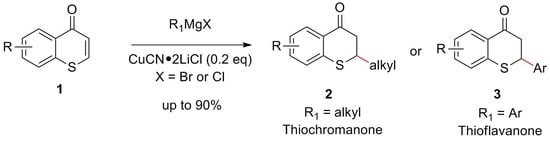
Conjugate Addition of Grignard Reagents to Thiochromones Catalyzed by Copper Salts: A Unified Approach to Both 2-Alkylthiochroman-4-One and Thioflavanone
Abstract
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. General Methods
3.2. Materials
3.3. General Procedure A: Conjugate Addition Reactions of Grignard Reagents (RMgX or ArMgX ; X = Cl or Br) to Thiochromones Catalyzed by CuCN∙2LiCl (0.2 eq)
3.4. Synthesis
3.4.1. Synthesis of 2-Cyclopropylthiochroman-4-one (2Ag)
3.4.2. Synthesis of 2-(9-Phenanthryl)thiochroman-4-one (3Ag)
3.4.3. Synthesis of 6,8-Difluoro-2-n-butylthiochroman-4-one (2Ka)
3.4.4. Synthesis of 6,8-Dichloro-2-n-butylthiochroman-4-one (2La)
3.4.5. Synthesis of 6,7-Dimethoxyl-2-n-butylthiochroman-4-one (2Na)
3.4.6. Synthesis of 6-Isopropyl-2-phenylthiochroman-4-one (3Ea)
3.4.7. Synthesis of 6,8-Dimethyl-2-phenylthiochroman-4-one (3Fa)
3.4.8. Synthesis of 6,8-Difluoro-2-phenylthiochroman-4-one (3Ka)
3.4.9. Synthesis of 6,8-Dichloro-2-phenylthiochroman-4-one (3La)
3.4.10. Synthesis of 8-Methoxy-2-phenylthiochroman-4-one (3Ma)
3.4.11. Synthesis of 2,3-Dihydro-2-phenyl-4H-naphtho[1,2-b]thiopyran-4-one (3Pa)
3.4.12. Synthesis of 3-Chloro-2-(2-naphthyl)-4H-thiochromen-4-one (4Af)
3.4.13. Synthesis of 2-(4-Methoxyphenyl)thiochroman-4-one 1,1-dioxide (5Ac)
3.4.14. Synthesis of 2-[3,5-(Trifluoromethyl)phenyl]thiochroman-4-one 1,1-dioxide (5Ae)
3.4.15. Synthesis of 2-(2-Methylphenyl)thiochroman-4-ol (7Ab)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ortiz, P.; Lanza, F.; Harutyunyan, S.R. 1,2- Versus 1,4-Asymmetric Addition of Grignard Reagents to Carbonyl Compounds. In Progress in Enantioselective Cu(I)-catalyzed Formation of Stereogenic Centers; Harutyunyan, S.R., Ed.; Springer Press: Basel, Switzerland, 2016; pp. 99–134. [Google Scholar]
- Kharasch, M.S.; Tawney, P.O. Factors Determining the Course and Mechanisms of Grignard Reactions. II. The Effect of Metallic Compounds on the Reaction between Isophorone and Methylmagenisum Bromide. J. Am. Chem. Soc. 1941, 63, 2308–2316. [Google Scholar] [CrossRef]
- Damani, L.A. (Ed.) Sulphur-Containing Drugs and Related Organic Compounds; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Clayden, J.; MacLellan, P. Asymmetric synthesis of tertiary thiols and thioethers. Beilstein J. Org. Chem. 2011, 7, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Ingall, A.H. Thiopyrans and Fused Thiopyrans. In Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon Press: Oxford, UK, 1984; Volume 3, p. 885. [Google Scholar]
- Schneller, S.W. Thiochromanones and Related Compounds. In Advances in Heterocyclic Chemistry; Katritzky, A.R., Boulton, A.J., Eds.; Academic Press: New York, USA, 1975; Volume 18, p. 59. [Google Scholar]
- Acton, A.Q. (Ed.) Sulfur Compounds: Advances in Research and Application; Scholarly Editions: Atlanta, GA, USA, 2012. [Google Scholar]
- Nielsen, S.F.E.; Nielsen, O.; Olsen, G.M.; Liljefors, T.; Peters, D. Novel Potent Novel Potent Ligands for the Central Nicotinic Acetylcholine Receptor: Synthesis, Receptor Binding, and 3D-QSAR Analysis. J. Med. Chem. 2000, 43, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Eastman, C.M.; Njardarson, J.T. Beyond C, H, O and N! Analysis of the Elemental Composition of U.S. FDA Approved Drug Architectures. J. Med. Chem. 2014, 57, 9764–9773. [Google Scholar] [CrossRef] [PubMed]
- Takimiya, K.; Osaka, I.; Mori, T.; Nakano, M. Organic Semiconductors Based on [1]Benzothieno[3,2-b][1]benzothiophene Substructure. Acc. Chem. Res. 2014, 47, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.I.; Eady, C.C.; Silcock, P.; Perry, N.B.; Van Klink, J.W. Fast Phenotyping of LFS-Silenced (Tearless) Onions by Desorption Electrospray Ionization Mass Spectrometry (DESI-MS). J. Agric. Food Chem. 2013, 61, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Ma, C.Q.; Bauerle, P. Functional Oligothiophenes: Molecular Design for Multidimensional Nanoarchitectures and Their Applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef]
- Lin, D.Y.; Zhang, S.Z.; Block, E.; Katz, L.C. Encoding social signals in the mouse main olfactory bulb. Nature 2005, 434, 470–477. [Google Scholar] [CrossRef]
- Wermuch, C.G. Molecular variations based on isosteric replacements. In The Practice of Medicinal Chemistry; Wermuth, C.G., Ed.; Academic Press: San Diego, CA, USA, 1996; pp. 203–237. [Google Scholar]
- Choi, E.J.; Lee, J.I.; Kim, G.-H. Evaluation of the anticancer activities of thioflavanone and thioflavone in human breast cancer cell lines. Int. J. Mol. Med. 2012, 29, 252–256. [Google Scholar]
- Vargas, E.; Echeverri, F.; Vélez, I.D.; Robledo, S.M.; Quiňones, W. Synthesis and Evaluation of Thiochroman-4-one Derivatives as Potential Leishmanicidal Agents. Molecules 2017, 22, 2041. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Li, H.-Z.; Suo, H.; Wang, Y.; Yang, C.; Ma, Z.; Liu, Y. Cytoxic effect of three novel thiochromanone derivatives on tumor cell in vitro and underlying mechanism. Glob. Adv. Res. J. Med. Sci. 2014, 3, 240–250. [Google Scholar]
- Harborne, J.B. The Flavonoids: Advances in Research Since 1980; Chapman and Hall: New York, NY, USA, 1988. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Anthocyanins and other flavonoids. Nat. Prod. Rep. 1995, 12, 639–657. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.M.; Markham, K.R. (Eds.) Flavonoids: Chemistry, Biochemistry and Applications; Taylor & Francis: London, UK, 2006. [Google Scholar]
- Philipp, A.; Jirkovsky, I.; Martel, R.R. Synthesis and antiallergic properties of some 4H, 5H-pyrano[3,2-c][1]benzopyran-4-one, 4H,5H-[1]benzothiopyrano[4,3-b]pyran-4-one, and 1,4-dihydro-5H-[1]benzothiopyrano[4,3-b]pyridine-4-one derivatives. J. Med. Chem. 1980, 23, 1372–1376. [Google Scholar] [CrossRef]
- Ramalingam, K.; Thyvelikakath, G.X.; Berlin, K.D.; Chesnut, R.W.; Brown, R.A.; Durham, N.N.; Ealick, S.E.; Van der Helm, D. Synthesis and biological activity of some derivatives of thiochroman-4-one and tetrahydrothiapyran-4-one. J. Med. Chem. 1977, 20, 847–850. [Google Scholar] [CrossRef]
- Wang, H.K.; Bastow, K.F.; Cosentino, L.M.; Lee, K.H. Antitumor Agents. 166. Synthesis and Biological Evaluation of 5,6,7,8-Substituted-2-phenylthiochromen-4-ones. J. Med. Chem. 1996, 39, 1975–1980. [Google Scholar] [CrossRef]
- Holshouser, M.H.; Loeffler, L.J.; Hall, I.H. Synthesis of peptides by the solid-phase method. 6. Neurotensin, fragments, and analogs. J. Med. Chem. 1981, 24, 853–858. [Google Scholar] [CrossRef]
- Dhanak, D.; Keenan, R.M.; Burton, G.; Kaura, A.; Darcy, M.G.; Shah, D.H.; Ridgers, L.H.; Breen, A.; Lavery, P.; Tew, D.G.; et al. Benzothiopyran-4-one based reversible inhibitors of the human cytomegalovirus (HCMV) protease. Bioorg. Med. Chem. Lett. 1998, 8, 3677–3682. [Google Scholar] [CrossRef]
- Nussbaumer, P.; Lehr, P.; Billich, A. 2-Substituted 4-(Thio)chromenone 6-O-Sulfamates: Potent Inhibitors of Human Steroid Sulfatase. J. Med. Chem. 2002, 45, 4310–4320. [Google Scholar] [CrossRef]
- Kataoka, T.; Watanabe, S.; Mori, E.; Kadomoto, R.; Tanimura, S.; Kohno, M. Synthesis and structure-activity relationships of thioflavone derivative as specific inhibitors of the ERK-MAP kinase signaling pathway. Bioorg. Med. Chem. 2004, 12, 2397–2407. [Google Scholar] [CrossRef]
- Soni, D.V.; Jacobberger, J.W. Gene modulation by Cox-1 and Cox-2 specific inhibitors in human colorectal carcinoma cancer cells. Cell Cycle 2004, 3, 349–357. [Google Scholar] [PubMed] [Green Version]
- Bondock, S.; Metwally, M.A. Thiochroman-4-ones: Synthesis and reactions. J. Sulfur Chem. 2008, 29, 623–653. [Google Scholar] [CrossRef]
- Dalla Via, L.; Marciani Magno, S.; Gia, O.; Marini, A.M.; Da Settimo, F.; Salerno, S.; La Motta, C.; Simorini, F.; Taliani, S.; Lavecchia, A.; et al. Benzothiopyranoindole-Based Antiproliferative Agents: Synthesis, Cytoxicity, Nucleic Acids Interaction, and Topoisomerases Inbibition Properties. J. Med. Chem. 2009, 52, 5429–5441. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.K.; Li, K. Stannous Chloride-Mediated Reductive Cyclization-Rearrangement of Nitroarenyl Ketones. J. Org. Chem. 2002, 67, 8662–8665. [Google Scholar] [CrossRef]
- Aramaki, Y.; Seto, M.; Okawa, T.; Oda, T.; Kanzaki, N.; Shiraishi, M. Synthesis of 1-Benzothiepine and 1-Benzazepine Derivatives as Orally Active CCR5 Antagonists. Chem. Pharm. Bull. 2004, 52, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Dike, S.Y.; Ner, D.H.; Kumar, A. A New Enantioselective Chemoenzymatic Synthesis of R-(−)Thiazesim Hydrochloride. Bioorg. Med. Chem. Lett. 1991, 1, 383–386. [Google Scholar] [CrossRef]
- Phippen, C.B.W.; McErlean, C.S.P. A 1,5-Benzothiazepine Synthesis. Tetrahedron Lett. 2011, 52, 1490–1492. [Google Scholar] [CrossRef]
- Li, W.; Schlepphorst, C.; Daniliuc, C.; Glorius, F. Asymmetric Hydrogenation of Vinylthioethers: Access to Optically Active 1,5-Benzothiazepine Derivatives. Angew. Chem. Int. Ed. 2016, 55, 3300–3303. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Wang, C.J. Organocatalytic Asymmetric Sulfa-Michael Addition of Thiols to α,β-Unsaturated Hexafluoroisopropyl Esters: Expeditious Access to (R)-Thiazesim. Org. Lett. 2013, 15, 3448–3451. [Google Scholar] [CrossRef]
- Fukata, Y.; Asano, K.; Matsubara, S. Facile Net Cycloaddition Approach to Optically Active 1,5-Benzothiazepines. J. Am. Chem. Soc. 2015, 137, 5320–5323. [Google Scholar] [CrossRef]
- Qi, X.; Xiang, H.; Yang, C. Synthesis of Functionalized Chromeno[2,3-b]pyrrol-4(1H)-ones by Silver-Catalyzed Cascade Reactions of Chromones/Thiochromones and Isocyanoacetates. Org. Lett. 2015, 17, 5590–5593. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ahmad, V.U.; Liebscher, J. Stereoselective Synthesis of Thiochroman-4-ones by Ring Transformation of Chiral 5-Ylidene-1,3-dioxan-4-ones with 2-Bromothiophenol via Bromo-Lithium Exchange. Eur. J. Org. Chem. 2001, 2001, 529–535. [Google Scholar] [CrossRef]
- Vaghoo, H.; Prakash, G.K.; Narayanan, A.; Choudhary, R.; Paknia, F.; Mathew, T.; Olah, G.A. Superelectrophilic Activation of Crotonic/Methacrylic Acids: Direct Access to Thiochroman-4-ones from Benzenethiols by Microwave-Assisted One-Pot Alkylation/Cyclic Acylation. Org. Lett. 2015, 17, 6170–6173. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.-M.; Kawamura, M.; Shimada, S.; Hayashi, T.; Tanaka, M. Synthesis of 1-Tetralones by Intramolecular Friedel-Crafts Reaction of 4-Arylbutyric Acids Using Lewis Acid Catalysts. Tetrahedron Lett. 2003, 44, 4007–4010. [Google Scholar] [CrossRef]
- Hoettecke, N.; Rotzoll, S.; Albrecht, U.; Lalk, M.; Fischer, C.; Langer, P. Synthesis and Antimicrobial Activity of 2-Alkenylchroman-4-ones, 2-Alkenylthiochroman-4-ones and 2-Alkenylquinol-4-ones. Bioorg. Med. Chem. 2008, 16, 10319–10325. [Google Scholar] [CrossRef]
- Dong, X.Q.; Fang, X.; Wang, C.J. Organocatalytic Asymmetric Sulfa-Michael Addition of Thiols to 4,4,4-Trifluorocrotonates. Org. Lett. 2011, 13, 4426–4429. [Google Scholar] [CrossRef]
- Xiao, W.-J.; Alper, H. Regioselective Carbonylative Heteroannulation of o-Iodothiophenols with Allenes and Carbon Monoxide Catalyzed by a Palladium Complex: A Novel and Efficient Access to Thiochroman-4-one Derivatives. J. Org. Chem. 1999, 64, 9646–9652. [Google Scholar] [CrossRef]
- Dawood, K.M.; Ishii, H.; Fuchigami, T. Electrolytic Partial Fluorination of Organic Compound. 54.1 Anodic Mono- and Trifluorination of Thiochroman-4-one Derivatives and the Factors Affecting Product Selectivity. J. Org. Chem. 2001, 66, 7030–7034. [Google Scholar] [CrossRef]
- Palani, T.; Park, K.; Song, K.H.; Lee, S. Palladium-Catalyzed Synthesis of (Z)-3-Arylthioacrylic Acids and Thiochromenones. Adv. Synth. Catal. 2013, 355, 1160–1168. [Google Scholar] [CrossRef]
- Han, X.; Yue, Z.; Zhang, X.; He, Q.; Yang, C. Copper-Mediated, Palladium-Catalyzed Cross-Coupling of 3-Iodochromones, Thiochromones, and Quinolones with Ethyl Bromodifluoroacetate. J. Org. Chem. 2013, 78, 4850–4856. [Google Scholar] [CrossRef]
- Inami, T.; Kurahashi, T.; Matsubara, S. Nickel-Catalyzed Reaction of Thioisatins and Alkynes: A Facile Synthesis of Thiochromones. Org. Lett. 2014, 16, 5660–5662. [Google Scholar] [CrossRef] [PubMed]
- Muthupandi, P.; Sundaravelu, N.; Sekar, G. Domino Synthesis of Thiochromenes through Cu-Catalyzed Incorporation of Sulfur Using Xanthate Surrogate. J. Org. Chem. 2017, 82, 1936–1942. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Jin, M.; Wang, J. Rh-Catalyzed Conjugate Addition of Arylzinc Chlorides to Thiochromones: A Highly Enantioselective Pathway for Accessing Chiral Thioflavanones. Org. Lett. 2016, 18, 4986–4989. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Zhou, W.; Lu, Z.; Zeng, S.; Wang, J. A highly enantioselective access to chiral chromanones and thiochromanones via copper-catalyzed asymmetric conjugated reduction of chromones and thiochromones. Chem. Comm. 2017, 53, 6844–6847. [Google Scholar] [CrossRef]
- Lee, J.I. New Synthesis of Thioflavanones by the Regioselective Cyclization of 1-(2-Benzylthio)phenyl-3-phenyl-2-propen-1-ones with Hydrobromic Acid. Bull. Korean Chem. Soc. 2020, 41, 484–487. [Google Scholar] [CrossRef]
- Lemke, M.K.; Schwab, P.; Fischer, P.; Tischer, S.; Witt, M.; Noehringer, L.; Rogachev, V.; Jager, A.; Kataeva, O.; Frohlich, R.; et al. A Practical Access to Highly Enantiomerically Pure Flavanones by Catalytic Asymmetric Transfer Hydrogenation. Angew. Chem. Int. Ed. 2013, 52, 11651–11655. [Google Scholar] [CrossRef]
- Zhao, D.-B.; Beiring, B.; Glorius, F. Ruthenium-NHC-catalyzed Asymmetric Hydrogenation of Flavones and Chromones: General Access to Enantiomerically Enriched Flavanones, Flavanols, Chromanones, and Chromanols. Angew. Chem. Int. Ed. 2013, 52, 8454–8458. [Google Scholar] [CrossRef]
- Kumar, P.; Rao, A.T.; Pandey, B. Chemoselective Reduction of Vinylogous Thioesters of Thiochromones. Synth. Commun. 1994, 24, 3297–3306. [Google Scholar] [CrossRef]
- Sakirolla, R.; Yaeghoobi, M.; Abd Rahman, N. Synthesis of Flavanones, Azaflavanones, and Thioflavanones Catalyzed by PMA-SiO2 as a Mild, Efficient, and Reusable Catalyst. Monatsh. Chem. 2012, 143, 797–800. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kobayashi, A.; Tanmatsu, M. A Facile Synthesis of 2-Arylthiochroman-4-ones by the Reaction of 3-Aryl-1-(2-halophenyl)prop-2-en-1-ones with Sodium Hydrogensulfide. Heterocycles 2012, 85, 919–925. [Google Scholar] [CrossRef]
- Sangeetha, S.; Muthupandi, P.; Sekar, G. Copper-Catalyzed Domino Synthesis of 2-Arylthiochromanones through Concomitant C–S Bond Formations Using Xanthate as Sulfur Source. Org. Lett. 2015, 17, 6006–6009. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, W.; Konieczny, M. Synthesis of Thioflavanone and Flavanone Derivatives by Cyclization of Chalcones. Synthesis 2009, 1811–1814. [Google Scholar] [CrossRef]
- Lee, J.I. A New Synthesis of Thioflavanones from Thiosalicylic Acid. Bull. Korean Chem. Soc. 2008, 29, 1263–1265. [Google Scholar]
- Zu, L.; Wang, J.; Li, H.; Xie, H.; Jiang, W.; Wang, W. Cascade Michael-Aldol Reactions Promoted by Hydrogen Bonding Mediated Catalysis. J. Am. Chem. Soc. 2007, 129, 1036–1037. [Google Scholar] [CrossRef]
- Konieczny, M.T.; Horowska, B.; Kunikowski, A.; Konopa, J.; Wierzba, K.; Yamada, Y.; Asao, T. Synthesis and Reactivity of 5,8-Dihydroxythioflavanone Derivatives. J. Org. Chem. 1999, 64, 359–364. [Google Scholar] [CrossRef]
- Kaye, P.T.; Mphahlele, M.J. Benzodiazepine Analogues. Part 8.1 Trimethylsilyl Azide Mediated Schmidt Rearrangement of Thioflavanone and Thiochromanone Precursors. Synth. Commun. 1995, 25, 1495–1509. [Google Scholar] [CrossRef]
- Bouisseau, A.; Glancy, J.; Willis, M.C. Two-Component Assembly of Thiochroman-4-ones and Tetrahydrothiopyran-4-ones Using Rhodium-Catalyzed Alkyne Hydroacylation/Thio-Conjugate-Addition Sequence. Org. Lett. 2016, 18, 5676–5679. [Google Scholar] [CrossRef]
- Bass, S.A.; Parker, D.M.; Bellinger, T.J.; Eaton, A.S.; Dibble, A.S.; Koroma, K.L.; Sekyi, S.A.; Pollard, D.A.; Guo, F. Development of Conjugate Addition of Lithium Dialkylcuprates to Thiochromones: Synthesis of 2-Alkylthiochroman-4-ones and Additional Synthetic Applications. Molecules 2018, 23, 1728. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.; Jeffries, M.C.; Graves, B.N.; Graham, S.A.; Pollard, D.A.; Pang, G.; Chen, H.Y. A Rapid Entry Into Thioflavanones via Conjugate Additions of Diarylcuprates to Thiochromones. Tetrahedron 2017, 73, 5745–5750. [Google Scholar] [CrossRef]
- Dike, S.Y.; Mahalingam, M. Efficient and Improved Procedure for the Synthesis of 3-Chloro Derivatives of Flavones, Chromones and their Sulfur Analogues. Synthetic Communications 1989, 19, 3443–3451. [Google Scholar] [CrossRef]
- Sangeetha, S.; Sekar, G. Copper-Catalyzed One-pot Synthesis of 2-Arylthiochromenones: An in situ Recycle of Waste Byproduct as Useful Reagent. Org. Lett. 2019, 21, 75–79. [Google Scholar] [CrossRef] [PubMed]
- French, K.L.; Angel, A.J.; Williams, A.R.; Hurst, D.R.; Beam, C.F. A new preparation of substituted 4H-1-benzothiopyran-4-ones from c(α)N-benzoylbydrazones or c(α)N-carboalkoxyhydrazones and methyl thiosalicylate. J. Heterocycl. Chem. 1998, 35, 45–48. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellinger, T.J.; Harvin, T.; Pickens-Flynn, T.; Austin, N.; Whitaker, S.H.; Tang Yuk Tutein, M.L.C.; Hukins, D.T.; Deese, N.; Guo, F. Conjugate Addition of Grignard Reagents to Thiochromones Catalyzed by Copper Salts: A Unified Approach to Both 2-Alkylthiochroman-4-One and Thioflavanone. Molecules 2020, 25, 2128. https://doi.org/10.3390/molecules25092128
Bellinger TJ, Harvin T, Pickens-Flynn T, Austin N, Whitaker SH, Tang Yuk Tutein MLC, Hukins DT, Deese N, Guo F. Conjugate Addition of Grignard Reagents to Thiochromones Catalyzed by Copper Salts: A Unified Approach to Both 2-Alkylthiochroman-4-One and Thioflavanone. Molecules. 2020; 25(9):2128. https://doi.org/10.3390/molecules25092128
Chicago/Turabian StyleBellinger, Tania J., Teavian Harvin, Ti’Bran Pickens-Flynn, Nataleigh Austin, Samuel H. Whitaker, Mai Ling C. Tang Yuk Tutein, Dabria T. Hukins, Nichele Deese, and Fenghai Guo. 2020. "Conjugate Addition of Grignard Reagents to Thiochromones Catalyzed by Copper Salts: A Unified Approach to Both 2-Alkylthiochroman-4-One and Thioflavanone" Molecules 25, no. 9: 2128. https://doi.org/10.3390/molecules25092128