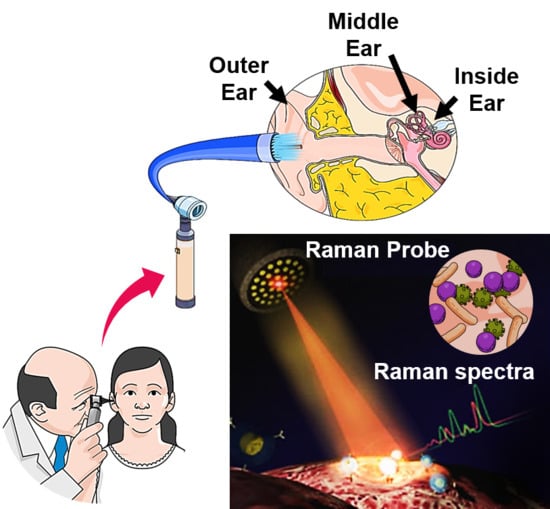
Optical Identification of Middle Ear Infection
Abstract
:1. Introduction
2. Clinical Imaging Techniques for the Identification of Otitis Media
3. Preclinical Imaging Techniques for the Identification of Otitis Media
4. Clinical Trials Using the Optical Imaging Techniques
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nguyen, C.T.; Jung, W.; Kim, J.; Chaney, E.J.; Novak, M.; Stewart, C.N.; Boppart, S.A. Noninvasive in vivo optical detection of biofilm in the human middle ear. Proc. Natl. Acad. Sci. USA 2012, 109, 9529–9534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, J.O. Otitis media. Clin. Infect. Dis. 1994, 19, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Rosenfeld, R.M.; Zeisel, S.A. Otitis media and speech and language: A meta-analysis of prospective studies. Pediatrics 2004, 113, e238–e248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, J.O. The burden of otitis media. Vaccine 2000, 19, S2–S8. [Google Scholar] [CrossRef]
- Harmes, K.; Blackwood, R.A.; Burrows, H.; Cooke, J.M.; Van Harrison, R.; Passamani, P. Otitis media: Diagnosis and treatment. Am. Fam. Physician 2013, 88, 435–440. [Google Scholar]
- Heikkinen, T.; Thint, M.; Chonmaitree, T. Prevalence of various respiratory viruses in the middle ear during acute otitis media. N. Engl. J. Med. 1999, 340, 260–264. [Google Scholar] [CrossRef]
- Stool, S.E.; Field, M.J. The impact of otitis media. Pediatric Infect. Dis. J. 1989, 8, S11–S14. [Google Scholar] [CrossRef]
- Ahmed, S.; Shapiro, N.L.; Bhattacharyya, N. Incremental health care utilization and costs for acute otitis media in children. Laryngoscope 2014, 124, 301–305. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, J.; Li, T.; Svanberg, S.; Svanberg, K. Optical detection of middle ear infection using spectroscopic techniques: Phantom experiments. J. Biomed. Opt. 2015, 20, 057001. [Google Scholar] [CrossRef]
- Bluestone, C.D.; Klein, J.O. Otitis Media in Infants and Children; PMPH: North Carolona, USA, 2007. [Google Scholar]
- Dicks, L.; Knoetze, H.; Van Reenen, C. Otitis media: A review, with a focus on alternative treatments. Probiotics Antimicrob. Proteins 2009, 1, 45–59. [Google Scholar] [CrossRef]
- Maxson, S.; Yamauchi, T. Acute otitis media. Pediatrics Rev. 1996, 17, 191. [Google Scholar] [CrossRef]
- Kubba, H.; Pearson, J.; Birchall, J. The aetiology of otitis media with effusion: A review. Clin. Otolaryngol. Allied Sci. 2000, 25, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Lieberthal, A.S.; Carroll, A.E.; Chonmaitree, T.; Ganiats, T.G.; Hoberman, A.; Jackson, M.A.; Joffe, M.D.; Miller, D.T.; Rosenfeld, R.M.; Sevilla, X.D. The diagnosis and management of acute otitis media. Pediatrics 2013, 131, e964–e999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonnaer, E.L.; Graamans, K.; Sanders, E.A.; Curfs, J.H. Advances in understanding the pathogenesis of pneumococcal otitis media. Pediatric Infect. Dis. J. 2006, 25, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.D.; Hong, W.; Dew, K.E.; Winn, D.R.; Pang, B.; Watt, J.; Glover, D.T.; Hollingshead, S.K.; Swords, W.E. Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J. Infect. Dis. 2009, 199, 786–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappelletty, D. Microbiology of bacterial respiratory infections. Pediatric Infect. Dis. J. 1998, 17, S55–S61. [Google Scholar] [CrossRef]
- Del Beccaro, M.A.; Mendelman, P.M.; Inglis, A.F.; Richardson, M.A.; Duncan, N.O.; Clausen, C.R.; Stull, T.L. Bacteriology of acute otitis media: A new perspective. J. Pediatrics 1992, 120, 81–84. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Hu, F.Z.; Gieseke, A.; Nistico, L.; Nguyen, D.; Hayes, J.; Forbes, M.; Greenberg, D.P.; Dice, B.; Burrows, A. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 2006, 296, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Ayala, O.D.; Wakeman, C.A.; Skaar, E.P.; Mahadevan-Jansen, A. Identification of bacteria causing acute otitis media using Raman microspectroscopy. In Biomedical Vibrational Spectroscopy 2016: Advances in Research and Industry; International Society for Optics and Photonics; San Francisco, CA, USA, 2016; p. 97040U. [Google Scholar]
- Holm, M.M.; Vanlerberg, S.L.; Sledjeski, D.D.; Lafontaine, E.R. The Hag protein of Moraxella catarrhalis strain O35E is associated with adherence to human lung and middle ear cells. Infect. Immun. 2003, 71, 4977–4984. [Google Scholar] [CrossRef] [Green Version]
- Ludman, H.S.; Bradley, P.J. ABC of Ear, Nose and Throat; John Wiley & Sons: Hoboken, NJ, USA, 2012; Volume 254. [Google Scholar]
- Mittal, R.; Lisi, C.V.; Gerring, R.; Mittal, J.; Mathee, K.; Narasimhan, G.; Azad, R.K.; Yao, Q.; Grati, M.H.; Yan, D. Current concepts in the pathogenesis and treatment of chronic suppurative otitis media. J. Med. Microbiol. 2015, 64, 1103. [Google Scholar] [CrossRef]
- Monroy, G.L.; Shelton, R.L.; Nolan, R.M.; Nguyen, C.T.; Novak, M.A.; Hill, M.C.; McCormick, D.T.; Boppart, S.A. Noninvasive depth-resolved optical measurements of the tympanic membrane and middle ear for differentiating otitis media. Laryngoscope 2015, 125, E276–E282. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.S.; Kaleida, P.H. How helpful is pneumatic otoscopy in improving diagnostic accuracy? Pediatrics 2003, 112, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Moshtaghi, O.; Sahyouni, R.; Haidar, Y.M.; Huang, M.; Moshtaghi, A.; Ghavami, Y.; Lin, H.W.; Djalilian, H.R. Smartphone-enabled otoscopy in neurotology/otology. Otolaryngol. Head Neck Surg. 2017, 156, 554–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhavana, K.; Ahmad, M.; Sharma, P. Smartphone Otoscopy Sans Attachment: A Paradigm Shift in Diagnosing Ear Pathologies. OTO Open 2018, 2, 2473974X18786496. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Oh, S.J.; Cheon, H. Potential clinical applications of terahertz radiation. J. Appl. Phys. 2019, 125, 190901. [Google Scholar] [CrossRef]
- D’Arco, A.; Di Fabrizio, M.; Dolci, V.; Petrarca, M.; Lupi, S. THz Pulsed Imaging in Biomedical Applications. Condens. Matter 2020, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Reid, C.B.; Fitzgerald, A.; Reese, G.; Goldin, R.; Tekkis, P.; O’Kelly, P.; Pickwell-MacPherson, E.; Gibson, A.P.; Wallace, V.P. Terahertz pulsed imaging of freshly excised human colonic tissues. Phys. Med. Biol. 2011, 56, 4333. [Google Scholar] [CrossRef] [Green Version]
- Teppo, H.; Revonta, M.; Lindén, H.; Palmu, A. Detection of middle-ear fluid in children with spectral gradient acoustic reflectometry: A screening tool for nurses? Scand. J. Prim. Health Care 2006, 24, 88–92. [Google Scholar] [CrossRef]
- Shekelle, P.; Takata, G.; Chan, L.S.; Mangione-Smith, R.; Corley, P.M.; Morphew, T.; Morton, S. Diagnosis, natural history, and late effects of otitis media with effusion. Evid. Rep./Technol. Assess. 2002, 55, 1. [Google Scholar]
- Palmu, A.; Puhakka, H.; Rahko, T.; Takala, A.K. Diagnostic value of tympanometry in infants in clinical practice. Int. J. Pediatric Otorhinolaryngol. 1999, 49, 207–213. [Google Scholar] [CrossRef]
- Watters, G.; Jones, J.; Freeland, A. The predictive value of tympanometry in the diagnosis of middle ear effusion. Clin. Otolaryngol. Allied Sci. 1997, 22, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Sundvall, P.D.; Papachristodoulou, C.E.; Nordeman, L. Diagnostic methods for acute otitis media in 1 to 12 year old children: A cross sectional study in primary health care. BMC Fam. Pract. 2019, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Tu, H.; Chaney, E.J.; Stewart, C.N.; Boppart, S.A. Non-invasive optical interferometry for the assessment of biofilm growth in the middle ear. Biomed. Opt. Express 2010, 1, 1104–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evers, D.J.; Nachabé, R.; Klomp, H.M.; van Sandick, J.W.; Wouters, M.W.; Lucassen, G.W.; Hendriks, B.H.; Wesseling, J.; Ruers, T.J. Diffuse reflectance spectroscopy: A new guidance tool for improvement of biopsy procedures in lung malignancies. Clin. Lung Cancer 2012, 13, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, J.; Nisha, G.; Manju, S.; Philip, E.; Jeemon, P.; Baiju, K.; Beena, V.; Subhash, N. Diffuse reflectance spectroscopy: Diagnostic accuracy of a non-invasive screening technique for early detection of malignant changes in the oral cavity. BMJ Open 2011, 1, e000071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucchi, N.W.; Demas, A.; Narayanan, J.; Sumari, D.; Kabanywanyi, A.; Kachur, S.P.; Barnwell, J.W.; Udhayakumar, V. Real-time fluorescence loop mediated isothermal amplification for the diagnosis of malaria. PLoS ONE 2010, 5, e13733. [Google Scholar] [CrossRef] [Green Version]
- Preciado, D.; Nolan, R.M.; Joshi, R.; Krakovsky, G.M.; Zhang, A.; Pudik, N.A.; Kumar, N.K.; Shelton, R.L.; Boppart, S.A.; Bauman, N.M. Otitis Media Middle Ear Effusion Identification and Characterization Using an Optical Coherence Tomography Otoscope. Otolaryngol. Head Neck Surg. 2020, 162, 367–374. [Google Scholar] [CrossRef]
- Große-Stoltenberg, A.; Hellmann, C.; Werner, C.; Oldeland, J.; Thiele, J. Evaluation of continuous VNIR-SWIR spectra versus narrowband hyperspectral indices to discriminate the invasive Acacia longifolia within a Mediterranean dune ecosystem. Remote Sens. 2016, 8, 334. [Google Scholar] [CrossRef] [Green Version]
- Gerger, A.; Koller, S.; Kern, T.; Massone, C.; Steiger, K.; Richtig, E.; Kerl, H.; Smolle, J. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J. Investig. Dermatol. 2005, 124, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Gerger, A.; Koller, S.; Weger, W.; Richtig, E.; Kerl, H.; Samonigg, H.; Krippl, P.; Smolle, J. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer 2006, 107, 193–200. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S.; Cavagna, R.; Bosis, S.; Droghetti, R.; Faelli, N.; Tosi, S.; Begliatti, E.; Group, S.S. Recurrent respiratory tract infections in pediatric age: A population-based survey of the therapeutic role of macrolides. J. Chemother. 2003, 15, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Nazzari, E.; Torretta, S.; Pignataro, L.; Marchisio, P.; Esposito, S. Role of biofilm in children with recurrent upper respiratory tract infections. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Sabharwal, V.; Okonkwo, O.S.; Shlykova, N.; Tong, R.; Lin, L.Y.; Wang, W.; Guo, S.; Rosowski, J.J.; Pelton, S.I. Treatment of otitis media by transtympanic delivery of antibiotics. Sci. Transl. Med. 2016, 8, ra120–ra356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.B.; Moon, I.S.; Bark, H.S.; Kim, S.H.; Park, D.W.; Noh, S.K.; Huh, Y.M.; Suh, J.S.; Oh, S.J.; Jeon, T.I. Terahertz otoscope and potential for diagnosing otitis media. Biomed. Opt. Express 2016, 7, 1201–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, E.; Kesser, B.W.; Peirce-Cottler, S.; Keeley, M. Development and validation of a novel ear simulator to teach pneumatic otoscopy. Simul. Healthc. 2012, 7, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, R.; Won, J.; Monroy, G.L.; Hill, M.C.; Porter, R.G.; Novak, M.A.; Boppart, S.A. In vivo detection of nanometer-scale structural changes of the human tympanic membrane in otitis media. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Chaichi, A.; Prasad, A.; Gartia, M. Raman Spectroscopy and Microscopy Applications in Cardiovascular Diseases: From Molecules to Organs. Biosensors 2018, 8, 107. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, R.M.; Goodacre, R. Discrimination of bacteria using surface-enhanced Raman spectroscopy. Anal. Chem. 2004, 76, 40–47. [Google Scholar] [CrossRef]
- Goodacre, R.; Timmins éadaoin, M.; Burton, R.; Kaderbhai, N.; Woodward, A.M.; Kell, D.B.; Rooney, P.J. Rapid identification of urinary tract infection bacteria using hyperspectral whole-organism fingerprinting and artificial neural networks. Microbiology 1998, 144, 1157–1170. [Google Scholar] [CrossRef] [Green Version]
- Maquelin, K.; Choo-Smith, L.P.I.; van Vreeswijk, T.; Endtz, H.P.; Smith, B.; Bennett, R.; Bruining, H.A.; Puppels, G.J. Raman spectroscopic method for identification of clinically relevant microorganisms growing on solid culture medium. Anal. Chem. 2000, 72, 12–19. [Google Scholar] [CrossRef]
- Schuster, K.C.; Reese, I.; Urlaub, E.; Gapes, J.R.; Lendl, B. Multidimensional information on the chemical composition of single bacterial cells by confocal Raman microspectroscopy. Anal. Chem. 2000, 72, 5529–5534. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Paidi, S.K.; Kang, J.W.; Spegazzini, N.; Dasari, R.R.; Valdez, T.A.; Barman, I. Discerning the differential molecular pathology of proliferative middle ear lesions using Raman spectroscopy. Sci. Rep. 2015, 5, 13305. [Google Scholar] [CrossRef] [PubMed]
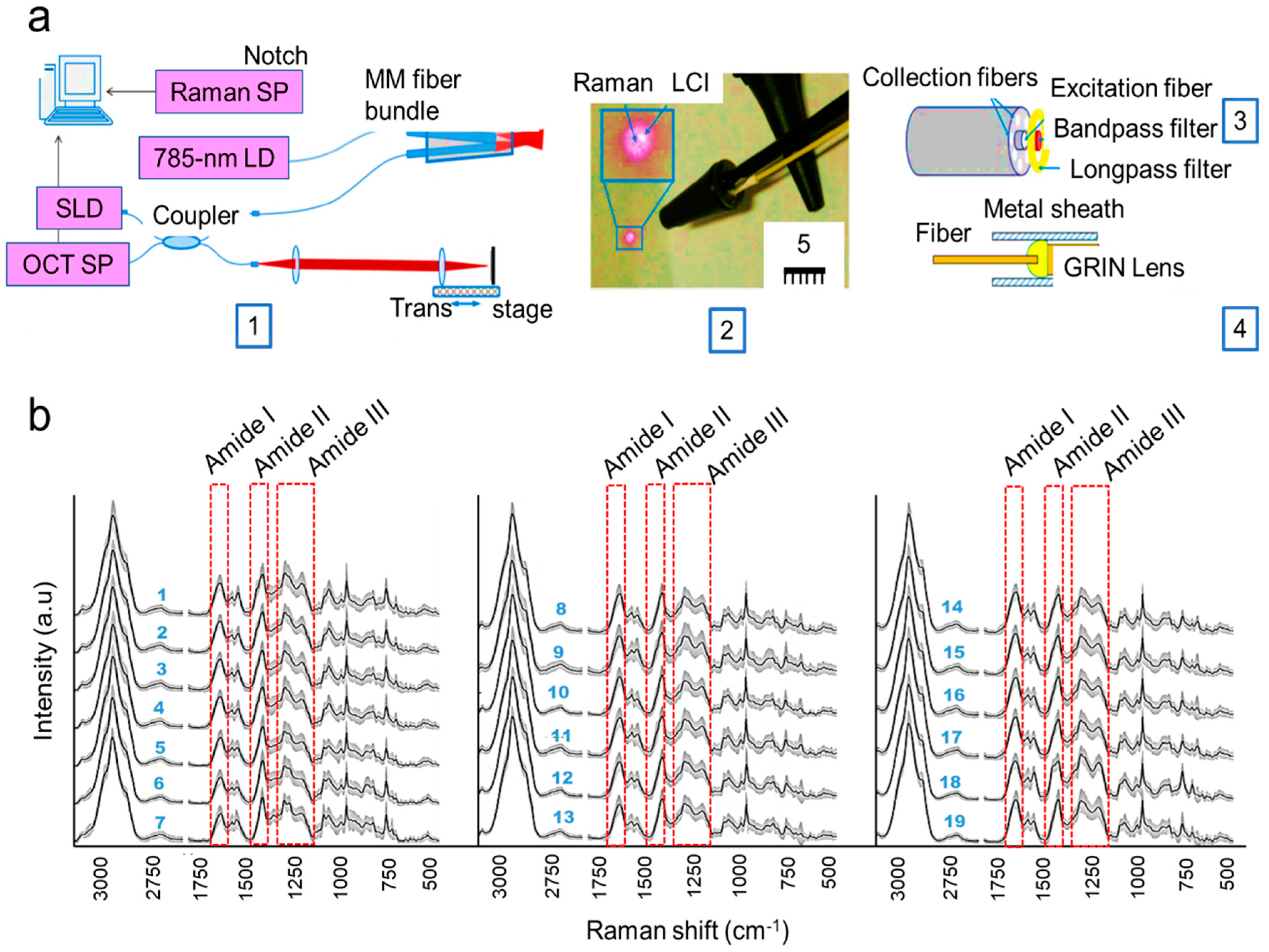
- Zhao, Y.; Monroy, G.L.; You, S.; Shelton, R.L.; Nolan, R.M.; Tu, H.; Chaney, E.J.; Boppart, S.A. Rapid diagnosis and differentiation of microbial pathogens in otitis media with a combined Raman spectroscopy and low-coherence interferometry probe: Toward in vivo implementation. J. Biomed. Opt. 2016, 21, 107005. [Google Scholar] [CrossRef] [PubMed]
- Rebrošová, K.; Šiler, M.; Samek, O.; Růžička, F.; Bernatová, S.; Holá, V.; Ježek, J.; Zemánek, P.; Sokolová, J.; Petráš, P. Rapid identification of staphylococci by Raman spectroscopy. Scientific reports 2017, 7, 14846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, G.B.; Nam, S.W.; Choi, S.; Lee, G.J.; Park, H.K. Evaluation of antibiotic effects on Pseudomonas aeruginosa biofilm using Raman spectroscopy and multivariate analysis. Biomed. Opt. Express 2014, 5, 3238–3251. [Google Scholar] [CrossRef]
- Kamińska, A.; Witkowska, E.; Kowalska, A.; Skoczyńska, A.; Ronkiewicz, P.; Szymborski, T.; Waluk, J. Rapid detection and identification of bacterial meningitis pathogens in ex vivo clinical samples by SERS method and principal component analysis. Anal. Methods 2016, 8, 4521–4529. [Google Scholar] [CrossRef] [Green Version]
- Pahlow, S.; Stöckel, S.; Pollok, S.; Cialla-May, D.; Rösch, P.; Weber, K.; Popp, J.R. Rapid identification of Pseudomonas spp. via Raman spectroscopy using pyoverdine as capture probe. Anal. Chem. 2016, 88, 1570–1577. [Google Scholar] [CrossRef]
- Dostál, L.; Misselwitz, R.; Laettig, S.; Alonso, J.C.; Welfle, H. Raman spectroscopy of regulatory protein Omega from Streptococcus pyogenes plasmid pSM19035 and complexes with operator DNA. J. Spectrosc. 2003, 17, 435–445. [Google Scholar] [CrossRef] [Green Version]
- Khalid, M.; Bora, T.; Al Ghaithi, A.; Thukral, S.; Dutta, J. Raman spectroscopy detects changes in bone mineral quality and collagen cross-linkage in staphylococcus infected human bone. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Sil, S.; Mukherjee, R.; Kumar, N.; Aravind, S.; Kingston, J.; Singh, U. Detection and classification of bacteria using Raman spectroscopy combined with multivariate analysis. Def. Life Sci. J. 2017, 2, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Meisel, S.; Stöckel, S.; Rösch, P.; Popp, J. Identification of meat-associated pathogens via Raman microspectroscopy. Food Microbiol. 2014, 38, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Hamasha, K.M. Raman Spectroscopy for the Microbiological Characterization and Identification of Medically Relevant Bacteria; Digital Commons@WayneState: Detroit, MI, USA, 2011. [Google Scholar]
- Pandey, R.; Valdez, T.A. Chemical Imaging in Middle Ear Pathology: Quo Vadis? J. Postdr. Res. Jan. 2016, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Ayala, O.D.; Wakeman, C.A.; Pence, I.J.; Gaddy, J.A.; Slaughter, J.C.; Skaar, E.P.; Mahadevan-Jansen, A. Drug-resistant Staphylococcus aureus strains reveal distinct biochemical features with Raman microspectroscopy. ACS Infect. Dis. 2018, 4, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Boppart, S.A.; Shelton, R.L. Handheld Device for Identification of Microbiological Constituents in the Middle Ear. U.S. Patent Application No. 16/012,201, 25 October 2018. [Google Scholar]
- Sundberg, M.; Peebo, M.; Öberg, P.Å.; Lundquist, P.G.; Strömberg, T. Diffuse reflectance spectroscopy of the human tympanic membrane in otitis media. Physiol. Meas. 2004, 25, 1473. [Google Scholar] [CrossRef] [PubMed]
- Keefe, D.H. Acoustical transmission-line model of the middle-ear cavities and mastoid air cells. J. Acoust. Soc. Am. 2015, 137, 1877–1887. [Google Scholar] [CrossRef] [Green Version]
- Ars, B.; Dirckx, J.; Ars-Piret, N.; Buytaert, J. Insights in the physiology of the human mastoid: Message to the surgeon. J. Int. Adv. Otol. 2012, 8, 296. [Google Scholar]
- Hu, L.; Li, W.; Lin, H.; Li, Y.; Zhang, H.; Svanberg, K.; Svanberg, S. Towards an optical diagnostic system for otitis media using a combination of otoscopy and spectroscopy. J. Biophotonics 2019, 12, e201800305. [Google Scholar] [CrossRef] [Green Version]
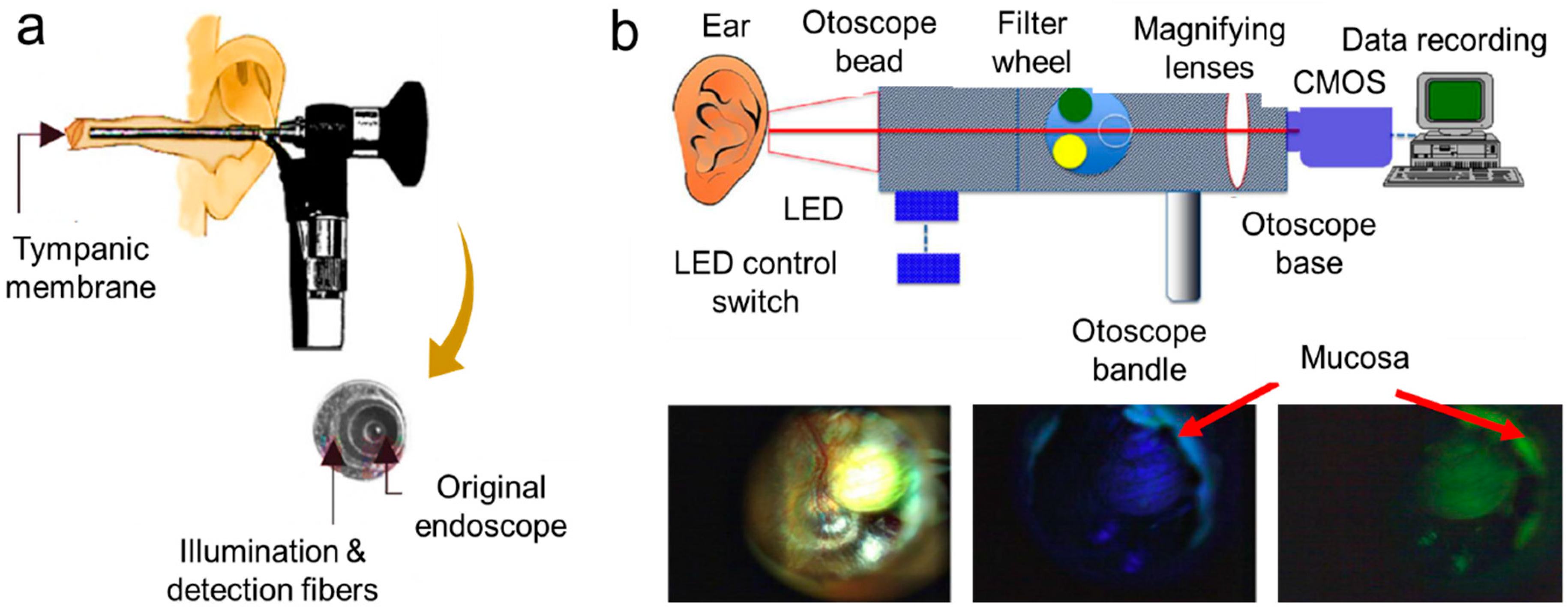
- Valdez, T.A.; Pandey, R.; Spegazzini, N.; Longo, K.; Roehm, C.; Dasari, R.R.; Barman, I. Multiwavelength fluorescence otoscope for video-rate chemical imaging of middle ear pathology. Anal. Chem. 2014, 86, 10454–10460. [Google Scholar] [CrossRef] [Green Version]
- Coticchia, J.M.; Chen, M.; Sachdeva, L.; Mutchnick, S. New paradigms in the pathogenesis of otitis media in children. Front. Pediatrics 2013, 1, 52. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, C.T.; Robinson, S.R.; Jung, W.; Novak, M.A.; Boppart, S.A.; Allen, J.B. Investigation of bacterial biofilm in the human middle ear using optical coherence tomography and acoustic measurements. Hear. Res. 2013, 301, 193–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, W.; Kim, J.; Jeon, M.; Chaney, E.J.; Stewart, C.N.; Boppart, S.A. Handheld optical coherence tomography scanner for primary care diagnostics. IEEE Trans. Biomed. Eng. 2011, 58, 741–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelton, R.L.; Jung, W.; Sayegh, S.I.; McCormick, D.T.; Kim, J.; Boppart, S.A. Optical coherence tomography for advanced screening in the primary care office. J. Biophotonics 2014, 7, 525–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fercher, A.F. Optical coherence tomography. J. Biomed. Opt. 1996, 1, 157–174. [Google Scholar] [CrossRef]
- Boppart, S.A.; Xi, C. Device and Method for Imaging the Ear Using Optical Coherence Tomography. U.S. Patent No. 8,115,934, 14 February 2012. [Google Scholar]
- Carr, J.A.; Valdez, T.A.; Bruns, O.T.; Bawendi, M.G. Using the shortwave infrared to image middle ear pathologies. Proc. Natl. Acad. Sci. USA 2016, 113, 9989–9994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, A.; Cook, N.; Tessaro, S.; Deregt, D.; Desroches, G.; Dubeski, P.; Tong, A.; Godson, D. Early detection and prediction of infection using infrared thermography. Can. J. Anim. Sci. 2004, 84, 73–80. [Google Scholar] [CrossRef]
- Tang, E.N.; Nair, A.; Baker, D.W.; Hu, W.; Zhou, J. In vivo imaging of infection using a bacteria-targeting optical nanoprobe. J. Biomed. Nanotechnol. 2014, 10, 856–863. [Google Scholar] [CrossRef] [Green Version]
- Hoa, M.; Tomovic, S.; Nistico, L.; Hall-Stoodley, L.; Stoodley, P.; Sachdeva, L.; Berk, R.; Coticchia, J.M. Identification of adenoid biofilms with middle ear pathogens in otitis-prone children utilizing SEM and FISH. Int. J. Pediatric Otorhinolaryngol. 2009, 73, 1242–1248. [Google Scholar] [CrossRef]
- Saafan, M.E.; Ibrahim, W.S.; Tomoum, M.O. Role of adenoid biofilm in chronic otitis media with effusion in children. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 2417–2425. [Google Scholar] [CrossRef]
- Boppart, S.A.; Richards-Kortum, R. Point-of-care and point-of-procedure optical imaging technologies for primary care and global health. Sci. Transl. Med. 2014, 6, rv252–rv253. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.R.; Gaylor, K.A.; Pilgrim, A.J. Comparison of traditional otoscope to iPhone otoscope in the pediatric ED. Am. J. Emerg. Med. 2015, 33, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Hoberman, A.; Paradise, J.L.; Wald, E.R.; Switze, G.E.; Kurs-Lasky, M.; Colborn, D.K.; Kearney, D.H.; Zoffel, L.M. Development and preliminary evaluation of a parent-reported outcome instrument for clinical trials in acute otitis media. Pediatric Infect. Dis. J. 2009, 28, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Hoberman, A.; Kaleida, P.H.; Rockette, H.E.; Kurs-Lasky, M.; Hoover, H.; Pichichero, M.E.; Roddey, O.F.; Harrison, C.; Hadley, J.A. Otoscopic signs of otitis media. Pediatric Infect. Dis. J. 2011, 30, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, R.M. Pneumatic otoscopy in healthy full-term infants. Pediatrics 1987, 79, 520–523. [Google Scholar] [PubMed]
- Uitti, J.M.; Laine, M.K.; Tähtinen, P.A.; Ruuskanen, O.; Ruohola, A. Symptoms and otoscopic signs in bilateral and unilateral acute otitis media. Pediatrics 2013, 131, e398–e405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiro, D.M.; King, W.D.; Arnold, D.H.; Johnston, C.; Baldwin, S. A randomized clinical trial to assess the effects of tympanometry on the diagnosis and treatment of acute otitis media. Pediatrics 2004, 114, 177–181. [Google Scholar] [CrossRef]
- Helenius, K.K.; Laine, M.K.; Tähtinen, P.A.; Lahti, E.; Ruohola, A. Tympanometry in discrimination of otoscopic diagnoses in young ambulatory children. Pediatric Infect. Dis. J. 2012, 31, 1003–1006. [Google Scholar] [CrossRef]
- Djalilian, H.R.; Ridgway, J.; Tam, M.; Sepehr, A.; Chen, Z.; Wong, B.J. Imaging the human tympanic membrane using optical coherence tomography in vivo. Otol. Neurotol. 2008, 29, 1091. [Google Scholar] [CrossRef] [Green Version]
- Cho, N.H.; Lee, S.H.; Jung, W.; Jang, J.H.; Kim, J. Optical coherence tomography for the diagnosis and evaluation of human otitis media. J. Korean Med. Sci. 2015, 30, 328–335. [Google Scholar] [CrossRef] [Green Version]



| Technology | Advantages | Limitations | Performance Parameters |
|---|---|---|---|
| Otoscope | Visually examines middle ear including coloration, transparency, and presence of liquid in the tympanic membrane. | Visual inspection may not be enough to detect the type of infection. | Sensitivity = 61–70%; Specificity = 61–70% [24,25]. |
| Cell Scope | Visually examines middle ear like the otoscope with the help of a cellphone. | Visual inspection with the help of a cellphone may not detect the exact type of infection. | Sensitivity = 70%; Specificity = 70% [26,27]. |
| Terahertz (THz) otoscope | Electromagnetic waves sensitively detect water molecules for feasible AOM diagnosis. | Terahertz waves are sensitive to membrane geometry. | * Sensitivity = 82–89%; * Specificity = 66–77% [28,29,30]. |
| Acoustic reflectometry | Measure the fluid formation in the middle ear. | Limited information for structural changes in tympanic membrane. | Sensitivity = 63.6–96%; Specificity = 79.7–87% [31,32]. |
| Tympanometry | Examine the movement of the eardrum by air pressure. | Structural changes in tympanic membrane cannot be detected accurately. | Sensitivity = 70–91%; Specificity = 71.7–98% [32,33,34]. |
| Pneumatic otoscopy | Examine the mobility in the tympanic membrane. | Misinterpretation of diagnosis and often performed by untrained personnel. | Sensitivity = 74–94%; Specificity = 79–80% [25,35]. |
| Raman spectrometer | Determine the unique chemical fingerprints of molecules responsible for ear infection. | Sometimes, Raman signals may need to be enhanced for better sensitivity of detection. | Sensitivity = 95.48%; Specificity = 99.06%. |
| Low-coherence interferometry (LCI) along with Raman scattering spectroscopy (RS) | Identify pathogens of ear infection. | Bacterial pathogens in body fluid cannot be detected. | Not available. |
| Low-coherence interferometry/optical coherence tomography | Non-invasive; causes no tissue damage, and suitable for in vivo applications. | Sometimes, the beam focus cannot reach to the long ear canal; thus, it delivers inadequate signal-to-noise ratio data. | Sensitivity = 68–86%; Specificity = 90–98% [1,36]. |
| Diffuse reflectance spectroscopy | Measure the hemoglobin content of the tympanic membrane. | May not be suitable for in vivo experiment. | * Sensitivity = 89–100%; Specificity = 79–100% [37,38]. |
| Reflectance and scattering absorption spectroscopy | Analyze the gases and also determine the oxygen flow in the eardrum. | Experiments were done on ear phantom and planning to be performed in clinical trials soon. | Not available. |
| Fluorescence otoscope | A platform for fluorescence imaging of congenital cholesteatomas (i.e., non-cancerous skin growth) found in the middle ear tissue. | Difficult to get proper signal-to-noise ratio for weak fluorescent features. | * Sensitivity = 96.7%; Specificity = 91.7% [39]. |
| Hand-held OCT | Provide quantitative information about the biofilm progression in cases of middle ear infection. | Acquiring full three-dimensional in vivo imaging is difficult. | Sensitivity = 68–90.9%; Specificity = 90.2–98% [1,40] |
| SWIR (short wavelength infrared) otoscope | Analyze the anatomical structures situated after the thin tissue membranes inside the ear such as an ear drum. | Some supplementary training is required for the medical practitioners. | Sensitivity = *67–100%; Specificity = 89–100% [41]. |
| Confocal laser scanning microscopy (CLSM) | Detect biofilm-related middle ear pathogens. | Detecting biofilm in adenoid is a challenging task. | * Sensitivity = 85.19–98.15%; Specificity = 97.6–99.26% [42,43]. |
| Range (cm−1) | Peak Assignment |
|---|---|
| 640–675 | Guanine (B-DNA), tyrosine valine |
| 713–740 | Adenine, glycoside |
| 745–790 | Cytosine, uracil, thymine, tryptophan |
| 800–815 | O–P–O (RNA) |
| 930–990 | C-C stretch (α-helix skeletal mode), C–N stretch |
| 1000–1010 | Phenylalanine, C–C aromatic ring stretch |
| 1025–1060 | C–C stretch (phospholipids, glucosidic rings), C–N stretch |
| 1080–1105 | PO2−/O–P–O (DNA), CO32−/C–C or C–O–C stretching (carbohydrates) |
| 1130–1145 | C–O–C (unsaturated fatty acids) |
| 1215–1295 | Amide III (random), thymine phenylalanine, tryptophan |
| 1330–1345 | Adenine, guanine, C–H stretch |
| 1390–1415 | COO– symmetric stretch |
| 1440–1475 | CH2 deformation |
| 1510–1560 | Amide II (C=C) |
| 1570–1595 | Adenine, guanine (ring stretching), nuclei acid bands |
| 1658–1700 | Amide I |
| 2890–2900 | C−H-stretching deformation vibrations of CH2 and CH3 |
| Technology | Objective | Main Observations | No. of Patients Observed | Authors |
|---|---|---|---|---|
| Otoscope with cellphone: CellScope Oto (CSO) |
| Physicians, patients, and parents favored CellScope Oto in comparison to the conventional otoscope as it was easy to use, had good diagnostic precision, with the benefit of image acquisition to track changes through the period of infection. | 51 (adults) | Richards et al. [87] |
| SWIR otoscope |
| SWIR facilitated non-invasive optical penetration to image deep ear tissues and identify fluid accumulation in the middle ear, which is otherwise difficult to visualize from a conventional otoscope. | 10 (adults) | Carr et al. [81] |
| Otoscope |
| Due to a lack of techniques to track early symptoms in children with AOM, a parent-reported AOM severity of symptoms (AOM-SOS) structured questionnaire was established to understand the AOM symptoms for better treatment trials. | 264 (children) | Shaikh et al. [88] |
| Otoscope |
| To understand AOM diagnosis, endoscopic still images of the tympanic membrane were examined by expert otoscopists. Preventive antibiotic treatment was the individual-advised diagnostic criteria. | 783 (children) | Shaikh et al. [89] |
| Pneumatic otoscope |
| This study revealed the general ear features in healthy newborns (~ 72 h of life), mostly having pink/red colored eardrums, with a dull gray/opaque tympanic membrane. | 81 (newborn) | Cavanaugh et al. [90] |
| Otoscope |
| This clinical trial helped to understand the severity and symptoms of uni-/bi-lateral AOM in children aged 6 to 35 months. Assessment revealed that bilateral AOM was more severe than unilateral AOM. | 232 (children) | Uitti et al. [91] |
| Tympanometry |
| A randomized trial was conducted to understand physician diagnosis and prescription for OM when using either a tympanometry (specific to the middle ear) or an otoscope (sees all the ear). The study revealed that antibiotics were prescribed for OM in both cases. | 698 (children) | Spiro et al. [92] |
| Tympanometry with otoscope |
| The clinical trial study showed that tympanometry could be used as an adjunctive device with pneumatic otoscopy and not as a standalone device. | 515 (children) | Helenius et al. [93] |
| Optical coherence tomography |
| The OCT clinical trials provided a non-invasive means to study the middle ear microstructure in vivo utilizing a safe near-infrared light source. Advantages include the ability to image diseased tissues with high resolution. | 10 (adults) | Djalilian et al. [94] |
| Optical coherence tomography |
| The biofilm thickness results from the OCT clinical trials revealed a statistically significant quantitative difference between normal, acute, and chronic otitis media (OM) infections. | 34 (children) | Monroy et al. [24] |
| Combination of low-coherence interferometry and optical coherence tomography |
| The clinical findings from the OCT image scans in adults with chronic OM indicated the formation of biofilms as opposed to no biofilms in healthy subjects. | 20 (adult) | Nguyen et al. [1] |
| Optical coherence tomography |
| Spectral domain-OCT (840 nm) was utilized to acquire axial depth scan images from normal and healthy ear to understand the OM infections. These OCT image databases could potentially serve as a means to upgrade the current otoscopic techniques. | 39 (non-specified) | Cho et al. [95] |
| Confocal laser scanning microscopy (CLSM) |
| The CLSM mucosal biofilm images, collected in this clinical study, revealed that chronic OM in humans is biofilm related. | 26 (children) | Hall Stoodley [19] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, A.; Hasan, S.M.A.; Gartia, M.R. Optical Identification of Middle Ear Infection. Molecules 2020, 25, 2239. https://doi.org/10.3390/molecules25092239
Prasad A, Hasan SMA, Gartia MR. Optical Identification of Middle Ear Infection. Molecules. 2020; 25(9):2239. https://doi.org/10.3390/molecules25092239
Chicago/Turabian StylePrasad, Alisha, Syed Mohammad Abid Hasan, and Manas Ranjan Gartia. 2020. "Optical Identification of Middle Ear Infection" Molecules 25, no. 9: 2239. https://doi.org/10.3390/molecules25092239