Crosslinked Silk Fibroin/Gelatin/Hyaluronan Blends as Scaffolds for Cell-Based Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
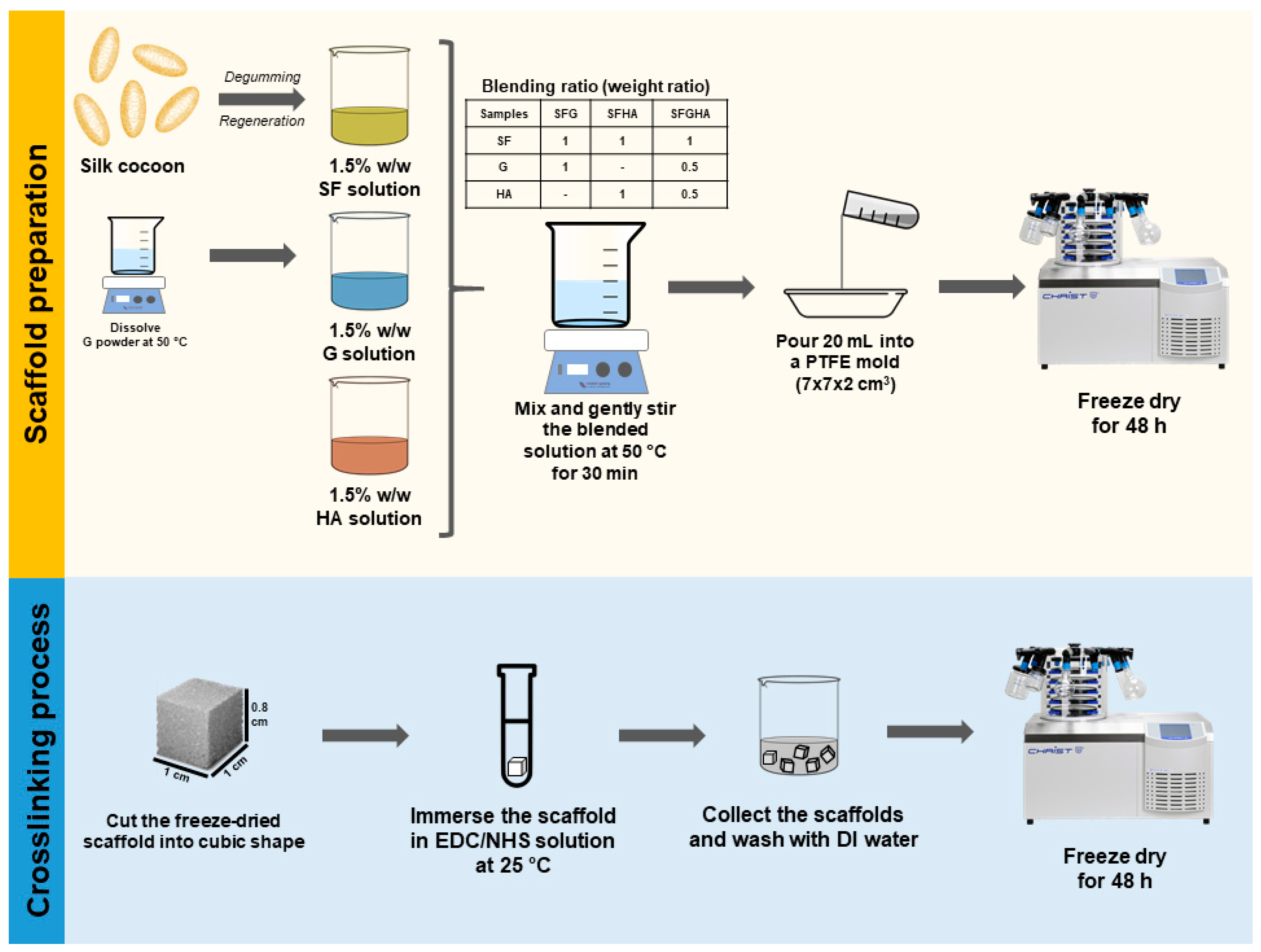
2.2. Scaffold Fabrication
2.3. Evaluation of Crosslinking Efficiency
2.4. Swellability and Degradability of the Scaffolds
2.5. Micromorphology of the Scaffolds
2.6. Adhesion and Proliferation of NIH/3T3 Cells on the Scaffolds
2.7. Visualization of Cell Morphology
2.8. Statistical Analysis
3. Results
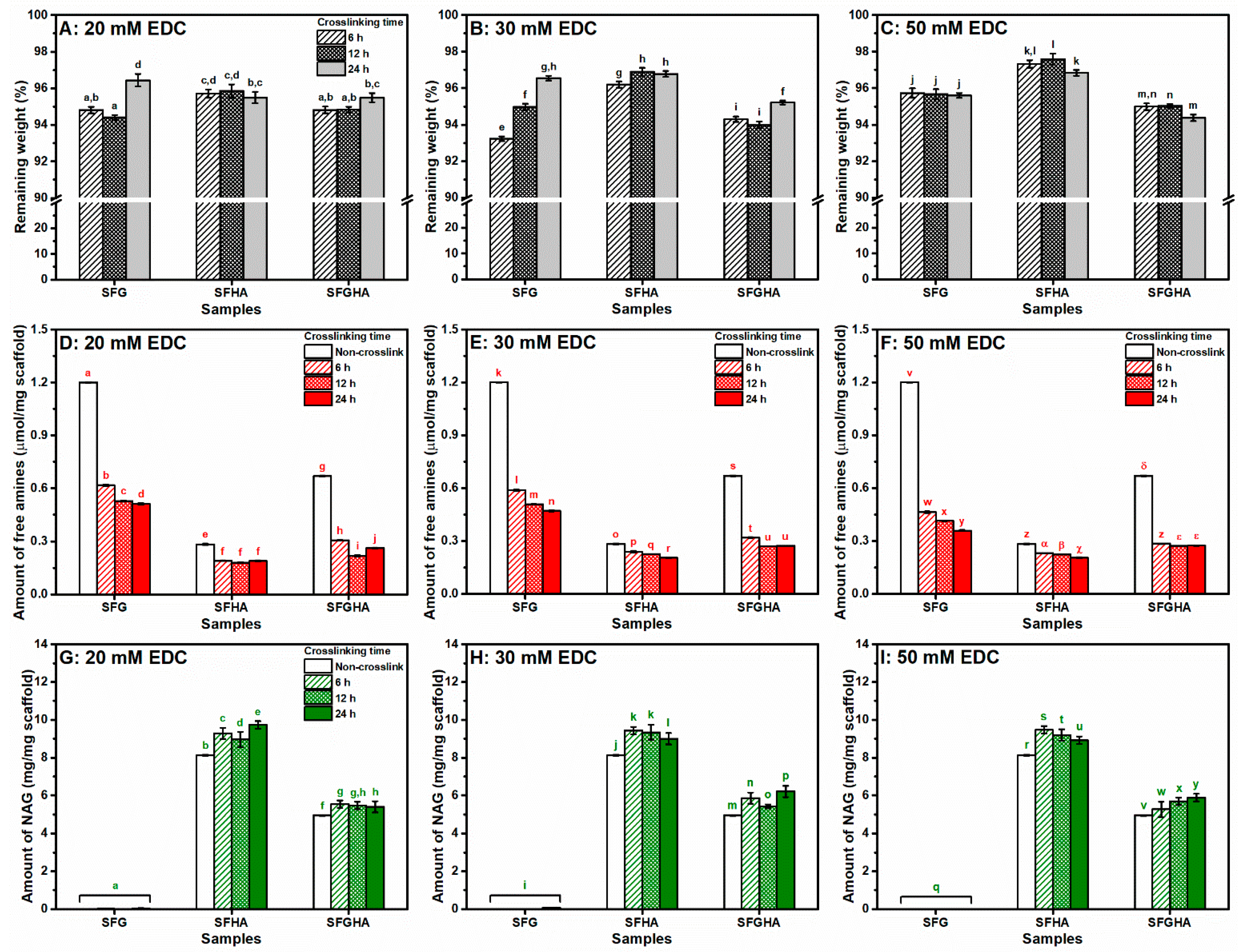
3.1. Crosslinking Efficiency
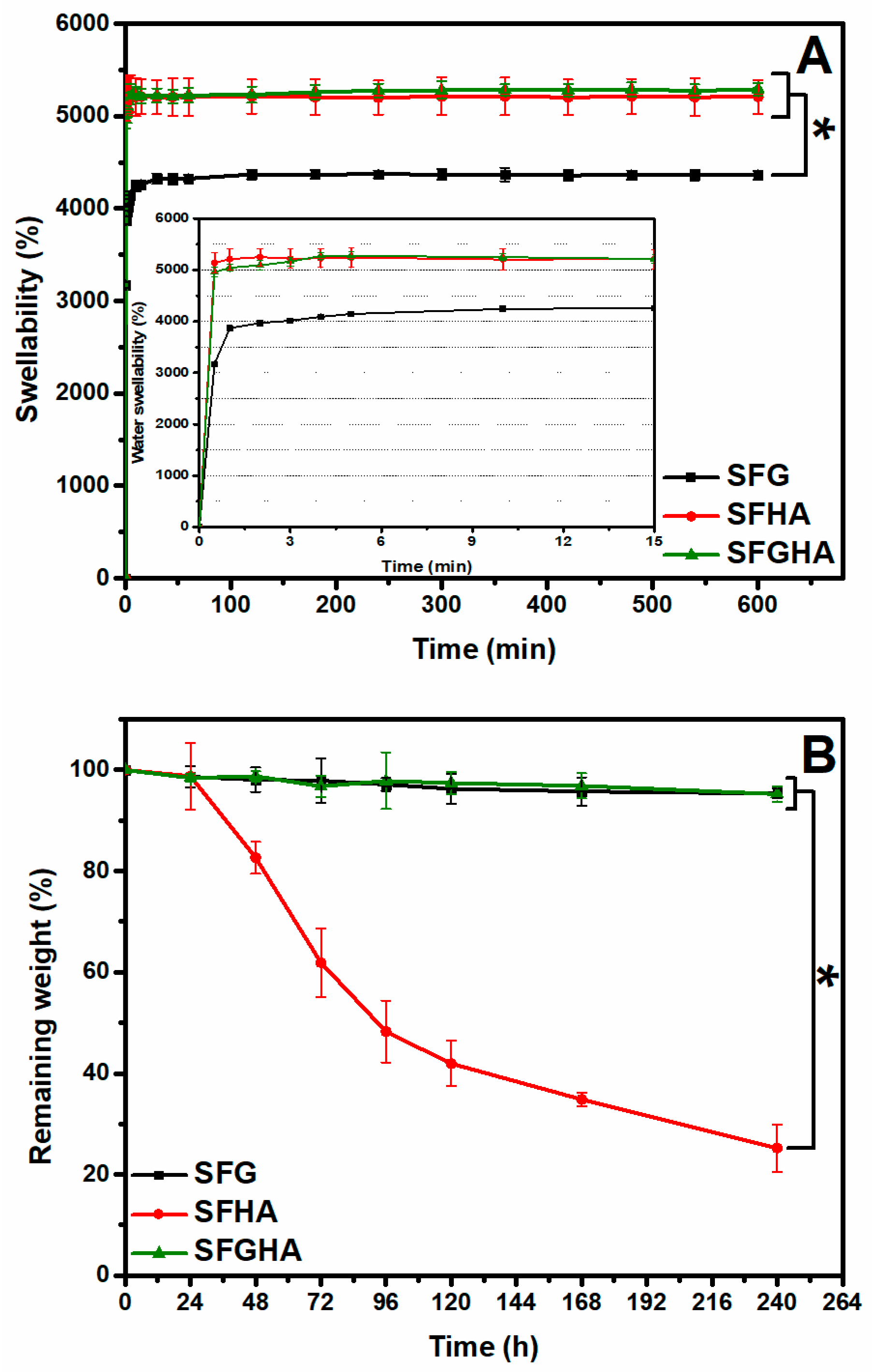
3.2. Swellability and Degradability of the Scaffolds
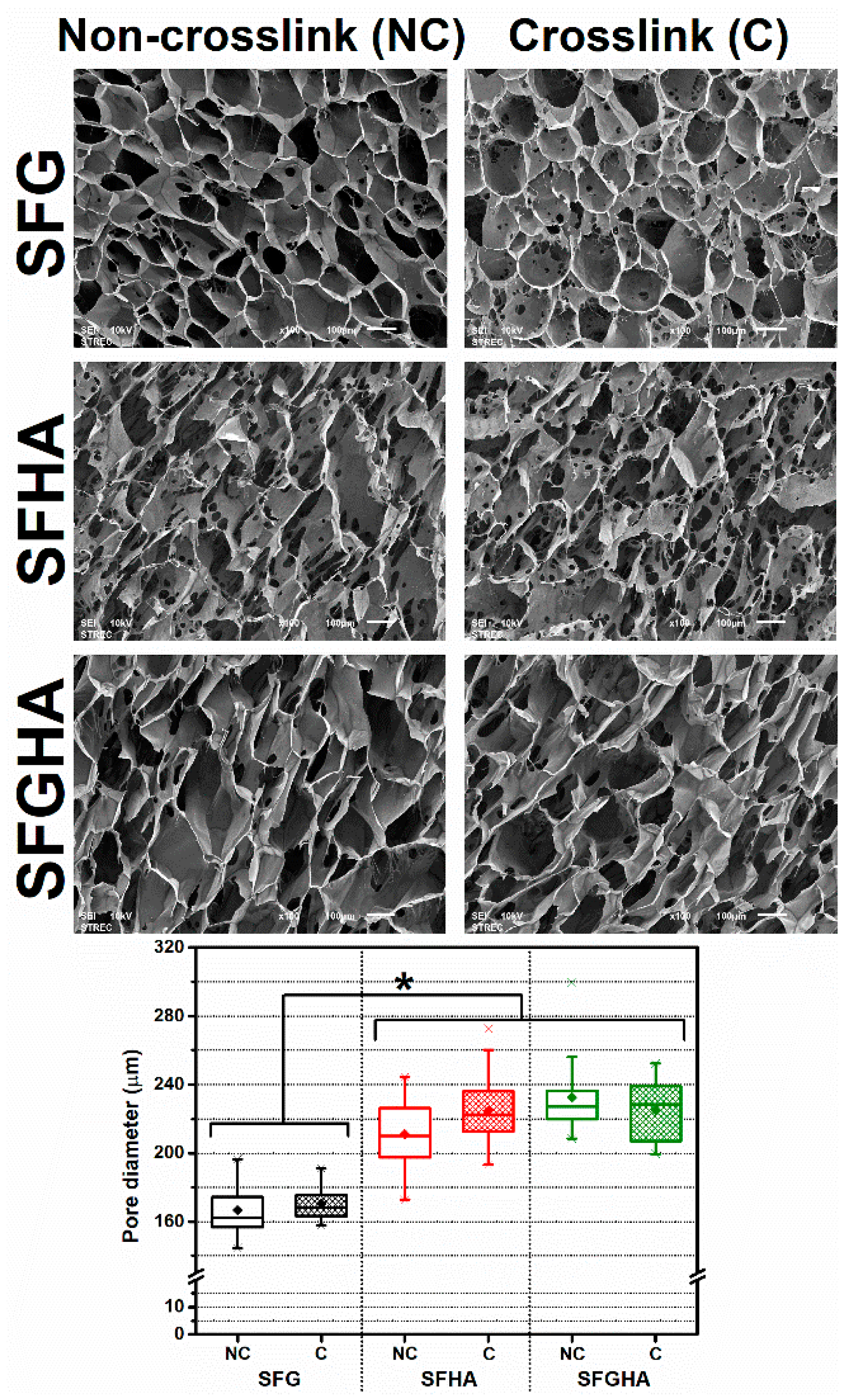
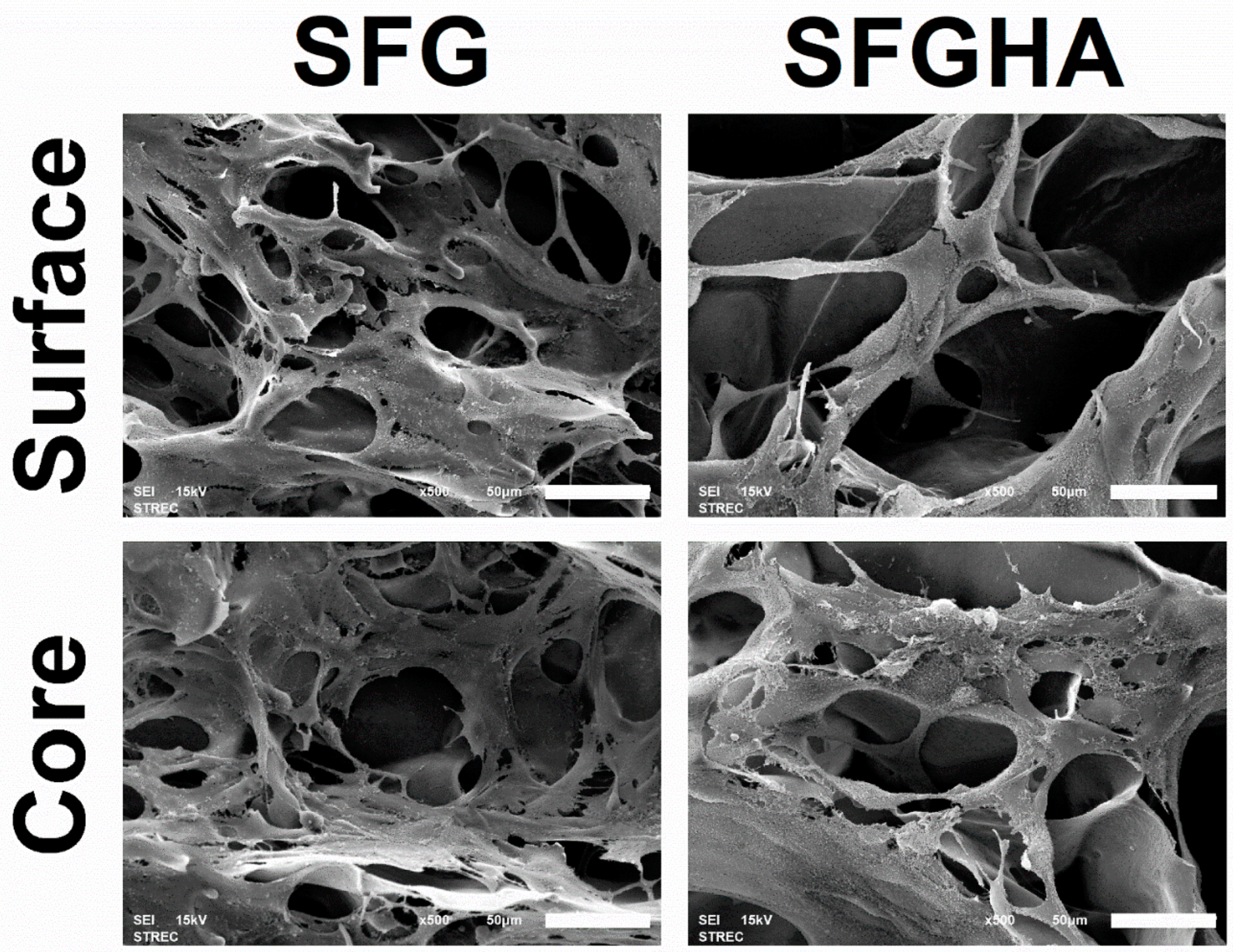
3.3. Micromorphology of the Freeze-Dried Scaffolds
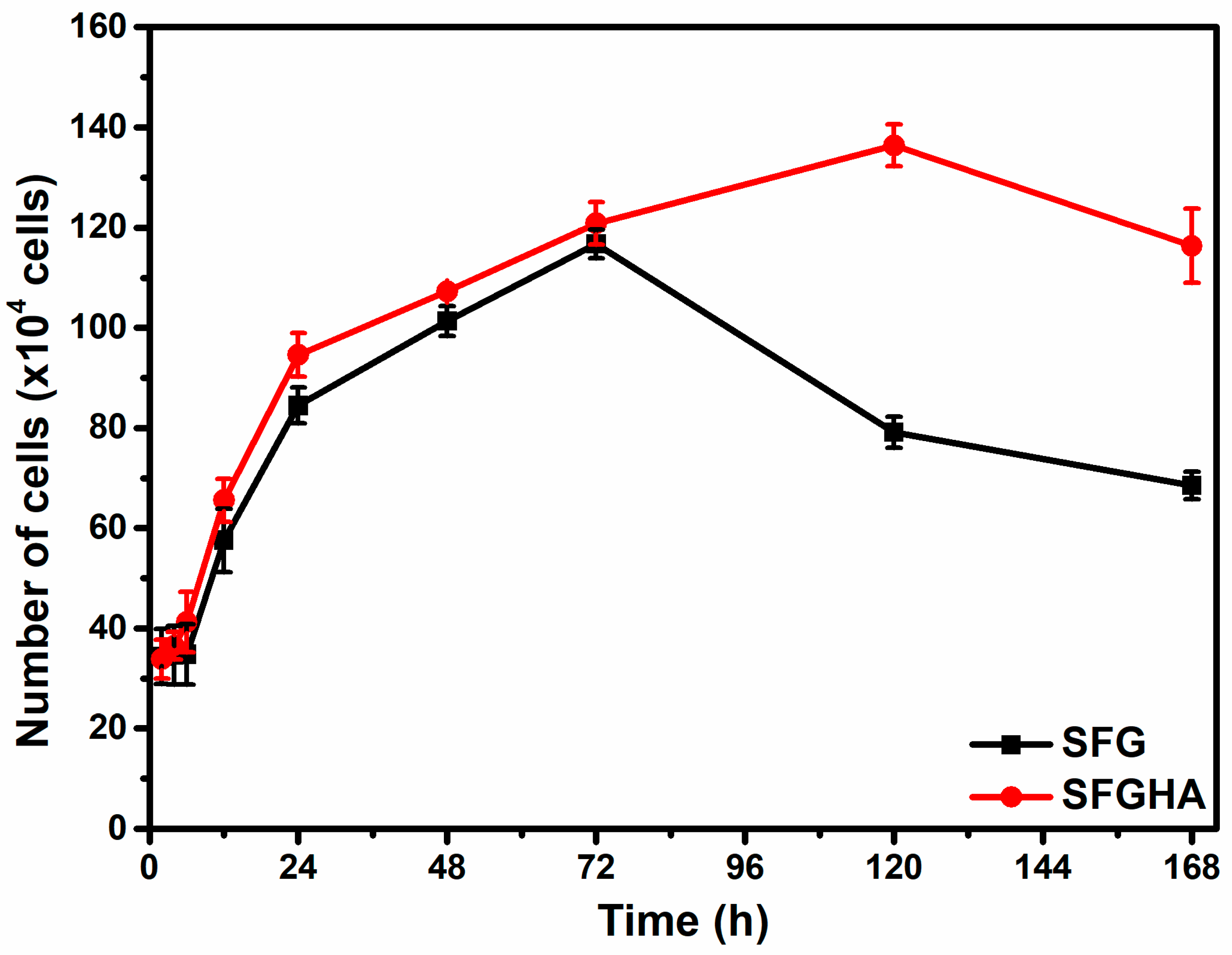
3.4. Cell Adhesion and Proliferation
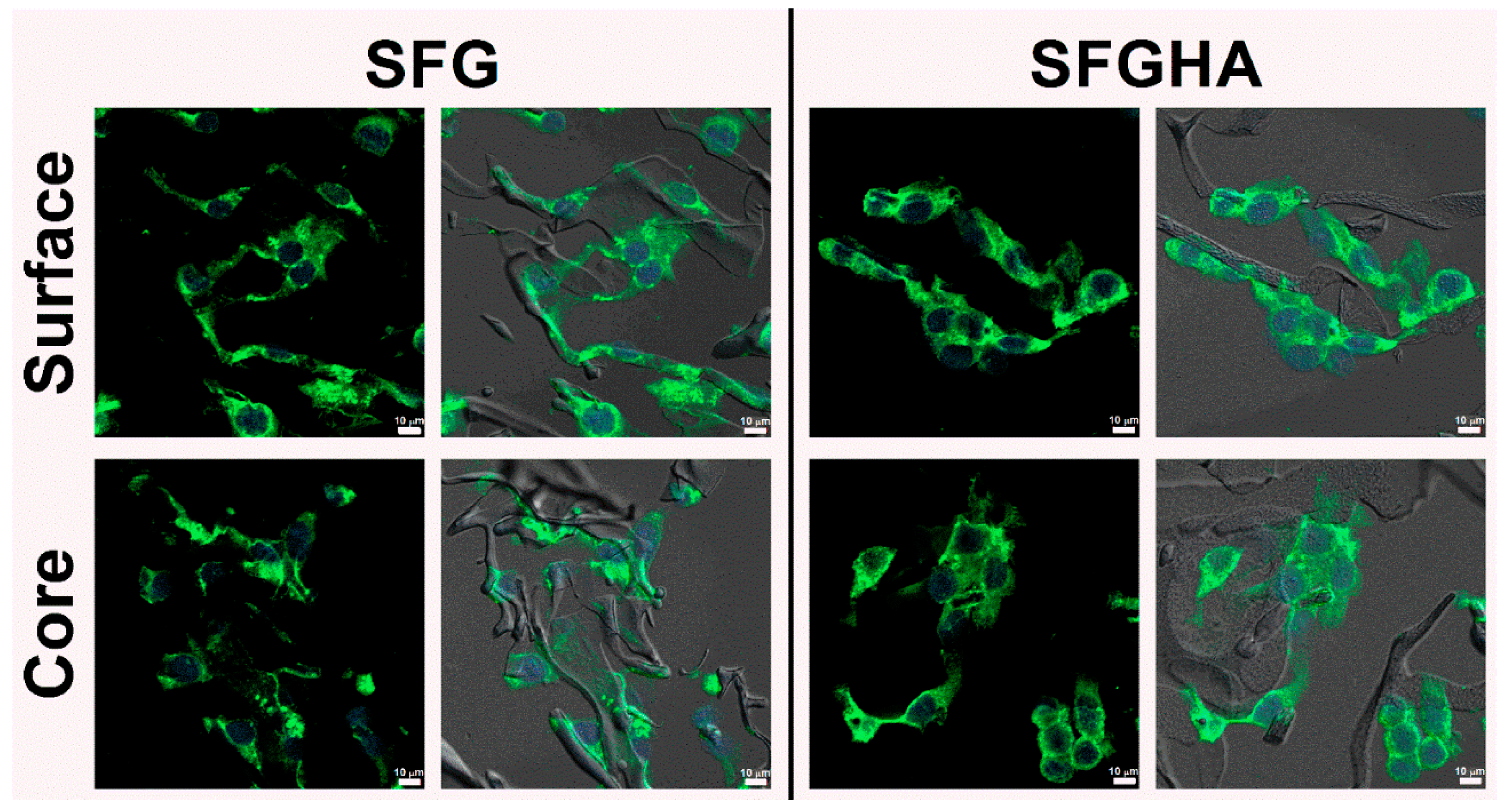
3.5. Cell Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.M.; O’Brien, F.J.; Little, D.G.; Schindeler, A. Cell-scaffold interactions in the bone tissue engineering triad. Eur. Cell Mater. 2013, 26, 120–132. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Mikos, A.G. From material to tissue: Biomaterial development, scaffold fabrication, and tissue engineering. AIChE J. 2008, 54, 3048–3067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The biomedical use of silk: Past, present, future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madden, P.W.; Lai, J.N.X.; George, K.A.; Giovenco, T.; Harkin, D.G.; Chirila, T.V. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials 2011, 32, 4076–4084. [Google Scholar] [CrossRef]
- Amornsudthiwat, P.; Mongkolnavin, R.; Kanokpanont, S.; Panpranot, J.; Wong, C.S.; Damrongsakkul, S. Improvement of early cell adhesion on Thai silk fibroin surface by low energy plasma. Colloids Surf. B Biointerfaces 2013, 111, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.; Ghosh, S.K.; Kundu, S.C. Silk fibroin protein from mulberry and non-mulberry silkworms: Cytotoxicity, biocompatibility and kinetics of L929 murine fibroblast adhesion. J. Mater. Sci. Mater. Med. 2008, 19, 2827–2836. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Jetbumpenkul, P.; Amornsudthiwat, P.; Kanokpanont, S.; Damrongsakkul, S. Balanced electrostatic blending approach–An alternative to chemical crosslinking of Thai silk fibroin/gelatin scaffold. Int. J. Biol. Macromol. 2012, 50, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Okhawilai, M.; Rangkupan, R.; Kanokpanont, S.; Damrongsakkul, S. Preparation of Thai silk fibroin/gelatin electrospun fiber mats for controlled release applications. Int. J. Biol. Macromol. 2010, 46, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, X.; Zhang, Z.; Wang, Y.; Huang, C.; Yang, C.; Liu, L.; Zhang, Q. Silk fibroin/collagen/hyaluronic acid scaffold incorporating pilose antler polypeptides microspheres for cartilage tissue engineering. Mater. Sci. Eng. C 2019, 94, 35–44. [Google Scholar] [CrossRef]
- Yang, W.; Xu, H.; Lan, Y.; Zhu, Q.; Liu, Y.; Huang, S.; Shi, S.; Hancharou, A.; Tang, B.; Guo, R. Preparation and characterisation of a novel silk fibroin/hyaluronic acid/sodium alginate scaffold for skin repair. Int. J. Biol. Macromol. 2019, 130, 58–67. [Google Scholar] [CrossRef]
- Yan, S.; Wang, Q.; Tariq, Z.; You, R.; Li, X.; Li, M.; Zhang, Q. Facile preparation of bioactive silk fibroin/hyaluronic acid hydrogels. Int. J. Biol. Macromol. 2018, 118, 775–782. [Google Scholar] [CrossRef]
- Grabarek, Z.; Gergely, J. Zero-length crosslinking procedure with the use of active esters. Anal. Biochem. 1990, 185, 131–135. [Google Scholar] [CrossRef]
- Vachiraroj, N.; Ratanavaraporn, J.; Damrongsakkul, S.; Pichyangkura, R.; Banaprasert, T.; Kanokpanont, S. A comparison of Thai silk fibroin-based and chitosan-based materials on in vitro biocompatibility for bone substitutes. Int. J. Biol. Macromol. 2009, 45, 470–477. [Google Scholar] [CrossRef]
- Bubnis, W.A.; Ofner, C.M. The determination of ϵ-amino groups in soluble and poorly soluble proteinaceous materials by a spectrophotometrie method using trinitrobenzenesulfonic acid. Anal. Biochem. 1992, 207, 129–133. [Google Scholar] [CrossRef]
- Blix, G. The determination of hexosamines according to Elson and Morgan. Acta Chem. Scand. 1948, 2, 467–473. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; DesRochers, T.M.; Burke, K.A.; Kaplan, D.L. The effect of sterilization on silk fibroin biomaterial properties. Macromol. Biosci. 2015, 15, 861–874. [Google Scholar] [CrossRef] [Green Version]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Kapoor, S.; Kundu, S.C. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef]
- Najberg, M.; Mansor, M.H.; Taillé, T.; Bouré, C.; Molina-Peña, R.; Boury, F.; Cenis, J.L.; Garcion, E.; Lorenzo-Alvarez, C. Aerogel sponges of silk fibroin, hyaluronic acid and heparin for soft tissue engineering: Composition-properties relationship. Carbohydr. Polym. 2020, 237, 116107. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; You, H.; Cai, J.; Zhang, Q.; Yan, S.; You, R. Physically crosslinked silk fibroin/hyaluronic acid scaffolds. Carbohydr. Polym. 2020, 239, 116232. [Google Scholar] [CrossRef]
- Yu, L.-M.; Liu, T.; Ma, Y.-L.; Zhang, F.; Huang, Y.-C.; Fan, Z.-H. Fabrication of silk-hyaluronan composite as a potential scaffold for tissue repair. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Das, S.K.; Yan, S.; You, R.; Li, X.; Luo, Z.; Li, M.; Zhang, Q.; Kaplan, D.L. Stability and biodegradation of silk fibroin/hyaluronic acid nerve conduits. Compos. Part B Eng. 2020, 200, 108222. [Google Scholar] [CrossRef]
- Yan, S.; Han, G.; Wang, Q.; Zhang, S.; You, R.; Luo, Z.; Xu, A.; Li, X.; Li, M.; Zhang, Q.; et al. Directed assembly of robust and biocompatible silk fibroin/hyaluronic acid composite hydrogels. Compos. Part B Eng. 2019, 176, 107204. [Google Scholar] [CrossRef]
- Yin, F.; Lin, L.; Zhan, S. Preparation and properties of cellulose nanocrystals, gelatin, hyaluronic acid composite hydrogel as wound dressing. J. Biomater. Sci. Polym. Ed. 2019, 30, 190–201. [Google Scholar] [CrossRef]
- Choi, Y.S.; Hong, S.R.; Lee, Y.M.; Song, K.W.; Park, M.H.; Nam, Y.S. Studies on gelatin-containing artificial skin: II. Preparation and characterization of cross-linked gelatin-hyaluronate sponge. J. Biomed. Mater. Res. 1999, 48, 631–639. [Google Scholar] [PubMed]
- Wang, T.-W.; Spector, M. Development of hyaluronic acid-based scaffolds for brain tissue engineering. Acta Biomater. 2009, 5, 2371–2384. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Senbanjo, L.T.; Chellaiah, M.A. Cd44: A multifunctional cell surface adhesion receptor is a regulator of progression and metastasis of cancer cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, R.M.; Iocono, J.A.; Ehrlich, H.P. Hyaluronic acid stimulates human fibroblast proliferation within a collagen matrix. J. Cell. Physiol. 1998, 177, 465–473. [Google Scholar] [CrossRef]
- Ruedinger, F.; Lavrentieva, A.; Blume, C.; Pepelanova, I.; Scheper, T. Hydrogels for 3D mammalian cell culture: A starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 2015, 99, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
| Samples | % Attachment | µ | PDT (h) | ||
|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | |||
| SFG | 73.87 ± 6.87 | 73.18 ± 11.04 | 74.18 ± 10.01 | 0.055 ± 0.008 | 12.88 ± 1.97 |
| SFGHA | 71.64 ± 3.06 | 75.79 ± 2.38 | 87.68 ± 8.58 | 0.050 ± 0.003 | 13.97 ± 0.72 |
| Scaffolding Materials | Crosslinking Method | Physical Properties | Biological Evaluation | Ref. |
|---|---|---|---|---|
| SFHA and SFHA with heparin (4%, 25% HA ratio) | EDC/NHS (blending) | Water uptake (93%), Pore size (20–140 µm), Young’s modulus (12.5–13.1 kPa) | Cytocompatible (NIH/3T3, 24 h) | [24] |
| SFHA (5%, 1.5-5% HA ratio) | Soft-freezing | Water solubility (4.0–4.6% with 1.5–5% HA ratio), Compressive modulus (32–57 kPa), 21-day degradation (~47% in PBS, ~72% in enzymes) | NA | [25] |
| SFHA (2.5% SF + 0.25% HA) | Ethanol evaporation | Swellability (20–30 times), PBS degradation (80–85% in 30 days), Compressive modulus (28–30 kPa) | SFHA scaffolds supported cell adhesion, proliferation, and migration (BMSCs, 16 days) | [26] |
| SFHA (2%, 5–20% HA ratio) | EDC/NHS (immersion) | Water solubility (6–8%), Swellability (5–10 times), 21-day degradation (10–12% in PBS, 75–95% in enzymes) | SFHA scaffolds supported cell adhesion, proliferation, and migration (mESCs, 9 days) | [27] |
| SFHA (10%, 20–40% HA ratio) | Ca2+-Formic acid | Water solubility (2–2.5%), Swellability (7–12 times), Compressive stress (0.01–0.2 MPa) | SFHA scaffolds supported cell adhesion and proliferation (L929, 9 days) | [28] |
| GHA (5%, 2% HA ratio) | EDC/NHS (blending) | Swellability (6–7 times), Elastic modulus (0.9–1 kPa), Young’s modulus (~140 kPa), Compressive modulus (~40 kPa) | GHA scaffolds supported cell proliferation (NIH/3T3, 7 days) | [29] |
| SFHA (2.5%, 20–60% HA ratio) | EDC/NHS (blending) | Pore size (50–100 µm), Compressive strength (3.2–11.9 kPa), 18–day degradation (6–10% in PBS, 65–90% in α-chymotrypsin) | SFHA scaffolds were biocompatible after 5-day implantation in rats | [16] |
| SFGHA (1.5%, 25–50% G ratio, 25–50% HA ratio) | EDC/NHS (immersion) | Water solubility (2–5%), Swellability (40–50 times), 10-day degradation (80% in culture medium), Pore size (160–240 µm) | SFG and SFGHA scaffolds supported cell adhesion, proliferation, and migration (NIH/3T3, 7 days) | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duangpakdee, A.; Laomeephol, C.; Jindatip, D.; Thongnuek, P.; Ratanavaraporn, J.; Damrongsakkul, S. Crosslinked Silk Fibroin/Gelatin/Hyaluronan Blends as Scaffolds for Cell-Based Tissue Engineering. Molecules 2021, 26, 3191. https://doi.org/10.3390/molecules26113191
Duangpakdee A, Laomeephol C, Jindatip D, Thongnuek P, Ratanavaraporn J, Damrongsakkul S. Crosslinked Silk Fibroin/Gelatin/Hyaluronan Blends as Scaffolds for Cell-Based Tissue Engineering. Molecules. 2021; 26(11):3191. https://doi.org/10.3390/molecules26113191
Chicago/Turabian StyleDuangpakdee, Anongnart, Chavee Laomeephol, Depicha Jindatip, Peerapat Thongnuek, Juthamas Ratanavaraporn, and Siriporn Damrongsakkul. 2021. "Crosslinked Silk Fibroin/Gelatin/Hyaluronan Blends as Scaffolds for Cell-Based Tissue Engineering" Molecules 26, no. 11: 3191. https://doi.org/10.3390/molecules26113191






