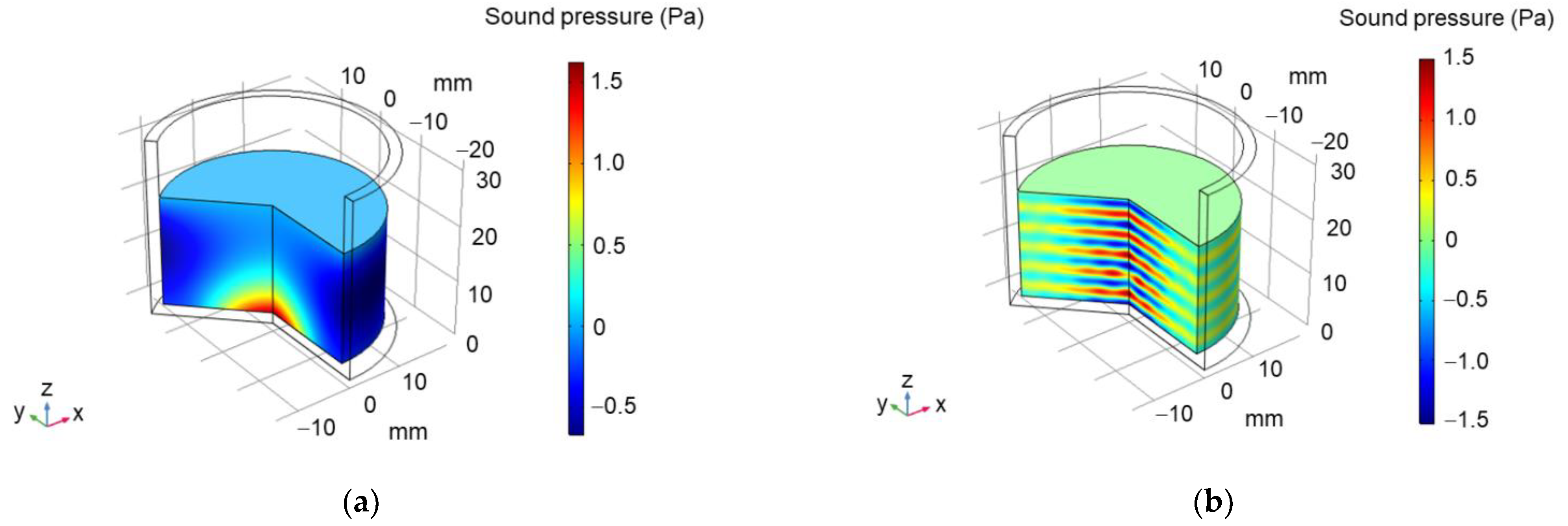
2.1. Designing Ultrasonic Transducer
2.2. Surface Processing and Photovoltage Measuring
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vinatoru, M.; Mason, T. Jean-Louis Luche and the Interpretation of Sonochemical Reaction Mechanisms. Molecules 2021, 26, 755. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Sáez, V.; Mason, T.J. Sonoelectrochemical Synthesis of Nanoparticles. Molecules 2009, 14, 4284–4299. [Google Scholar] [CrossRef]
- Xu, H.; Zeiger, B.W.; Suslick, K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013, 42, 2555–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gedanken, A.; Perelshtein, I. Power ultrasound for the production of nanomaterials. In Power Ultrasonics. Applications of High-Intensity Ultrasound; Gallego-Juárez, J.A., Graff, K.F., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2015; pp. 543–576. [Google Scholar] [CrossRef]
- Taha, A.; Ahmed, E.; Ismaiel, A.; Ashokkumar, M.; Xu, X.; Pan, S.; Hu, H. Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends Food Sci. Technol. 2020, 105, 363–377. [Google Scholar] [CrossRef]
- Sandhya, M.; Ramasamy, D.; Sudhakar, K.; Kadirgama, K.; Harun, W. Ultrasonication an intensifying tool for preparation of stable nanofluids and study the time influence on distinct properties of graphene nanofluids—A systematic overview. Ultrason. Sonochem. 2021, 73, 105479. [Google Scholar] [CrossRef]
- Maisonhaute, E.; Prado, C.; White, P.C.; Compton, R.G. Surface acoustic cavitation understood via nanosecond electrochemistry. Part III: Shear stress in ultrasonic cleaning. Ultrason. Sonochem. 2002, 9, 297–303. [Google Scholar] [CrossRef]
- Cobley, A.; Mason, T.; Cobley, A.; Mason, T.J. The evaluation of sonochemical techniques for sustainable surface modification in electronic manufacturing. Circuit World 2007, 33, 29–34. [Google Scholar] [CrossRef]
- Paniwnyk, L.; Cobley, A. Ultrasonic Surface Modification of Electronics Materials. Phys. Procedia 2010, 3, 1103–1108. [Google Scholar] [CrossRef] [Green Version]
- Arruda, L.B.; Orlandi, M.; Lisboa-Filho, P. Morphological modifications and surface amorphization in ZnO sonochemically treated nanoparticles. Ultrason. Sonochem. 2013, 20, 799–804. [Google Scholar] [CrossRef]
- Savkina, R.K.; Gudymenko, A.I.; Kladko, V.P.; Korchovyi, A.; Nikolenko, A.S.; Smirnov, A.; Stara, T.; Strelchuk, V.V. Silicon Substrate Strained and Structured via Cavitation Effect for Photovoltaic and Biomedical Application. Nanoscale Res. Lett. 2016, 11, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skorb, E.V.; Möhwald, H. Ultrasonic approach for surface nanostructuring. Ultrason. Sonochem. 2016, 29, 589–603. [Google Scholar] [CrossRef]
- Bai, F.; Wang, L.; Saalbach, K.-A.; Twiefel, J. A Novel Ultrasonic Cavitation Peening Approach Assisted by Water Jet. Appl. Sci. 2018, 8, 2218. [Google Scholar] [CrossRef] [Green Version]
- Nadtochiy, A.; Korotchenkov, O.; Schlosser, V. Sonochemical Modification of SiGe Layers for Photovoltaic Applications. Phys. Status Solidi A 2019, 216, 1900154. [Google Scholar] [CrossRef]
- Angermann, H.; Henrion, W.; Roseler, A. Wet-chemical conditioning of silicon: Electronic properties correlated with the surface morphology. In Silicon-Based Materials and Devices; Nalwa, H.S., Ed.; Academic Press: San Diego, CA, USA, 2001; Volume 1, pp. 267–298. [Google Scholar]
- Fuchs, F. Ultrasonic cleaning and washing of surfaces. In Power Ultrasonics. Applications of High-Intensity Ultrasound; Gallego-Juárez, J.A., Graff, K.F., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2015; pp. 577–609. [Google Scholar] [CrossRef]
- Yasui, K. Influence of ultrasonic frequency on multibubble sonoluminescence. J. Acoust. Soc. Am. 2002, 112, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Joyce, E.M.; Mason, T.J. Evaluation of the mechanisms of the effect of ultrasound on Microcystis aeruginosa at different ultrasonic frequencies. Water Res. 2012, 46, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Joyce, E.; Phull, S.; Lorimer, J.; Mason, T.J. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrason. Sonochem. 2003, 10, 315–318. [Google Scholar] [CrossRef]
- Koda, S.; Miyamoto, M.; Toma, M.; Matsuoka, T.; Maebayashi, M. Inactivation of Escherichia coli and Streptococcus mutans by ultrasound at 500kHz. Ultrason. Sonochem. 2009, 16, 655–659. [Google Scholar] [CrossRef]
- Calimli, M.H.; Nas, M.S.; Acidereli, H.; Sen, F. Sonochemical methods and their leading properties for chemical synthesis. In Green Sustainable Process for Chemical and Envi-Ronmental Engineering and Science, 1st ed.; Asiri, A.M., Kanchi, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 13; pp. 355–365. [Google Scholar]
- Bussemaker, M.J.; Zhang, D. A phenomenological investigation into the opposing effects of fluid flow on sonochemical activity at different frequency and power settings. 1. Overhead stirring. Ultrason. Sonochem. 2014, 21, 436–445. [Google Scholar] [CrossRef]
- Monnier, H.; Wilhelm, A.; Delmas, H. The influence of ultrasound on micromixing in a semi-batch reactor. Chem. Eng. Sci. 1999, 54, 2953–2961. [Google Scholar] [CrossRef]
- Van Der Sluis, L.W.M.; Versluis, M.; Wu, M.K.; Wesselink, P.R. Passive ultrasonic irrigation of the root canal: A review of the literature. Int. Endod. J. 2007, 40, 415–426. [Google Scholar] [CrossRef]
- Dashtimoghadam, E.; Johnson, A.; Fahimipour, F.; Malakoutian, M.; Vargas, J.; Gonzalez, J.; Ibrahim, M.; Baeten, J.; Tayebi, L. Vibrational and sonochemical characterization of ultrasonic endodontic activating devices for translation to clinical efficacy. Mater. Sci. Eng. C 2020, 109, 110646. [Google Scholar] [CrossRef] [PubMed]
- Podolian, A.; Nadtochiy, A.; Kuryliuk, V.; Korotchenkov, O.; Schmid, J.; Drapalik, M.; Schlosser, V. The potential of sonicated water in the cleaning processes of silicon wafers. Sol. Energy Mater. Sol. Cells 2011, 95, 765–772. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Applied Sonochemistry: Uses of Power Ultrasound in Chemistry and Processing; Wiley: Weinheim, Germany, 2002. [Google Scholar]
- Son, Y.; No, Y.; Kim, J. Geometric and operational optimization of 20-kHz probe-type sonoreactor for enhancing sonochemical activity. Ultrason. Sonochem. 2020, 65, 105065. [Google Scholar] [CrossRef]
- Ensminger, D.; Bond, L.J. Ultrasonics Fundamentals, Technologies, and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Ostrovskii, I.V.; Nadtochiy, A.B. Domain resonance in two-dimensional periodically poled ferroelectric resonator. Appl. Phys. Lett. 2005, 86, 222902. [Google Scholar] [CrossRef]
- Royer, D.; Dieulesaint, E. Elastic Waves in Solids I, 1st ed.; Springer: Berlin, Germany, 2010. [Google Scholar]
- Nadtochiy, A.; Shmid, V.; Korotchenkov, O. Miniature ultrasonic transducer for lab-on-a-chip applications. In Proceedings of the 2020 IEEE 40th International Conference on Electronics and Nanotechnology (ELNANO), Kyiv, Ukraine, 22–24 April 2020; Institute of Electrical and Electronics Engineers (IEEE): New York, NY, USA, 2020; pp. 425–429. [Google Scholar]
- Koda, S.; Kimura, T.; Kondo, T.; Mitome, H. A standard method to calibrate sonochemical efficiency of an individual reaction system. Ultrason. Sonochem. 2003, 10, 149–156. [Google Scholar] [CrossRef]
- Barchouchi, A.; Molina-Boisseau, S.; Gondrexon, N.; Baup, S. Sonochemical activity in ultrasonic reactors under heterogeneous conditions. Ultrason. Sonochem. 2021, 72, 105407. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Y.; Wei, H.; Li, K.-B.; He, Q.; Xie, J.-C.; Zhang, J.-T. Iodine-enhanced ultrasound degradation of sulfamethazine in water. Ultrason. Sonochem. 2018, 42, 759–767. [Google Scholar] [CrossRef]
- Jovanovski, V.; Orel, B.; Ješe, R.; Vuk, A.Š.; Mali, G.; Hocčevar, S.B.; Grdadolnik, J.; Stathatos, E.; Lianos, P. Novel Polysilsesquioxane−I-/I3-Ionic Electrolyte for Dye-Sensitized Photoelectrochemical Cells. J. Phys. Chem. B 2005, 109, 14387–14395. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, A.; Margalias, A.; Stathatos, E. Functional quasi-solid-state electrolytes for dye sensitized solar cells prepared by amine alkylation reactions. RSC Adv. 2015, 5, 58307–58315. [Google Scholar] [CrossRef]
- Nomura, S.; Mukasa, S.; Kuroiwa, M.; Okada, Y.; Murakami, K. Cavitation Bubble Streaming in Ultrasonic-Standing-Wave Field. Jpn. J. Appl. Phys. 2005, 44, 3161–3164. [Google Scholar] [CrossRef]
- Yasuda, K.; Nguyen, T.T.; Asakura, Y. Measurement of distribution of broadband noise and sound pressures in sonochemical reactor. Ultrason. Sonochem. 2018, 43, 23–28. [Google Scholar] [CrossRef]
- Jamshidi, R.; Pohl, B.; Peuker, U.; Brenner, G. Numerical investigation of sonochemical reactors considering the effect of inhomogeneous bubble clouds on ultrasonic wave propagation. Chem. Eng. J. 2012, 189-190, 364–375. [Google Scholar] [CrossRef]
- Sasmal, S.; Goud, V.V.; Mohanty, K. Ultrasound Assisted Lime Pretreatment of Lignocellulosic Biomass toward Bioethanol Production. Energy Fuels 2012, 26, 3777–3784. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Hunger, R.; Fritsche, R.; Jaeckel, B.; Jaegermann, W.; Webb, L.J.; Lewis, N.S. Chemical and electronic characterization of methyl-terminated Si(111) surfaces by high-resolution synchrotron photoelectron spectroscopy. Phys. Rev. B 2005, 72, 045317. [Google Scholar] [CrossRef] [Green Version]
- Johansson, E.; Hurley, P.T.; Brunschwig, B.S.; Lewis, N.S. Infrared Vibrational Spectroscopy of Isotopically Labeled Ethyl-Terminated Si(111) Surfaces Prepared Using a Two-Step Chlorination/Alkylation Procedure. J. Phys. Chem. C 2009, 113, 15239–15245. [Google Scholar] [CrossRef] [Green Version]
- Podolian, A.; Kozachenko, V.; Nadtochiy, A.; Borovoy, N.; Korotchenkov, O. Photovoltage transients at fullerene-metal interfaces. J. Appl. Phys. 2010, 107, 93706. [Google Scholar] [CrossRef]
- Angermann, H. Characterization of wet-chemically treated silicon interfaces by surface photovoltage measurements. Anal. Bioanal. Chem. 2002, 374, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.J.; Cuevas, A. Very low bulk and surface recombination in oxidized silicon wafers. Semicond. Sci. Technol. 2001, 17, 35–38. [Google Scholar] [CrossRef]
- Gaubas, E.; Simoen, E.; Vanhellemont, J. Review—Carrier Lifetime Spectroscopy for Defect Characterization in Semiconductor Materials and Devices. ECS J. Solid State Sci. Technol. 2016, 5, P3108–P3137. [Google Scholar] [CrossRef]
- Podolian, A.; Nadtochiy, A.; Korotchenkov, O.; Romanyuk, B.; Melnik, V.; Popov, V. Enhanced photoresponse of Ge/Si nanostructures by combining amorphous silicon deposition and annealing. J. Appl. Phys. 2018, 124, 095703. [Google Scholar] [CrossRef]
- Shmid, V.; Podolian, A.; Nadtochiy, A.; Yazykov, D.; Semenko, M.; Korotchenkov, O. Photovoltaic Characterization of Si and SiGe Surfaces Sonochemically Treated in Dichloromethane. J. Nano Electron. Phys. 2020, 12, 1023. [Google Scholar] [CrossRef]
- Royea, W.J.; Juang, A.; Lewis, N.S. Preparation of air-stable, low recombination velocity Si(111) surfaces through alkyl termination. Appl. Phys. Lett. 2000, 77, 1988–1990. [Google Scholar] [CrossRef] [Green Version]
- Klute, C.H.; Walters, W.D. The Thermal Decomposition of Tetrahydrofuran. J. Am. Chem. Soc. 1946, 68, 506–511. [Google Scholar] [CrossRef]
- Lifshitz, A.; Bidani, M.; Bidani, S. Thermal reactions of cyclic ethers at high temperatures. Part 3. Pyrolysis of tetrahydrofuran behind reflected shocks. J. Phys. Chem. 1986, 90, 3422–3429. [Google Scholar] [CrossRef]
- Verdicchio, M.; Sirjean, B.; Tran, L.-S.; Glaude, P.-A.; Battin-Leclerc, F. Unimolecular decomposition of tetrahydrofuran: Carbene vs. diradical pathways. Proc. Combust. Inst. 2015, 35, 533–541. [Google Scholar] [CrossRef]
- Schmidt, J.; Peibst, R.; Brendel, R. Surface passivation of crystalline silicon solar cells: Present and future. Sol. Energy Mater. Sol. Cells 2018, 187, 39–54. [Google Scholar] [CrossRef]
- Gude, C.; Rettig, W. Radiative and Nonradiative Excited State Relaxation Channels in Squaric Acid Derivatives Bearing Differently Sized Donor Substituents: A Comparison of Experiment and Theory. J. Phys. Chem. A 2000, 104, 8050–8057. [Google Scholar] [CrossRef]
- Hokenek, S.; Kuhn, J.N. Methanol Decomposition over Palladium Particles Supported on Silica: Role of Particle Size and Co-Feeding Carbon Dioxide on the Catalytic Properties. ACS Catal. 2012, 2, 1013–1019. [Google Scholar] [CrossRef]
- Huisken, F.; Kulcke, A.; Laush, C.; Lisy, J.M. Dissociation of small methanol clusters after excitation of the O–H stretch vibration at 2.7 μ. J. Chem. Phys. 1991, 95, 3924–3929. [Google Scholar] [CrossRef]
- Chowdhury, P. Infrared depletion spectroscopy suggests fast vibrational relaxation in the hydrogen-bonded aniline–tetrahydrofuran (C6H5–NH2…OC4H8) complex. Chem. Phys. Lett. 2000, 319, 501–506. [Google Scholar] [CrossRef]
- Janeckova, R.; May, O.; Milosavljević, A.; Fedor, J. Partial cross sections for dissociative electron attachment to tetrahydrofuran reveal a dynamics-driven rich fragmentation pattern. Int. J. Mass Spectrom. 2014, 365-366, 163–168. [Google Scholar] [CrossRef] [Green Version]
- De Bruycker, R.; Tran, L.-S.; Carstensen, H.-H.; Glaude, P.-A.; Monge, F.; Alzueta, M.U.; Battin-Leclerc, F.; Van Geem, K.M. Experimental and modeling study of the pyrolysis and combustion of 2-methyl-tetrahydrofuran. Combust. Flame 2017, 176, 409–428. [Google Scholar] [CrossRef] [Green Version]
- Kaur, S.P.; Ramachandran, C. Hydrogen-tetrahydrofuran mixed hydrates: A computational study. Int. J. Hydrogen Energy 2018, 43, 19559–19566. [Google Scholar] [CrossRef]



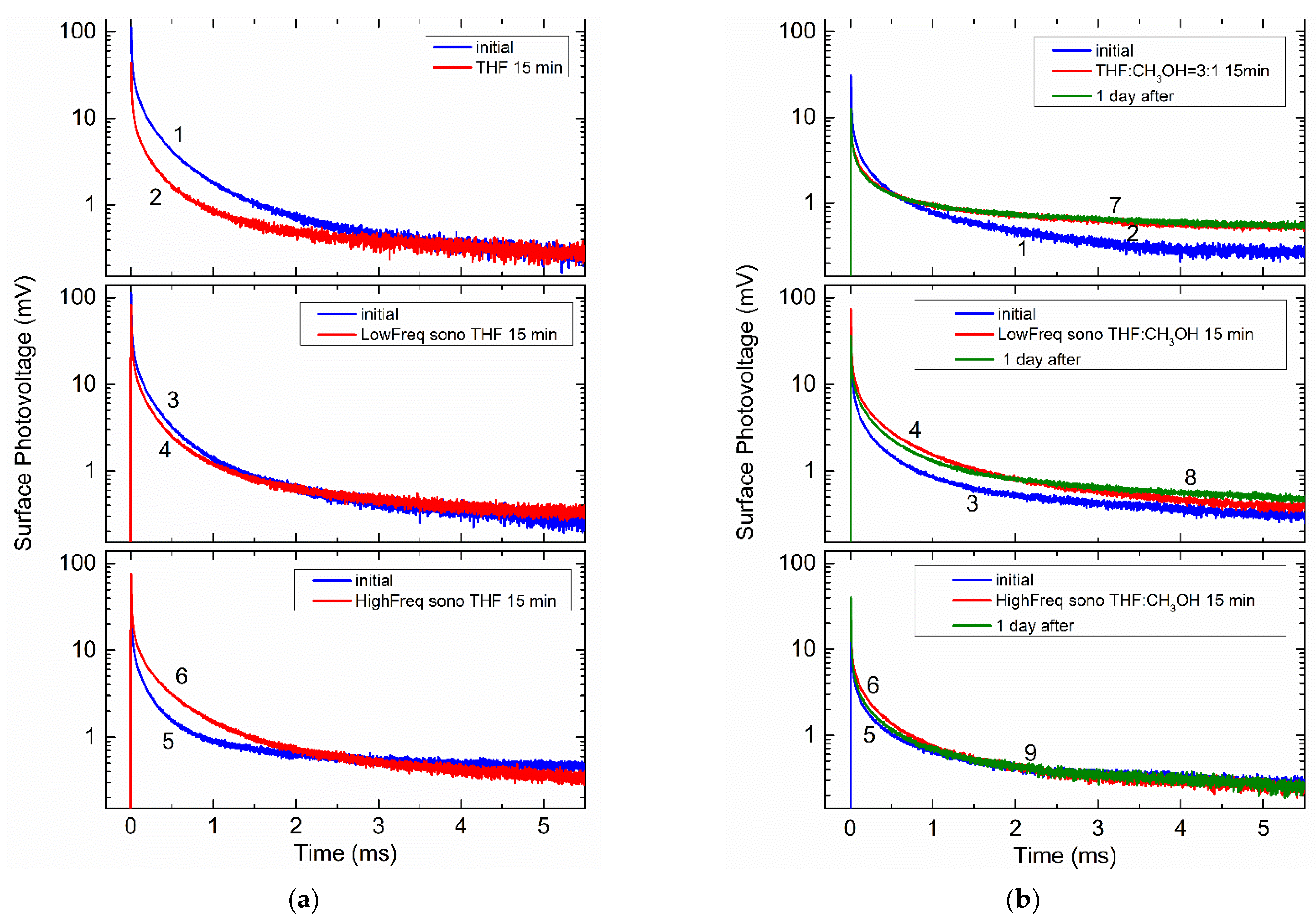

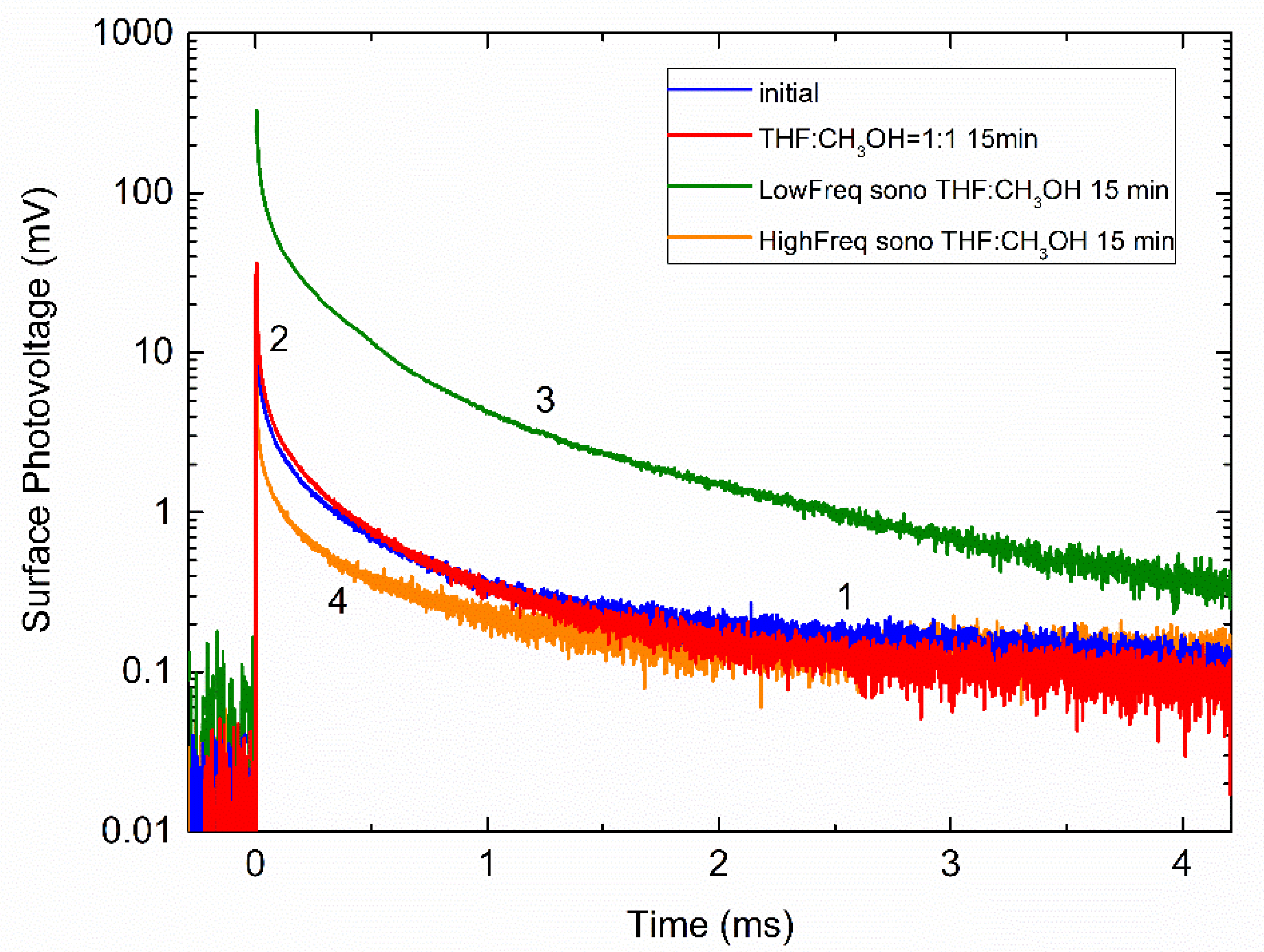
| Treatment | Peak Amplitude (mV) | ||
|---|---|---|---|
| THF Figure 4a | 112.8 (curve 1) | 6.0 | 0.294 |
| 44.4 (curve 2) | 11.1 | 0.329 | |
| 111.5 (curve 3) | 9.0 | 0.321 | |
| 83.6 (curve 4) | 5.1 | 0.288 | |
| 41.9 (curve 5) | 10.8 | 0.335 | |
| 75.6 (curve 6) | 8.4 | 0.296 | |
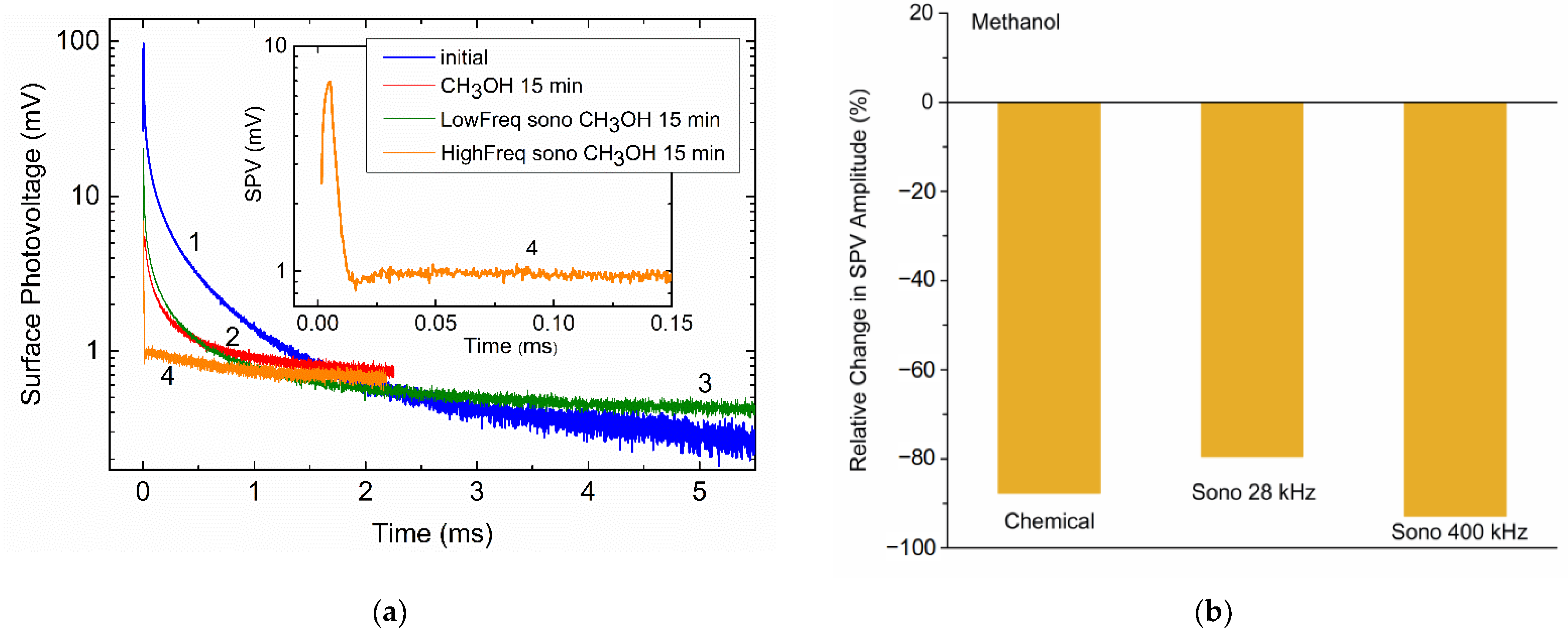
| THF/Methanol (3/1) Figure 4b | 14.6 (curve 1) | 6.4 | 0.302 |
| 15.4 (curve 2) | 3.0 | 0.247 | |
| 41.4 (curve 3) | 7.3 | 0.307 | |
| 74.6 (curve 4) | 5.3 | 0.275 | |
| 20.0 (curve 5) | 10.3 | 0.309 | |
| 34.4 (curve 6) | 5.7 | 0.274 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podolian, A.; Nadtochiy, A.; Korotchenkov, O.; Schlosser, V. Frequency-Dependent Sonochemical Processing of Silicon Surfaces in Tetrahydrofuran Studied by Surface Photovoltage Transients. Molecules 2021, 26, 3756. https://doi.org/10.3390/molecules26123756
Podolian A, Nadtochiy A, Korotchenkov O, Schlosser V. Frequency-Dependent Sonochemical Processing of Silicon Surfaces in Tetrahydrofuran Studied by Surface Photovoltage Transients. Molecules. 2021; 26(12):3756. https://doi.org/10.3390/molecules26123756
Chicago/Turabian StylePodolian, Artem, Andriy Nadtochiy, Oleg Korotchenkov, and Viktor Schlosser. 2021. "Frequency-Dependent Sonochemical Processing of Silicon Surfaces in Tetrahydrofuran Studied by Surface Photovoltage Transients" Molecules 26, no. 12: 3756. https://doi.org/10.3390/molecules26123756