Antimicrobial Potential of Biosynthesized Silver Nanoparticles by Aaronsohnia factorovskyi Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. The GC-MS of A. factorovskyi Showed Highly Active Antimicrobial Components
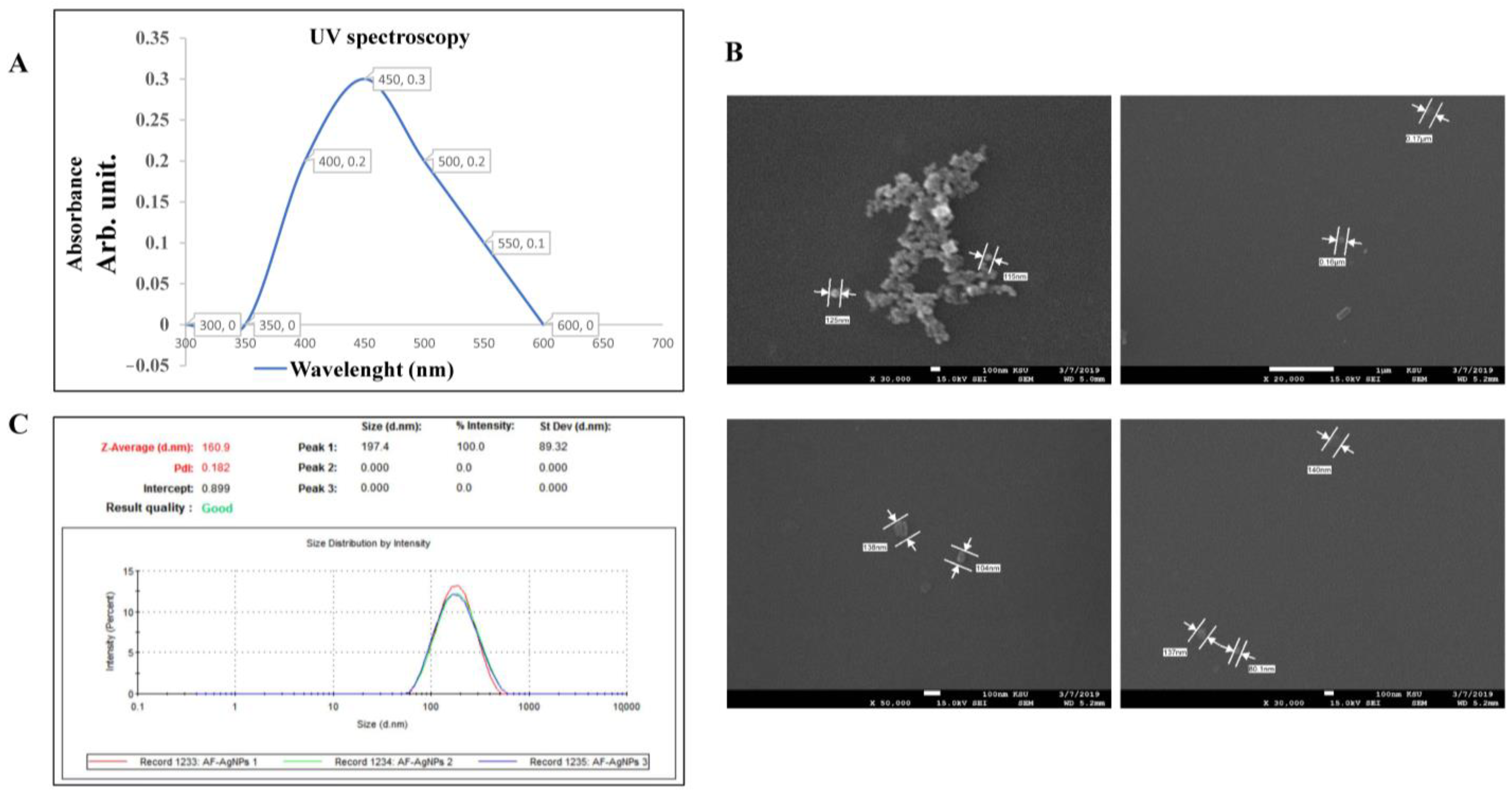
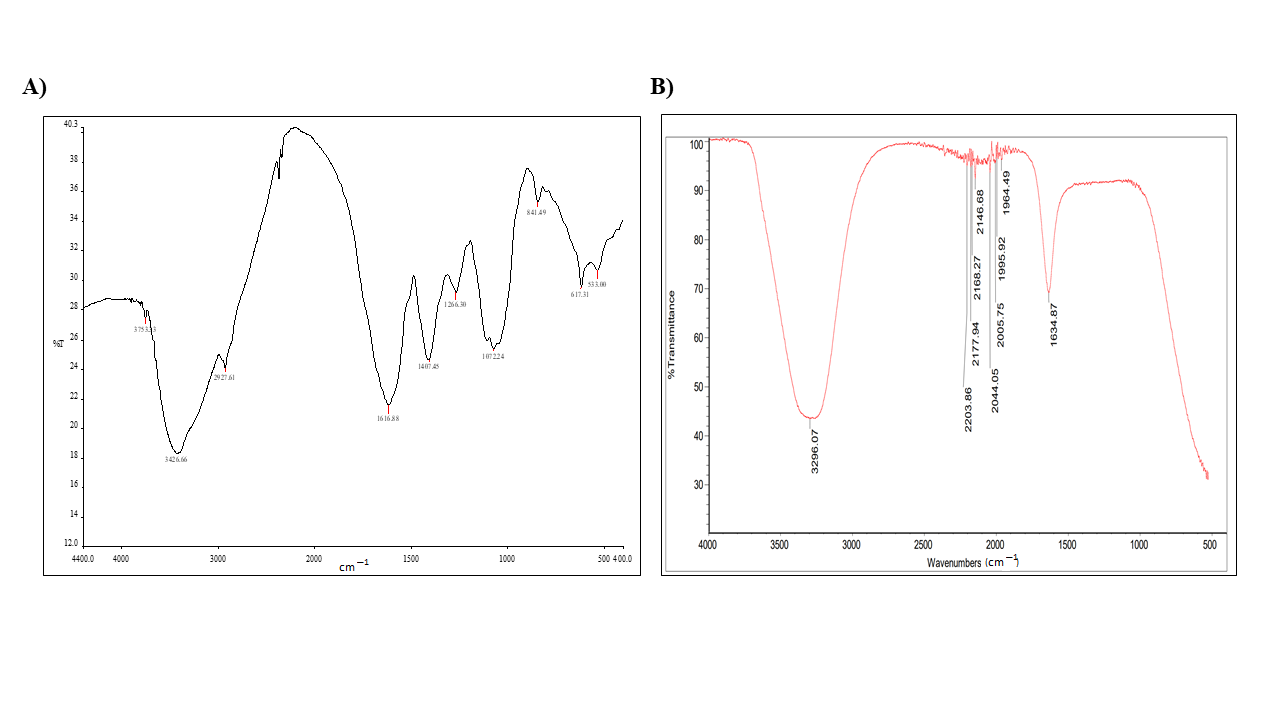
2.2. The Biogenic Properties of A. factorovskyi Silver Nanoparticles (AF-AgNPs)
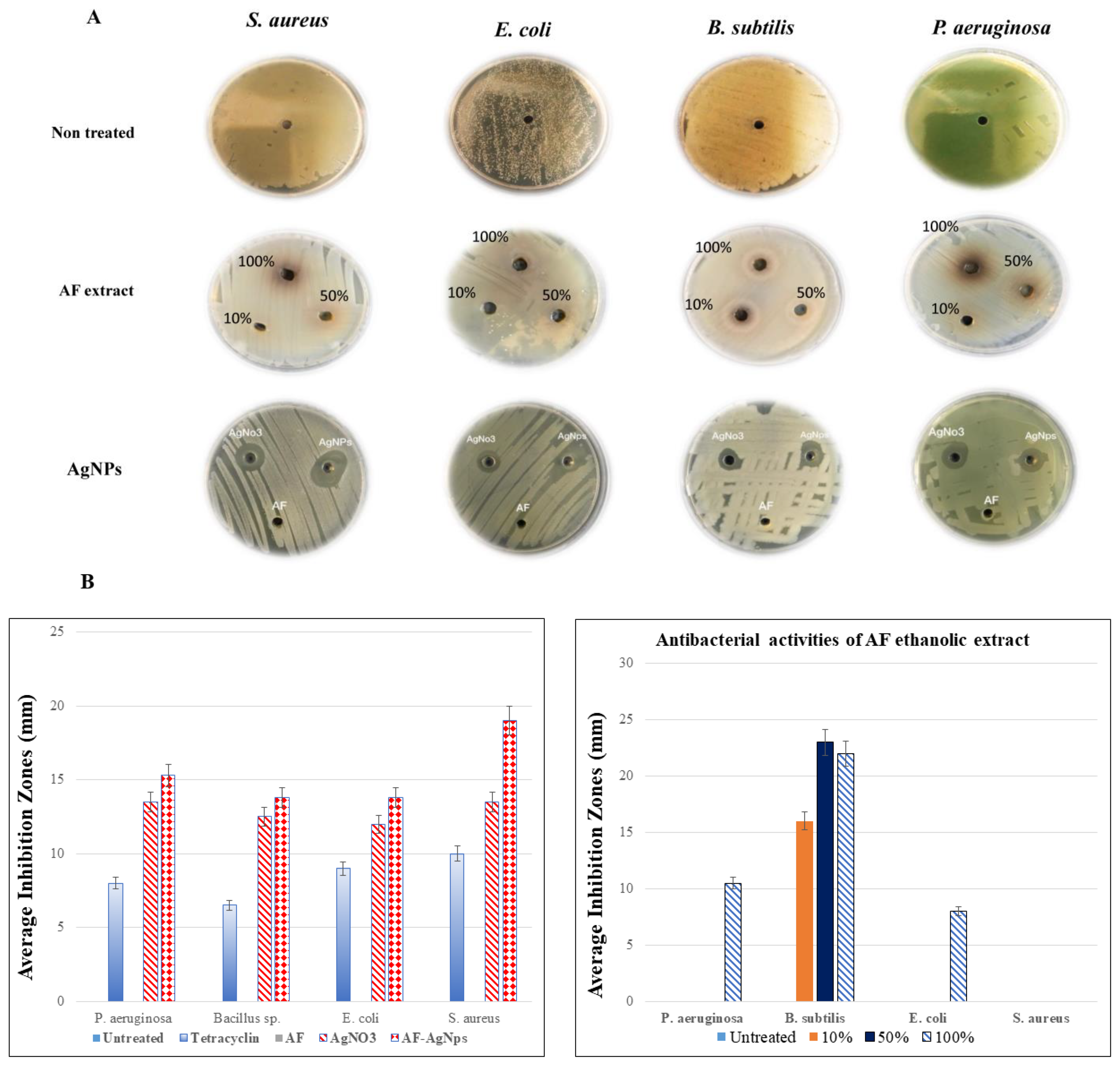
2.3. The Antibacterial Activity of A. factorovskyi Extract and Silver Nanoparticles
2.4. The Antifungal Activity of A. factorovskyi Extracts and Silver Nanoparticles
3. Materials and Method
3.1. Chemicals and Reagents
3.2. Plant Material Collection, Classification, and Preparation of Ethanolic Extract
3.3. Microorganisms
3.4. Preparation and Biogenic Characterization of AF-AgNPs
3.5. The Antibacterial Activity Evaluation
3.6. Determination of Antifungal Activity
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alotibi, F.O.; Ashour, E.H.; Al-Basher, G. Evaluation of the antifungal activity of Rumex vesicarius L. and Ziziphus spina-christi (L) Desf. Aqueous extracts and assessment of the morphological changes induced to certain myco-phytopathogens. Saudi J. Biol. Sci. 2020, 27, 2818–2828. [Google Scholar] [CrossRef] [PubMed]
- Barreda, V.D.; Palazzesi, L.; Tellería, M.C.; Olivero, E.B.; Raine, J.I.; Forest, F. Early evolution of the angiosperm clade Asteraceae in the Cretaceous of Antarctica. Proc. Natl. Acad. Sci. USA 2015, 112, 10989–10994. [Google Scholar] [CrossRef] [Green Version]
- Bader, A.; Abdallah, Q.M.A.; Abdelhady, M.; Tommasi, N.; Malafronte, N.; Shaheen, U.; Bkhaitan, M.M.; Cotugno, R. Cytotoxicity of Some Plants of the Asteraceae Family: Antiproliferative Activity of Psiadia punctulata Root Sesquiterpenes. Rec. Nat. Prod. 2019, 13, 307–315. [Google Scholar] [CrossRef]
- Abalkhil, T.A.; Alharbi, S.A.; Salmen, S.H.; Wainwright, M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotechnol. Biotechnol. Equip. 2017, 31, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Sre, P.R.R.; Reka, M.; Poovazhagi, R.; Kumar, M.A.; Murugesan, K. Antibacterial and cytotoxic effect of biologically synthesized silver nanoparticles using aqueous root extract of Erythrina indica lam. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 135, 1137–1144. [Google Scholar] [CrossRef]
- Mohammed, A.E. Arta (Calligonum Comosum, L’Her.) Shoot Extracts: Bio-Mediator in Silver Nanoparticles Formation and Antimycotic Potential. Nano Biomed. Eng. 2016, 8, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Jha, A.K.; Prasad, K.; Prasad, K.; Kulkarni, A.R. Plant system: Nature’s nanofactory. Colloids Surf. B 2009, 73, 219–223. [Google Scholar] [CrossRef]
- Ouerghemmi, I.; Rebey, I.B.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Tounsi, M.S. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown Tunisian Ruta chalepensis. J. Food Drug. Anal. 2017, 25, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules 2019, 24, 3770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.D.; Rao, G.B.; Bommaka, M.K.; Raghavendra, N.M.; Aleti, S. Eco-sustainable synthesis and biological evaluation of 2-phenyl 1,3-benzodioxole derivatives as anticancer, DNA binding and antibacterial agents. Arab. J. Chem. 2016, 9, S1875–S1883. [Google Scholar] [CrossRef] [Green Version]
- Tataringa, G.; Zbancioc, A.M. Coumarin Derivatives with Antimicrobial and Antioxidant Activities. In Phytochemicals in Human Health; Rao, V., Mans, D., Rao, L., Eds.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/books/phytochemicals-in-human-health/coumarin-derivatives-with-antimicrobial-and-antioxidant-activities (accessed on 7 June 2020). [CrossRef] [Green Version]
- McCann, M.; Curran, R.; Ben-Shoshan, M.; McKee, V.; Tahir, A.A.; Devereux, M.; Kavanagh, K.; Creaven, B.S.; Kellett, A. Silver(I) complexes of 9-anthracenecarboxylic acid and imidazoles: Synthesis, structure and antimicrobial activity. Dalton Trans. 2012, 7, 6516–6527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curran, R.; Lenehan, J.; McCann, M.; Kavanagh, K.; Devereux, M.; Egan, D.A.; Clifford, G.; Keane, K.; Creaven, B.S.; McKee, V. [Ag2(aca)2]n and [Ag4(aca)4(NH3)2] (acaH=9-anthracenecarboxylic acid): Synthesis, X-ray crystal structures, antimicrobial and anti-cancer activities. Inorg. Chem. Commun. 2007, 10, 1149–1153. [Google Scholar] [CrossRef] [Green Version]
- Usman, H.; Abdulrahman, F.I.; Ahmed, L.A.; Kaita, A.H.; Khan, I.Z. Antibacterial effects of cyanogenic glucoside isolated from the stem bark of Bauhinia rufescens Lam. Int. J. Biol. Chem. Sci. 2013, 7, 2139–2150. [Google Scholar] [CrossRef] [Green Version]
- Tagousop, C.N.; Tamokou, J.D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Alter. Med. 2018, 18, 252. [Google Scholar] [CrossRef]
- Jemal, K.; Sandeep, B.V.; Pola, S. Synthesis, Characterization, and Evaluation of the Antibacterial Activity of Allophylus serratus Leaf and Leaf Derived Callus Extracts Mediated Silver Nanoparticles. J. Nanomater. 2017, 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Rautela, A.; Rani, J.; Debnath (Das), M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: Characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Dadashpour, M.; Firouzi-Amandi, A.; Pourhassan-Moghaddam, M.; Maleki, M.J.; Soozangar, N.; Jeddi, F.; Nouri, M.; Zarghami, N.; Pilehvar-Soltanahmadi, Y. Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Mater. Sci. Eng. C 2018, 92, 902–912. [Google Scholar] [CrossRef]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PloS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Li, F.; Rong, M.; Lin, L.; Yao, Q.; Huang, Y.; Chen, X.; Wang, X. Chapter 1 – Introduction. In Novel Nanomaterials for Biomedical, Environmental and Energy Applications, Micro and Nano Technologies; Wang, X., Chen, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–36. [Google Scholar] [CrossRef]
- Sadeghi, B.; Gholamhoseinpoor, F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2015, 134, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Hameed, R.H.; Mohammed, G.J.; Hameed, I.H. Matricaria chamonbmilla: Bioactive Compounds of Methanolic Fruit Extract Using GC-MS and FTIR Techniques and Determination of its Antimicrobial Properties. Indian J. Public Health Res. Dev. 2018, 9, 223–228. [Google Scholar] [CrossRef]
- Rostami-Vartooni, A.; Moradi-Saadatmand, A.; Bagherzadeh, M. Green synthesis of Ag/Fe 3 O 4 /ZrO 2 nanocomposite using aqueous Centaurea cyanus flower extract and its catalytic application for reduction of organic pollutants. Iran. J. Catal. 2019, 9, 27–35. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Christenson, J.C.; Korgenski, E.K.; Relich, R.F. Laboratory Diagnosis of Infection Due to Bacteria, Fungi, Parasites, and Rickettsiae. In Principles and Practice of Pediatric Infectious Diseases, 5th ed.; Long, S.S., Prober, C.G., Fischer, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1422–1434.e3. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Ekennia, A.C.; Katata-Seru, L. Biosynthesis, characterization, and antimicrobial effect of silver nanoparticles obtained using Lavandula × intermedia. Res. Chem. Intermed. 2017, 43, 1383–1394. [Google Scholar] [CrossRef]
- Salman, H.D. Evaluation and Comparison the Antibacterial Activity of Silver Nano Particles (AgNPs) and Silver Nitrate (AgNO3) on Some Pathogenic Bacteria. J. Glob. Pharma Technol. 2017, 9, 238–248. [Google Scholar]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [Green Version]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef] [Green Version]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.; Hossain, K.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2017, 9, 1–16. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Metrouh-Amir, H.; Duarte, C.M.M.; Maiza, F. Solvent effect on total phenolic contents, antioxidant, and antibacterial activities of Matricaria pubescens. Ind. Crops. Prod. 2015, 67, 249–256. [Google Scholar] [CrossRef]
- Göger, G.; Demirci, B.; Ilgın, S.; Demirci, F. Antimicrobial and toxicity profiles evaluation of the Chamomile (Matricaria recutita L.) essential oil combination with standard antimicrobial agents. Ind. Crops. Prod. 2018, 120, 279–285. [Google Scholar] [CrossRef]
- Elgorban, A.M.; El-Samawaty, A.M.; Yassin, M.A.; Sayed, S.R.; Adil, S.F.; Elhindi, K.M.; Bakri, M.; Khan, M. Antifungal silver nanoparticles: Synthesis, characterization and biological evaluation. Biotechnol. Biotechnol. Equip. 2016, 30, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Yan, M.; Duan, H.; Bi, Y.; Cheng, X.; Yu, H. Synergistic antifungal activity of green synthesized silver nanoparticles and epoxiconazole against Setosphaeria turcica. J. Nanomater. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.-H.; Ma, Q.-H.; Li, S.-Y.; Zhang, D.-Q.; Cong, L.; Tian, Y.-L.; Yang, R.-Y. The antifungal effect of silver nanoparticles on Trichosporon asahii. J. Microbiol. Immunol. Infect. 2016, 49, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Naik, K.; Kowshik, M. The silver lining: Towards the responsible and limited usage of silver. J. Appl. Microbiol. 2017, 123, 1068–1087. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Jung, J.H.; Lamsal, K.; Kim, Y.S.; Min, J.S.; Lee, Y.S. Antifungal Effects of Silver Nanoparticles (AgNPs) against Various Plant Pathogenic Fungi. Mycobiology 2012, 40, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Henam, S.; Ahmad, F.; Shah, M.A.; Parveen, S.; Wani, A. Microwave synthesis of nanoparticles and their antifungal activities. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 213, 337–341. [Google Scholar] [CrossRef]
- Das, S.; Horváth, B.; Šafranko, S.; Jokić, S.; Széchenyi, A.; Kőszegi, T. Antimicrobial Activity of Chamomile Essential Oil: Effect of Different Formulations. Molecules. 2019, 24, 4321. [Google Scholar] [CrossRef] [Green Version]
- Painuli, S.; Rai, N.; Kumar, N. Gas chromatography and mass spectrometry analysis of methanolic extract of leaves of Rhododendron arboreum. Asian J. Pharm. Clin. Res. 2016, 9, 101–104. [Google Scholar]
- Mladenova, B.; Diankov, S.; Karsheva, M.; Stankov, S.; Hinkov, I. Plant mediated synthesis of silver nanoparticles using extracts from Tilia cordata, Matricaria chamomilla, Calendula officinalis and Lavandula angustifolia flowers. J. Chem. Technol. Metall. 2018, 53, 623–630. [Google Scholar]
- Pingale, S.S.; Rupanar, S.V.; Chaskar, M.G. Plant- mediated biosynthesis of Silver nanoparticles from Gymnema sylvestre and their use in phtodegradation of Methyl orange dye. J. Water Environ. Nanotechnol. 2018, 3, 106–115. [Google Scholar] [CrossRef]
- Owuama, C.I. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using a novel dilution tube method. Afr. J. Microbiol. Res. 2017, 11, 977–980. [Google Scholar] [CrossRef] [Green Version]
- Andleeba, S.; Alsalmeb, A.; Al-Zaqrib, N.; Waradd, I.; Alkahtanib, J.; Bukhari, S.M. In-vitro antibacterial and antifungal properties of the organic solvent extract of Argemone mexicana L. J. King Saud Univ. Sci. 2020, 32, 2053–2058. [Google Scholar] [CrossRef]


| Phenolic Compound | Formula | Chemical Structure | Molecular Weight | MS Fragments (m/z) | Relative Intensities% |
|---|---|---|---|---|---|
| Cyanogenic glycosides (2’-Epimer) | C16H19NO8 | 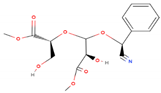 | 353.327 | 353.00 | 13.78 |
| 1,3-benzodioxol-4-ol | C7H6O3 | 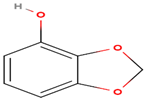 | 138.122 | 138.00 | 43.51 |
| Valeric acid | C5H10O2 | 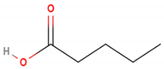 | 102.133 | 102.00 | 48.46 |
| 9-Anthracenecarboxylic acid | C15H10O2 | 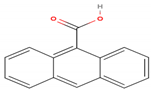 | 222.243 | 222.00 | 13.97 |
| Coumarin | C9H6O2 |  | 146.145 | 146.00 | 36.22 |
| Tested Material | Absorption (cm−1) | Appearance | Group | Compound Class |
|---|---|---|---|---|
| AF extract | 3753 | Medium, sharp | O-H stretching | Alcohol |
| 3426 | Strong, broad | O-H stretching | Alcohol | |
| 2927 | Weak, broad | O-H stretching | Alcohol | |
| 1616 | Strong | C=C stretching | α, β-unsaturated ketone | |
| 1407 | Medium | O-H bending | Carboxylic acid | |
| 1266 | Strong | C-N stretching | Aromatic amine | |
| 1072 | None | |||
| 841 | Strong | C-Cl stretching | Halo compound | |
| 617 | Strong | C-Br stretching | Halo compound | |
| 533 | Strong | C-I stretching | Halo compound | |
| AF-AgNPs | 3269 | Strong, broad | O-H stretching | Carboxylic acid |
| 2203 | Weak | CΞC stretching | Alkyne | |
| 2177, 2168 | Strong | S-CΞN stretching | Thiocyanate | |
| 2146 | Weak | CΞC stretching | Alkyne | |
| 1995, 1964 | Weak | C-H bending | Aromatic compound | |
| 2044, 2005 | Strong | N=C=S stretching | Isothiocyanate | |
| 1634 | Medium | C=C stretching | Alkene |
| A. factorovskyi Concentration of the Ethanolic Extract | S. aureus | E. coli | B. subtilis | P. aeruginosa |
|---|---|---|---|---|
| 10% | - | - | 16 | - |
| 50% | - | - | 23 | - |
| 100% | - | 8 | 22 | 10.5 |
| Treatment | S. aureus | E. coli | B. subtilis | P. aeruginosa |
|---|---|---|---|---|
| Untreated | - | - | - | - |
| Tetracycline | 8.00 ± 0.98 | 6.50 ± 0.15 | 9.00 ± 1.90 | 10 ± 0.66 |
| A. factorovskyi | - | - | - | - |
| AgNO3 | 14.17 ± 0.76 | 12.00 ± 0.00 | 12.50 ± 1.50 | 13.50 ± 0.50 |
| AF-AgNPs | 19.00 ± 2.94 | 13.83 ± 0.85 | 13.83 ± 1.43 | 15.33 ± 0.47 |
| Treatment | F. solani | H.rostratum | A. alternata | F. oxysporum |
|---|---|---|---|---|
| Untreated | 8.80 ± 0.00 | 8.80 ± 0.00 | 8.80 ± 0.00 | 8.80 ± 0.00 |
| Fluconazole | 6.00 ± 0.01 | 1.00 ± 0.50 * | 1.80 ± 0.10 * | 1.50 ± 0.83 * |
| A. factorovskyi | 4.50 ± 0.00 | 6.25 ± 0.00 | 7.10 ± 0.10 | 5.40 ± 0.00 |
| AgNO3 | 8.00 ± 0.01 | 5.00 ± 0.09 | 5.00 ± 0.22 | 2.40 ± 0.08 * |
| AF-AgNPs | 1.50 ± 0.00 * | 1.00 ± 0.00 * | 1.35 ± 0.15 * | 2.00 ± 0.00 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Otibi, F.; Al-Ahaidib, R.A.; Alharbi, R.I.; Al-Otaibi, R.M.; Albasher, G. Antimicrobial Potential of Biosynthesized Silver Nanoparticles by Aaronsohnia factorovskyi Extract. Molecules 2021, 26, 130. https://doi.org/10.3390/molecules26010130
Al-Otibi F, Al-Ahaidib RA, Alharbi RI, Al-Otaibi RM, Albasher G. Antimicrobial Potential of Biosynthesized Silver Nanoparticles by Aaronsohnia factorovskyi Extract. Molecules. 2021; 26(1):130. https://doi.org/10.3390/molecules26010130
Chicago/Turabian StyleAl-Otibi, Fatimah, Reem A. Al-Ahaidib, Raedah I. Alharbi, Rana M. Al-Otaibi, and Gadah Albasher. 2021. "Antimicrobial Potential of Biosynthesized Silver Nanoparticles by Aaronsohnia factorovskyi Extract" Molecules 26, no. 1: 130. https://doi.org/10.3390/molecules26010130






