Beneficial Impact of Semicarbazide-Sensitive Amine Oxidase Inhibition on the Potential Cytotoxicity of Creatine Supplementation in Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Creatine Supplementation and Type 2 Diabetes Mellitus
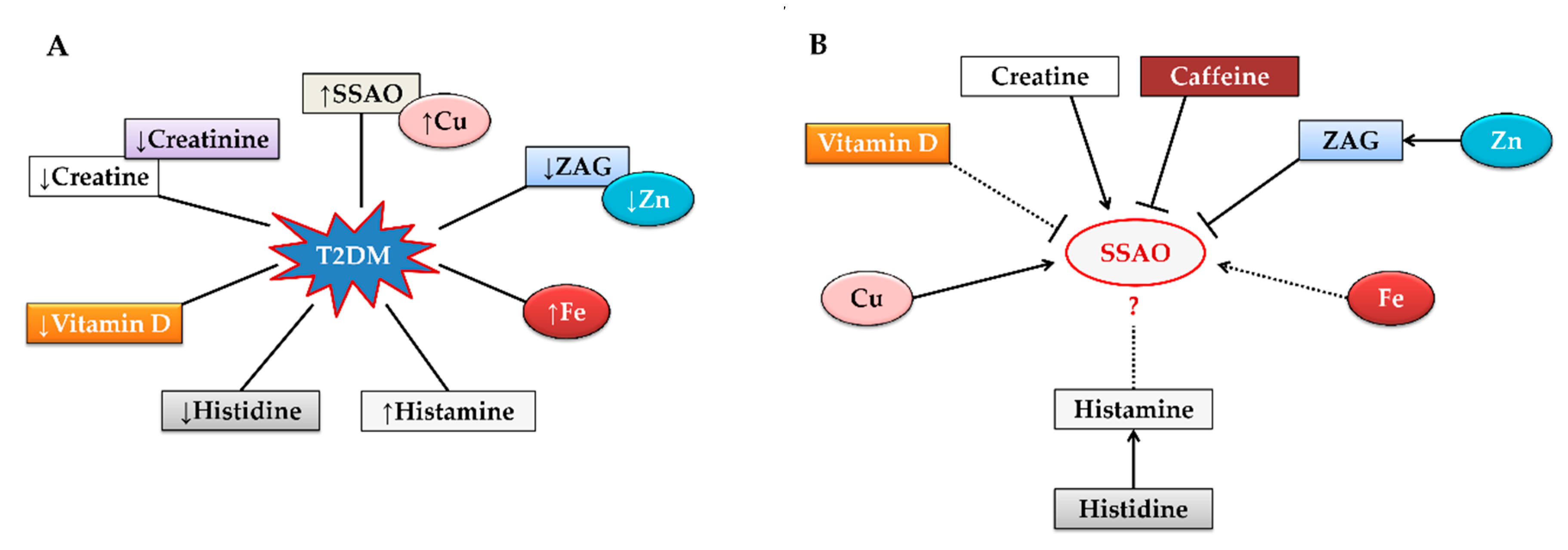
3. Why Is Semicarbazide-Sensitive Amine Oxidase Activity Elevated in Type 2 Diabetes Mellitus?
4. Role of Semicarbazide-Sensitive Amine Oxidase in Creatine Metabolism
5. Semicarbazide-Sensitive Amine Oxidase Mediated Creatine Impact on Type 2 Diabetes Mellitus
6. Promising Agents Involved in Regulating Semicarbazide-Sensitive Amine Oxidase Activity
6.1. Ability of Caffeine to Inhibit Semicarbazide-Sensitive Amine Oxidase and Its Simultaneous Administration with Creatine
6.2. Histamine/Histidine- Good or Bad for Type 2 Diabetes Mellitus?
6.3. Zinc-α2-glycoprotein/Zinc
6.4. Copper
6.5. Role of Dietary Iron Along with Creatine Supplementation in Type 2 Diabetes Mellitus
6.6. Vitamin D
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharroubi, A.T. Diabetes Mellitus: The Epidemic of the Century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Jialal, I. Diabetes Mellitus Type 2. Stat. Pearls 2020.
- Xu, G.; Liu, B.; Sun, Y.; Du, Y.; Snetselaar, L.G.; Hu, F.B.; Bao, W. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: Population based study. BMJ 2018, 362, k1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mainous, A.G.; Diaz, V.A.; Everett, S.J. Assessing Risk for Development of Diabetes in Young Adults. Ann. Fam. Med. 2007, 5, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Yanai, H.; Adachi, H.; Masui, Y.; Katsuyama, H.; Kawaguchi, A.; Hakoshima, M.; Waragai, Y.; Harigae, T.; Hamasaki, H.; Sakoa, A. Exercise Therapy for Patients with Type 2 Diabetes: A Narrative Review. J. Clin. Med. Res. 2018, 10, 365–369. [Google Scholar] [CrossRef] [Green Version]
- Colberg, S.R.; Sigal, R.J.; Fernhall, B.; Regensteiner, J.G.; Blissmer, B.J.; Rubin, R.R.; Chasan-Taber, L.; Albright, A.L.; Braun, B. Exercise and Type 2 Diabetes. Diabetes Care 2010, 33, e147–e167. [Google Scholar] [CrossRef] [Green Version]
- Schoch, R.D.; Willoughby, D.; Greenwood, M. The Regulation and Expression of the Creatine Transporter: A Brief Review of Creatine Supplementation in Humans and Animals. J. Int. Soc. Sports Nutr. 2006, 3, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Arlin, J.B.; Bhardwaj, R.M.; Johnston, A.; Miller, G.J.; Bardin, J.; Macdougall, F.; Fernandes, P.; Shankland, K.; William, I.F.D.; Florence, A.J. Structure and Stability of Two Polymorphs of Creatine and Its Monohydrate. Cryst. Eng. Comm. 2014, 16, 8197–8204. [Google Scholar] [CrossRef] [Green Version]
- Allen, P.J. Creatine Metabolism and Psychiatric Disorders: Does Creatine Supplementation Have Therapeutic Value? Neurosci. Biobehav. Rev. 2012, 36, 1442–1462. [Google Scholar] [CrossRef] [Green Version]
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine Supplementation with Specific View to Exercise/Sports Performance: An Update. J. Int. Soc. Sports. Nutr. 2012, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torok, Z.A.; Busekrus, R.B.; Hydock, D.S. Effects of Creatine Supplementation on Muscle Fatigue in Rats Receiving Doxorubicin Treatment. Nutr. Cancer. 2019, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; De Salles Painneli, V.; Roschel, H.; Artioli, G.G.; Neves, M.; De Sá Pinto, A.L.; Da Silva, M.E.; Cunha, M.R.; Otaduy, M.C.; Leite, C.; et al. Creatine in Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Med. Sci. Sports. Exerc. 2011, 43, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.; New, D. How the use of creatine supplements can elevate serum creatinine in the absence of underlying kidney pathology. BMJ Case Rep. 2014, 2014, bcr2014204754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bemben, M.G.; Lamont, H.S. Creatine Supplementation and Exercise Performance: Recent Findings. Sports Med. 2005, 35, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Meamar, R.; Maracy, M.; Nematollahi, S.; Yeroshalmi, S.; Zamani-Moghaddam, A.; Ghazvini, M.R. Effect of taking dietary supplement on hematological and biochemical parameters in male bodybuilders an equation model. Iran. J. Nurs. Midwifery. Res. 2015, 20, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Butts, J.; Jacobs, B.; Silvis, M. Creatine Use in Sports. Sports Health 2018, 10, 31–34. [Google Scholar] [CrossRef]
- Casey, A.; Greenhaff, P.L. Does Dietary Creatine Supplementation Play a Role in Skeletal Muscle Metabolism and Performance? Am. J. Clin. Nutr. 2000, 72, 607S–617S. [Google Scholar] [CrossRef]
- Van Loon, L.J.; Oosterlaar, A.M.; Hartgens, F.; Hesselink, M.K.; Snow, R.J.; Wagenmakers, A.J. Effects of Creatine Loading and Prolonged Creatine Supplementation on Body Composition, Fuel Selection, Sprint and Endurance Performance in Humans. Clin. Sci (Lond) 2003, 104, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Rawson, E.S.; Venezia, A.C. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids 2011, 40, 1349–1362. [Google Scholar] [CrossRef]
- Yu, P.H.; Deng, Y. Potential Cytotoxic Effect of Chronic Administration of Creatine, a Nutrition Supplement to Augment Athletic Performance. Med. Hypotheses 2000, 54, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Poortmans, J.R.; Kumps, A.; Duez, P.; Fofonka, A.; Carpentier, A.; Francaux, M. Effect of Oral Creatine Supplementation on Urinary Methylamine, Formaldehyde, and Formate. Med. Sci Sports Exerc 2005, 37, 1717–1720. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, C.K.; Carpentier, A.; Poortmans, J.R. Studies on the Safety of Creatine Supplementation. Amino Acids 2011, 40, 1409–1418. [Google Scholar] [CrossRef]
- Francaux, M.; Poortmans, J.R. Side Effects of Creatine Supplementation in Athletes. Int. J. Sports. Physiol. Perform. 2006, 1, 311–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sale, C.; Harris, R.C.; Florance, J.; Kumps, A.; Sanvura, R.; Poortmans, J.R. Urinary Creatine and Methylamine Excretion Following 4 X 5 G X day(-1) or 20 X 1 G X day(-1) of Creatine Monohydrate for 5 Days. J. Sports Sci. 2009, 27, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yu, P.H. Simultaneous Determination of Formaldehyde and Methylglyoxal in Urine: Involvement of Semicarbazide-Sensitive Amine Oxidase-Mediated Deamination in Diabetic Complications. J. Chromatogr. Sci. 1999, 37, 317–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noonan, T.; Lukas, S.; Peet, G.W.; Pelletier, J.; Panzenbeck, M.; Hanidu, A.; Mazurek, S.; Wasti, R.; Rybina, I.; Roma, T.; et al. The Oxidase Activity of Vascular Adhesion Protein-1 (VAP-1) Is Essential for Function. Am. J. Clin. Exp. Immunol. 2013, 2, 172–185. [Google Scholar]
- İnci, M.; Zararsız, İ.; Davarcı, M.; Görür, S. Toxic Effects of Formaldehyde on the Urinary System. Turk. J. Urol. 2013, 39, 48–52. [Google Scholar] [CrossRef]
- Wong, M.Y.W.; Saad, S.; Pollock, C.; Wong, M.G. Semicarbazide-Sensitive Amine Oxidase and Kidney Disease. Am. J. Physiol. Renal. Physiol. 2013, 305, F1637–F1644. [Google Scholar] [CrossRef] [Green Version]
- Lugaresi, R.; Leme, M.; de Salles Painelli, V.; Murai, I.H.; Roschel, H.; Sapienza, M.T.; Lancha Junior, A.H.; Gualano, B. Does long-term creatine supplementation impair kidney function in resistance-trained individuals consuming a high-protein diet? J. Int. Soc. Sports. Nutr. 2013, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Taes, Y.E.; Delanghe, J.R.; Wuyts, B.; van de Voorde, J.; Lameire, N.H. Creatine supplementation does not affect kidney function in an animal model with pre-existing renal failure. Nephrol. Dial. Transplant. 2003, 18, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Garpenstrand, H.; Ekblom, J.; Bäcklund, L.B.; Oreland, L.; Rosenqvist, U. Elevated Plasma Semicarbazide-Sensitive Amine Oxidase (SSAO) Activity in Type 2 Diabetes Mellitus Complicated by Retinopathy. Diabet. Med. 1999, 16, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lin, H.; Nien, F.; Wu, V.; Jiang, Y. Serum Vascular Adhesion Protein-1 Predicts End-Stage Renal Disease in Patients with Type 2 Diabetes. PLoS ONE 2016, 11, e0147981. [Google Scholar] [CrossRef] [PubMed]
- Karadi, I.; Meszaros, Z.; Csanyi, A.; Szombathy, T.; Hosszufalusi, N.; Romics, L.; Magyar, K. Serum semicarbazide-sensitive amine oxidase (SSAO) activity is an independent marker of carotid atherosclerosis. Clin. Chim. Acta 2002, 323, 139–146. [Google Scholar] [CrossRef]
- Nunes, S.F.; Figueiredo, I.V.; Soares, P.J.; Costa, N.E.; Lopes, M.C.; Caramona, M.M. Semicarbazide-sensitive amine oxidase activity and total nitrite and nitrate concentrations in serum: Novel biochemical markers for type 2 diabetes? Acta Diabetol. 2009, 46, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.F.; Figueiredo, I.V.; Pereira, J.S.; Soares, P.J.; Caramona, M.M.; Callingham, B. Changes in the activities of semicarbazide-sensitive amine oxidase in inferior mesenteric artery segments and in serum of patients with type 2 diabetes. Acta Diabetol. 2010, 47, 179–182. [Google Scholar] [CrossRef]
- Atari-Hajipirloo, S.; Valizadeh, N.; Khadem-Ansari, M.H.; Rasmi, Y.; Kheradmand, F. Altered Concentrations of Copper, Zinc, and Iron are Associated With Increased Levels of Glycated Hemoglobin in Patients With Type 2 Diabetes Mellitus and Their First-Degree Relatives. Int. J. Endocrinol. Metab. 2016, 14, e33273. [Google Scholar] [CrossRef] [Green Version]
- Papukashvili, D.; Rcheulishvili, N.; Deng, Y. Attenuation of Weight Gain and Prevention of Associated Pathologies by Inhibiting SSAO. Nutrients 2020, 12, 184. [Google Scholar] [CrossRef] [Green Version]
- Adhihetty, P.J.; Beal, M.F. Creatine and Its Potential Therapeutic Value for Targeting Cellular Energy Impairment in Neurodegenerative Diseases. Neuromol. Med. 2008, 10, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Duarte-Silva, S.; Neves-Carvalho, A.; Soares-Cunha, C.; Silva, J.M.; Teixeira-Castro, A.; Vieira, R.; Silva-Fernandes, A.; Maciel, P. Neuroprotective Effects of Creatine in the CMVMJD135 Mouse Model of Spinocerebellar Ataxia Type 3. Mov. Disord. 2018, 33, 815–826. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp. Gerontol. 2018, 108, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Hultman, E.; Kaijser, L.; Kristjansson, S.; Rolf, C.J.; Nyquist, O.; Sylvén, C. Creatine Supplementation in Chronic Heart Failure Increases Skeletal Muscle Creatine Phosphate and Muscle Performance. Cardiovasc. Res. 1995, 30, 413–418. [Google Scholar] [CrossRef]
- Sales, L.P.; Pinto, A.J.; Rodrigues, S.F.; Alvarenga, J.C.; Gonçalves, N.; Sampaio-Barros, M.M.; Benatti, F.B.; Gualano, B.; Rodrigues Pereira, R.M. Creatine supplementation (3g/day) and bone health in older women: A 2-year, randomized, placebo-controlled trial. J. Gerontol. A. Biol. Sci. Med. Sci. 2020, 75, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Galvan, E.; Walker, D.K.; Simbo, S.Y.; Dalton, R.; Levers, K.; O’Connor, A.; Goodenough, C.; Barringer, N.D.; Greenwood, M.; Rasmussen, C.; et al. Acute and Chronic Safety and Efficacy of Dose Dependent Creatine Nitrate Supplementation and Exercise Performance. J. Int. Soc. Sports. Nutr. 2016, 13, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, M.; Trojian, T.H. Creatine supplementation. Curr. Sports. Med. Rep. 2013, 12, 240–244. [Google Scholar] [CrossRef]
- Post, A.; Tsikas, D.; Bakker, S.J.L. Creatine is a Conditionally Essential Nutrient in Chronic Kidney Disease: A Hypothesis and Narrative Literature Review. Nutrients 2019, 11, 1044. [Google Scholar] [CrossRef] [Green Version]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports. Nutr 2017, 14, 18. [Google Scholar] [CrossRef]
- Inácio, S.G.; de Oliveira, G.V.; Alvares, T.S. Caffeine and Creatine Content of Dietary Supplements Consumed by Brazilian Soccer Players. Int. J. Sport. Nutr. Exerc. Metab. 2016, 26, 323–329. [Google Scholar] [CrossRef]
- Buford, T.W.; Kreider, R.B.; Stout, J.R.; Greenwood, M.; Campbell, B.; Spano, M.; Ziegenfuss, T.; Lopez, H.; Landis, J.; Antonio, J. International Society of Sports Nutrition position stand: Creatine supplementation and exercise. J. Int. Soc. Sports. Nutr. 2007, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Gufford, B.T.; Sriraghavan, K.; Miller, N.J.; Miller, D.W.; Gu, X.; Vennerstrom, J.L.; Robinson, D.H. Physicochemical characterization of creatine N-methylguanidinium salts. J. Diet. Suppl. 2010, 7, 240–252. [Google Scholar] [CrossRef]
- Gufford, B.T.; Ezell, E.L.; Robinson, D.H.; Miller, D.W.; Miller, N.J.; Gu, X.; Vennerstrom, J.L. pH-Dependent Stability of Creatine Ethyl Ester: Relevance to Oral Absorption. J. Diet. Suppl. 2013, 10, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Jagim, A.R.; Oliver, J.M.; Sanchez, A.; Galvan, E.; Fluckey, J.; Riechman, S.; Greenwood, M.; Kelly, K.; Meininger, C.; Rasmussen, C.; et al. A buffered form of creatine does not promote greater changes in muscle creatine content, body composition, or training adaptations than creatine monohydrate. J. Int. Soc. Sports. Nutr. 2012, 9, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, N.D.; Hall, R.D.; Blazevich, A.J. Creatine serum is not as effective as creatine powder for improving cycle sprint performance in competitive male team-sport athletes. J. Strength. Cond. Res. 2004, 18, 272–275. [Google Scholar] [PubMed]
- Selsby, J.T.; DiSilvestro, R.A.; Devor, S.T. Mg2+-creatine chelate and a low-dose creatine supplementation regimen improve exercise performance. J. Strength. Cond. Res. 2004, 18, 311–315. [Google Scholar] [PubMed]
- Davani-Davari, D.; Karimzadeh, I.; Ezzatzadegan-Jahromi, S.; Mahdi Sagheb, M. Potential Adverse Effects of Creatine Supplement on the Kidney in Athletes and Bodybuilders. Iran. J. Kidney. Dis. 2018, 12, 253–260. [Google Scholar]
- Jonas, S.K.; Riley, P.A.; Willson, R.L. Hydrogen Peroxide Cytotoxicity. Low-Temperature Enhancement by Ascorbate or Reduced Lipoate. Biochem. J. 1989, 264, 651–655. [Google Scholar] [CrossRef]
- Quievryn, G.; Zhitkovich, A. Loss of DNA-protein crosslinks from formaldehyde-exposed cells occurs through spontaneous hydrolysis and an active repair process linked to proteosome function. Carcinogenesis 2000, 21, 1573–1580. [Google Scholar] [CrossRef]
- Hjelmesæth, J.; Røislien, J.; Nordstrand, N.; Hofsø, D.; Hager, H.; Hartmann, A. Low serum creatinine is associated with type 2 diabetes in morbidly obese women and men: A cross-sectional study. BMC Endocr. Disord. 2010, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Harita, N.; Hayashi, T.; Kogawa Sato, K.; Nakamura, Y.; Yoneda, T.; Endo, G.; Kambe, H. Lower Serum Creatinine Is a New Risk Factor of Type 2 Diabetes. The Kansai Healthcare Study. Diabetes Care 2009, 32, 424–426. [Google Scholar] [CrossRef] [Green Version]
- Nie, Q.; Chen, H.; Hu, J.; Gao, H.; Fan, L.; Long, Z.; Nie, S. Arabinoxylan Attenuates Type 2 Diabetes by Improvement of Carbohydrate, Lipid, and Amino Acid Metabolism. Mol. Nutr. Food. Res. 2018, 62, e1800222. [Google Scholar] [CrossRef]
- Gualano, B.; De Salles Painelli, V.; Roschel, H.; Lugaresi, R.; Dorea, E.; Artioli, G.G.; Lima, F.R.; da Silva, M.E.; Cunha, M.R.; Seguro, A.C.; et al. Creatine supplementation does not impair kidney function in type 2 diabetic patients: A randomized, double-blind, placebo-controlled, clinical trial. Eur. J. Appl. Physiol. 2011, 111, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Op’t Eijnde, B.; Jijakli, H.; Hespel, P.; Malaisse, W.J. Creatine supplementation increases soleus muscle creatine content and lowers the insulinogenic index in an animal model of inherited type 2 diabetes. Int. J. Mol. Med. 2006, 17, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.R.; Ferreira, J.C.; De Siqueira-Filho, M.A.; Carvalho, C.R.; Lancha, A.H., Jr.; Gualano, B. Creatine-induced glucose uptake in type 2 diabetes: A role for AMPK-α? Amino Acids 2012, 43, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Ročić, B.; Znaor, A.; Ročić, P.; Weber, D.; Vučić Lovrenčić, M. Comparison of antihyperglycemic effects of creatine and glibenclamide in type II diabetic patients. Wien. Med. Wochenschr. 2011, 161, 519–523. [Google Scholar]
- Zorzano, A.; Abella, A.; Marti, L.; Carpéné, C.; Palacín, M.; Testar, X. Semicarbazide-sensitive amine oxidase activity exerts insulin-like effects on glucose metabolism and insulin-signaling pathways in adipose cells. Biochim. Biophys. Acta. 2003, 1647, 3–9. [Google Scholar] [CrossRef]
- Stolen, C.M.; Madanat, R.; Marti, L.; Kari, S.; Gennady, G.; Yegutkin, H.S.; Zorzano, A.; Jalkanen, S. Semicarbazide-sensitive amine oxidase overexpression has dual consequences: Insulin mimicry and diabetes-like complications. FASEB J. 2004, 18, 702–704. [Google Scholar] [CrossRef]
- Stolen, C.M.; Yegutkin, G.G.; Kurkijärvi, R.; Bono, P.; Alitalo, K.; Jalkanen, S. Origins of Serum Semicarbazide-Sensitive Amine Oxidase. Circ. Res. 2004, 95, 50–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baoquan, C.; Wang, L.; Zhang, Z.; Zhang, Y.; Deng, Y. Distribution and Accumulation of Caffeine in Rat Tissues and Its Inhibition on Semicarbazide-Sensitive Amine Oxidase. Neurotoxicology 2012, 33, 1248–1253. [Google Scholar]
- Wang, L.; Xiao, S.; Li, Y.; Wang, L.; Che, B.; Zhao, X.; Deng, Y. Potential Toxicity of Chronic Creatine Supplementation in Mice. In Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering (ICBBE 2009), Beijing, China, 11–13 June 2009; IEEE: Beijing, China, 2009. [Google Scholar] [CrossRef]
- Gualano, B.; Novaes, R.B.; Artioli, G.G.; Freire, T.O.; Coelho, D.F.; Scagliusi, F.B.; Rogeri, P.S.; Roschel, H.; Ugrinowitsch, C.; Lancha, A.H. Effects of creatine supplementation on glucose tolerance and insulin sensitivity in sedentary healthy males undergoing aerobic training. Amino Acids 2008, 34, 245–250. [Google Scholar] [CrossRef]
- Reinehr, T. Inflammatory markers in children and adolescents with type 2 diabetes mellitus. Clin. Chim. Acta 2019, 496, 100–107. [Google Scholar] [CrossRef]
- Yu, P.H.; Wang, M.; Deng, Y.; Fan, H.; Shira-Bock, L. Involvement of Semicarbazide-Sensitive Amine Oxidase-Mediated Deamination in Atherogenesis in KKAy Diabetic Mice Fed with High Cholesterol Diet. Diabetologia 2002, 45, 1255–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, T.; Zhang, Y.; Luo, W.; Lv, J.; Han, C.; Hamlin, J.N.R.; Luo, H.; Li, H.; Wan, Y.; Yang, X.; et al. Formaldehyde induces diabetes-associated cognitive impairments. FASEB J. 2018, 32, 3669–3679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boor, P.J.; Trent, M.B.; Lyles, G.A.; Tao, M.; Ansari, G.A. Methylamine Metabolism to Formaldehyde by Vascular Semicarbazide-Sensitive Amine Oxidase. Toxicology 1992, 73, 251–258. [Google Scholar] [CrossRef]
- Obata, T. Diabetes and Semicarbazide-Sensitive Amine Oxidase (SSAO) Activity: A Review. Life Sci. 2006, 79, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Magyar, K.; Mészáros, Z. Semicarbazide-Sensitive Amine Oxidase (SSAO): Present and Future. Inflammopharmacology 2003, 11, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Nilnumkhum, A.; Rattiyaporn, K.; Sunisa, Y.; Visith, T. Caffeine Inhibits Hypoxia-Induced Renal Fibroblast Activation by Antioxidant Mechanism. Cell Adh. Migr. 2019, 13, 260–272. [Google Scholar] [CrossRef] [Green Version]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, A.; Tipton, K. Inhibition of Bovine Plasma Semicarbazide-Sensitive Amine Oxidase by Caffeine. J. Biochem. Mol. Toxicol. 2011, 25, 26–27. [Google Scholar] [CrossRef]
- Urry, E.; Jetter, A.; Holst, S.C.; Berger, W.; Spinas, G.A.; Langhans, W.; Landolt, H.P. A case-control field study on the relationships among type 2 diabetes, sleepiness and habitual caffeine intake. J. Psychopharmacol. 2017, 31, 233–242. [Google Scholar] [CrossRef]
- Urry, E.; Jetter, A.; Landolt, H.P. Assessment of CYP1A2 enzyme activity in relation to type-2 diabetes and habitual caffeine intake. Nutr. Metab. (Lond) 2016, 13, 66. [Google Scholar] [CrossRef] [Green Version]
- Chrysant, S.G. The impact of coffee consumption on blood pressure, cardiovascular disease and diabetes mellitus. Expert Rev. Cardiovasc. Ther. 2017, 15, 151–156. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, Y.; Ge, S.; Lu, H.; Chen, R.; Fang, P.; Shen, Y.; Wang, C.; Jia, W. Coffee consumption is positively related to insulin secretion in the Shanghai High-Risk Diabetic Screen ( SHiDS) Study. Nutr. Metab. (Lond) 2018, 15, 84. [Google Scholar] [CrossRef] [Green Version]
- Neves, J.S.; Leitão, L.; Magriço, R.; Vieira, M.B.; Dias, C.V.; Oliveira, A.; Carvalho, D.; Claggett, B. Caffeine consumption and mortality in diabetes: An analysis of NHANES 1999-2010. Front. Endocrinol. (Lausanne) 2018, 9, 547. [Google Scholar] [CrossRef]
- Duarte, J.M.N.; Skoug, C.; Silva, H.B.; Carvalho, R.A.; Gruetter, R.; Cunha, R.A. Impact of Caffeine Consumption on Type 2 Diabetes-Induced Spatial Memory Impairment and Neurochemical Alterations in the Hippocampus. Front. Neurosci. 2019, 12, 1015. [Google Scholar] [CrossRef] [PubMed]
- Rustenbeck, I.; Lier-Glaubitz, V.; Willenborg, M.; Eggert, F.; Engelhardt, U.; Jörns, A. Effect of chronic coffee consumption on weight gain and glycaemia in a mouse model of obesity and type 2 diabetes. Nutr. Diabetes. 2014, 30, e123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes. Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, L.A.; de Freitas, L.; Medeiros, T.E.; Osiecki, R.; Garcia Michel, R.; Snak, A.L.; Malfatti, C.R. Caffeine modifies blood glucose availability during prolonged low-intensity exercise in individuals with type-2 diabetes. Colomb. Med. (Cali) 2014, 45, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry. 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erukainure, O.L.; Ijomone, O.M.; Oyebode, O.A.; Chukwuma, C.I.; Aschner, M.; Islam, M.S. Hyperglycemia-Induced Oxidative Brain Injury: Therapeutic Effects of Cola Nitida Infusion against Redox Imbalance, Cerebellar Neuronal Insults, and Upregulated Nrf2 Expression in Type 2 Diabetic Rats. Food Chem. Toxicol. 2019, 127, 206–217. [Google Scholar] [CrossRef]
- Dewar, L.; Heuberger, R. The effect of acute caffeine intake on insulin sensitivity and glycemic control in people with diabetes. Diabetes Metab. Syndr. 2017, 2, S631–S635. [Google Scholar] [CrossRef]
- Lane, J.D.; Barkauskas, C.E.; Surwit, R.S.; Feinglos, M.N. Caffeine impairs glucose metabolism in type 2 diabetes. Diabetes Care 2004, 27, 2047–2048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Xue, W.; Liang, S.; Zhao, J.; Zhang, X. Acute caffeine ingestion reduces insulin sensitivity in healthy subjects: A systematic review and meta-analysis. Nutr. J. 2016, 15, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinto, C.L.; Botelho, P.B.; Pimentel, G.D.; Campos-Ferraz, P.L.; Mota, J.F. Creatine supplementation and glycemic control: A systematic review. Amino Acids 2016, 48, 2103–2129. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of Caffeine on Human Health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Smith, P.M.; Davison, R.C.; Hughes, M.G. Caffeine is ergogenic after supplementation of oral creatine monohydrate. Med. Sci. Sports. Exerc. 2002, 34, 1785–1792. [Google Scholar] [CrossRef]
- Lee, C.L.; Lin, J.C.; Cheng, C.F. Effect of caffeine ingestion after creatine supplementation on intermittent high-intensity sprint performance. Eur. J. Appl. Physiol. 2011, 111, 1669–1677. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [Green Version]
- Trexler, E.T.; Smith-Ryan, A.E.; Roelofs, E.J.; Hirsch, K.R.; Persky, A.M.; Mock, M.G. Effects of Coffee and Caffeine Anhydrous Intake During Creatine Loading. J. Strength. Cond. Res. 2016, 30, 1438–1446. [Google Scholar] [CrossRef] [Green Version]
- Trexler, E.T.; Smith-Ryan, A.E. Creatine and caffeine: Considerations for concurrent supplementation. Int. J. Sport. Nutr. Exerc. Metab. 2015, 25, 607–623. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Caffeine and creatine use in sport. Ann. Nutr. Metab. 2010, 57, 1–8. [Google Scholar] [CrossRef]
- Franco, F.S.C.; Costa, N.M.; Ferreira, S.A.; Carneiro-junior, M.A.; Natali, A.J. The effects of a high dosage of creatine and caffeine supplementation on the lean body mass composition of rats submitted to vertical jumping training. J. Int. Soc. Sports. Nutr. 2011, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akash, M.S.H.; Rehman, K.; Chen, S. Effects of coffee on type 2 diabetes mellitus. Nutrition 2014, 30, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hudson, R.; Kilpatrick, K.; Graham, T.E.; Ross, R. Caffeine ingestion is associated with reductions in glucose uptake independent of obesity and type 2 diabetes before and after exercise training. Diabetes Care 2005, 28, 566–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutz, M.R.; Gunter, M.J. Creatine monohydrate supplementation on body weight and percent body fat. Strength. Cond. Res. 2003, 17, 817–821. [Google Scholar]
- Schäfer, L.U.; Hayes, M.; Dekerle, J. Creatine supplementation improves performance above critical power but does not influence the magnitude of neuromuscular fatigue at task failure. Exp. Physiol. 2019, 104, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Icken, D.; Feller, S.; Engeli, S.; Mayr, A.; Müller, A.; Hilbert, A.; de Zwaan, M. Caffeine intake is related to successful weight loss maintenance. Eur. J. Clin. Nutr. 2016, 70, 532–534. [Google Scholar] [CrossRef]
- Seal, A.D.; Bardis, C.N.; Gavrieli, A.; Grigorakis, P.; Adams, J.D.; Arnaoutis, G.; Yannakoulia, M.; Kavouras, S.A. Coffee with High but Not Low Caffeine Content Augments Fluid and Electrolyte Excretion at Rest. Front. Nutr. 2017, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Jodra, P.; Lago-Rodríguez, A.; Sánchez-Oliver, A.J.; López-Samanes, A.; Pérez-López, A.; Veiga-Herreros, P.; San Juan, A.F.; Domínguez, R. Effects of caffeine supplementation on physical performance and mood dimensions in elite and trained-recreational athletes. J. Int. Soc. Sports. Nutr. 2020, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Sacramento, J.F.; Ribeiro, M.J.; Yubero, S.; Melo, B.F.; Obeso, A.; Guarino, M.P.; Gonzalez, C.; Conde, S.V. Disclosing caffeine action on insulin sensitivity: Effects on rat skeletal muscle. Eur. J. Pharm. Sci. 2015, 70, 107–116. [Google Scholar] [CrossRef]
- Urzúa, Z.; Trujillo, X.; Huerta, M.; Trujillo-Hernández, B.; Ríos-Silva, M.; Onetti, C.; Ortiz-Mesina, M.; Sánchez-Pastor, E. Effects of chronic caffeine administration on blood glucose levels and on glucose tolerance in healthy and diabetic rats. J. Int. Med. Res. 2012, 40, 2220–2230. [Google Scholar] [CrossRef]
- Jørgensen, E.A.; Knigge, U.; Warberg, J.; Kjaer, A. Histamine and the regulation of body weight. Neuroendocrinology 2007, 86, 210–214. [Google Scholar] [CrossRef]
- Iffiú-Soltész, Z.; Wanecq, E.; Prévot, D.; Grès, S.; Carpéné, C. Histamine oxidation in mouse adipose tissue is controlled by the AOC3 gene-encoded amine oxidase. Inflamm. Res. 2010, 59, S227–S229. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; McCarty, M.F.; OKeefe, J.H. Role of dietary histidine in the prevention of obesity and metabolic syndrome. Open Heart 2018, 5, e000676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, K.; Nakamura, Y.; Inaba, Y.; Matsumoto, M.; Kido, Y.; Asahara, S.; Matsuda, T.; Watanabe, H.; Maeda, A.; Inagaki, F.; et al. Histidine augments the suppression of hepatic glucose production by central insulin action. Diabetes 2013, 62, 2266–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppari, R.; Bjørbæk, C. Leptin revisited: Its mechanism of action and potential for treating diabetes. Nat. Rev. Drug. Discov. 2012, 11, 692–708. [Google Scholar] [CrossRef] [Green Version]
- Pini, A.; Verta, R.; Grange, C.; Gurrieri, M.; Rosa, A.C. Histamine and diabetic nephropathy: An up-to-date overview. Clin. Sci. (Lond). 2019, 133, 41–54. [Google Scholar] [CrossRef]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961.e17. [Google Scholar] [CrossRef] [Green Version]
- Romauch, M. Zinc-α2-glycoprotein is an inhibitor of amine oxidase copper-containing 3. Open Biol. 2019, 9, 190035. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.I.; Waheed, A.; Yadav, S.; Singh, T.P.; Ahmad, F. Zinc alpha 2-glycoprotein: A multidisciplinary protein. Mol. Cancer. Res. 2008, 6, 892–906. [Google Scholar] [CrossRef] [Green Version]
- Gong, F.Y.; Zhang, S.J.; Deng, J.Y.; Zhu, H.J.; Pan, H.; Li, N.S.; Shi, Y.F. Zinc-alpha2-glycoprotein is involved in regulation of body weight through inhibition of lipogenic enzymes in adipose tissue. Int. J. Obes. (Lond) 2009, 33, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Mracek, T.; Gao, D.; Tzanavari, T.; Bao, Y.; Xiao, X.; Stocker, C.; Trayhurn, P.; Bing, C. Downregulation of zinc-{alpha}2-glycoprotein in adipose tissue and liver of obese ob/ob mice and by tumour necrosis factor-alpha in adipocytes. J. Endocrinol. 2010, 204, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.H.; Qi, X.Y.; Li, J.; Wang, Y.B.; Wang, Y.D.; Liao, Z.Z.; Yang, J.; Ran, L.; Wen, G.B.; Liu, J.H. Serum zinc-α2-glycoprotein levels are elevated and correlated with thyroid hormone in newly diagnosed hyperthyroidism. BMC Endocr. Disord. 2019, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhu, H.; Dai, Y.; Pan, H.; Li, N.; Wang, L.; Yang, H.; Yan, K.; Gong, F. Zinc-α2-Glycoprotein Is Associated with Obesity in Chinese People and HFD-Induced Obese Mice. Front. Physiol. 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Li, J.; Chen, Y.; Zhong, X.; Kang, M. Effect of curcumin on visfatin and zinc-α2-glycoprotein in a rat model of non-alcoholic fatty liver disease. Acta. Cir. Bras. 2016, 31, 706–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Luo, X.; Li, Z.H.; Wu, J.C.; Luo, S.Z.; Xu, M.Y. Zinc-α2-glycoprotein 1 attenuates non-alcoholic fatty liver disease by negatively regulating tumour necrosis factor-α. World J. Gastroenterol. 2019, 25, 5451–5468. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, M.; Yamasandhi, P.G. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Indian. J. Endocrinol. Metab. 2018, 22, 421–428. [Google Scholar] [CrossRef]
- Yang, M.; Liu, R.; Li, S.; Luo, Y.; Zhang, Y.; Zhang, L.; Liu, D.; Wang, Y.; Xiong, Z.; Boden, G.; et al. Zinc-α2-glycoprotein is associated with insulin resistance in humans and is regulated by hyperglycemia, hyperinsulinemia, or liraglutide administration: Cross-sectional and interventional studies in normal subjects, insulin-resistant subjects, and subjects with newly diagnosed diabetes. Diabetes Care 2013, 36, 1074–1082. [Google Scholar]
- Tian, M.; Liang, Z.; Liu, R.; Li, K.; Tan, X.; Luo, Y.; Yang, M.; Gu, H.F.; Liu, H.; Li, L.; et al. Effects of sitagliptin on circulating zinc-α2-glycoprotein levels in newly diagnosed type 2 diabetes patients: A randomized trial. Eur. J. Endocrinol. 2016, 174, 147–155. [Google Scholar] [CrossRef]
- Bing, C.; Mracek, T.; Gao, D.; Trayhurn, P. Zinc-α2-glycoprotein: An adipokine modulator of body fat mass? Int. J. Obes. (Lond) 2010, 34, 1559–1565. [Google Scholar] [CrossRef] [Green Version]
- Carpéné, C.; Boulet, N.; Chaplin, A.; Mercader, J. Past, Present and Future Anti-Obesity Effects of Flavin-Containing and/or Copper-Containing Amine Oxidase Inhibitors. Medicines 2019, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Severo, J.S.; Morais, J.B.S.; Beserra, J.B.; Dos Santos, L.R.; de Sousa Melo, S.R.; de Sousa, G.S.; de Matos Neto, E.M.; Henriques, G.S.; do Nascimento Marreiro, N.D. Role of Zinc in Zinc-α2-Glycoprotein Metabolism in Obesity: A Review of Literature. Biol. Trace. Elem. Res. 2020, 193, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Al-Timimi, D.J.; Sulieman, D.M.; Hussen, K.R. Zinc status in type 2 diabetic patients: Relation to the progression of diabetic nephropathy. J. Clin. Diagn. Res. 2014, 8, CC04–CC08. [Google Scholar] [CrossRef] [PubMed]
- Fukunaka, A.; Fujitani, Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Ranasinghe, P.; Pigera, S.; Galappatthy, P.; Katulanda, P.; Constantine, G.R. Zinc and diabetes mellitus: Understanding molecular mechanisms and clinical implications. Daru 2015, 17, 44. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.; Zhang, F.; Zhu, W.; Wu, J.; Liang, M. Copper in Diabetes Mellitus: A Meta-Analysis and Systematic Review of Plasma and Serum Studies. Biol. Trace. Elem. Res. 2017, 177, 53–63. [Google Scholar] [CrossRef]
- Yang, H.; Ralle, M.; Wolfgang, M.J.; Dhawan, N.; Burkhead, J.L.; Rodriguez, S.; Kaplan, J.H.; Wong, G.W.; Haughey, N.; Lutsenko, S. Copper-dependent amino oxidase 3 governs selection of metabolic fuels in adipocytes. PLoS Biol. 2018, 16, e2006519. [Google Scholar] [CrossRef]
- Tanaka, A.; Kaneto, H.; Miyatsuka, T.; Yamamoto, K.; Yoshiuchi, K.; Yamasaki, Y.; Shimomura, I.; Matsuoka, T.A.; Matsuhisa, M. Role of copper ion in the pathogenesis of type 2 diabetes. Endocr. J. 2009, 56, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Squitti, R.; Mende, A.J.; Simonelli, I.; Ricordi, C. Diabetes and Alzheimer’s Disease: Can Elevated Free Copper Predict the Risk of the Disease? J. Alzheimers. Dis. 2017, 56, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Rajpathak, S.N.; Crandall, J.P.; Wylie-Rosett, J.; Kabat, G.C.; Rohan, T.E.; Hu, F.B. The role of iron in type 2 diabetes in humans. Biochim. Biophys. Acta 2009, 1790, 671–681. [Google Scholar] [CrossRef]
- Dutra, F.; Knudsen, F.S.; Curi, D.; Bechara, E.J. Aerobic oxidation of aminoacetone, a threonine catabolite: Iron catalysis and coupled iron release from ferritin. Chem. Res. Toxicol. 2001, 14, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.; Ma, J.; Manson, J.; Willett, W.C.; Hu, F.B. Iron intake and the risk of type 2 diabetes in women: A prospective cohort study. Diabetes Care 2006, 29, 1370–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Suhara, T.; Baba, Y.; Kawasaki, N.K.; Higa, J.K.; Matsui, T. Pathological Roles of Iron in Cardiovascular Disease. Curr. Drug. Targets. 2018, 19, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Aigner, E.; Feldman, A.; Datz, C. Obesity as an emerging risk factor for iron deficiency. Nutrients 2014, 6, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; Lu, M.; Liu, L.; Shi, L. Association between serum Fe levels and obesity: A meta-analysis. Nutr. Hosp. 2015, 31, 2451–2454. [Google Scholar] [PubMed]
- Hu, Z.; Chen, J.; Sun, X.; Wang, L.; Wang, A. Efficacy of vitamin D supplementation on glycemic control in type 2 diabetes patients: A meta-analysis of interventional studies. Medicine (Baltimore) 2019, 98, e14970. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D deficiency and diabetes. Biochem. J. 2017, 474, 1321–1332. [Google Scholar] [CrossRef]
- Nakashima, A.; Yokoyama, K.; Yokoo, T.; Urashima, M. Role of vitamin D in diabetes mellitus and chronic kidney disease. World J. Diabetes 2016, 7, 89–100. [Google Scholar]
- Sturza, A.; Văduva, A.; Uțu, D.; Rațiu, C.; Pop, N.; Duicu, O.; Popoiu, C.; Boia, E.; Matusz, P.; Muntean, D.M.; et al. Vitamin D. improves vascular function and decreases monoamine oxidase A expression in experimental diabetes. Mol. Cell. Biochem. 2019, 453, 33–40. [Google Scholar] [CrossRef]
- Carpéné, C.; Mercader, J.; Le Gonidec, S.; Schaak, S.; Mialet-Perez, J.; Jakaroff-Girard, A.; Galitzky, J. Body fat reduction without cardiovascular changes in mice after oral treatment with the MAO inhibitor phenelzine. Br. J. Pharmacol. 2018, 175, 2428–2440. [Google Scholar]
- Carpéné, C.; Gómez-Zorita, S.; Chaplin, A.; Mercader, J. Metabolic Effects of Oral Phenelzine Treatment on High-Sucrose-Drinking Mice. Int J. Mol. Sci. 2018, 19, 2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercader, J.; Sabater, A.G.; Le Gonidec, S.; Decaunes, P.; Chaplin, A.; Gómez-Zorita, S.; Milagro, F.I.; Carpéné, C. Oral Phenelzine Treatment Mitigates Metabolic Disturbances in Mice Fed a High-Fat Diet. J. Pharmacol. Exp. Ther. 2019, 371, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Khan, S.; Naseem, I. Antioxidant Role of Vitamin D in Mice With Alloxan-Induced Diabetes. Can. J. Diabetes 2018, 42, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Saedisomeolia, A.; Taheri, E.; Djalali, M.; Djazayeri, A.; Qorbani, M.; Rajab, A.; Larijani, B. Vitamin D status and its association with antioxidant profiles in diabetic patients: A cross-sectional study in Iran. Indian J. Med. Sci. 2013, 67, 29–37. [Google Scholar] [PubMed] [Green Version]
- Góth, L.; Nagy, T. Acatalasemia and diabetes mellitus. Arch. Biochem. Biophys. 2012, 525, 195–200. [Google Scholar] [CrossRef]
- Goth, L. Catalase Deficiency and Type 2 Diabetes. Diabetes Care 2008, 31, 2008. [Google Scholar] [CrossRef] [Green Version]
- Sozmen, B.; Delen, Y.; Girgin, F.K.; Sozmen, E.Y. Catalase and Paraoxonase in Hypertensive Type 2 Diabetes Mellitus: Correlation with Glycemic Control. Clin. Biochem. 1999, 32, 423–427. [Google Scholar] [CrossRef]
- Kechrid, Z.; Hamdi, M.; Nazıroğlu, M.; Flores-Arce, M. Vitamin D supplementation modulates blood and tissue zinc, liver glutathione and blood biochemical parameters in diabetic rats on a zinc-deficient diet. Biol. Trace Elem. Res. 2012, 148, 371–377. [Google Scholar] [CrossRef]
- Jakobsen, J.; Knuthsen, P. Stability of vitamin D in foodstuffs during cooking. Food Chem. 2014, 148, 170–175. [Google Scholar] [CrossRef]
- Jayasena, D.D.; Jung, S.; Kim, S.H.; Kim, H.J.; Alahakoon, A.U.; Lee, J.H.; Jo, C. Endogenous functional compounds in Korean native chicken meat are dependent on sex, thermal processing and meat cut. J. Sci. Food. Agric. 2015, 95, 771–775. [Google Scholar] [CrossRef]
- The U.S. Department of Agriculture’s (USDA’s). FoodData Central. National Nutrient Database for Standard Reference Legacy, Nutrients: Caffeine. 2018. Available online: https://fdc.nal.usda.gov (accessed on 14 April 2020).
- Foodb. Compound L-Histidine FDB011856. 2019. Available online: https://foodb.ca/compounds/FDB011856 (accessed on 14 April 2020).
- The U.S. Department of Agriculture’s (USDA’s). FoodData Central. Zinc. 2019. Available online: https://fdc.nal.usda.gov (accessed on 14 April 2020).
- The U.S. Department of Agriculture’s (USDA’s). FoodData Central. Copper. 2019. Available online: https://fdc.nal.usda.gov (accessed on 14 April 2020).
- The U.S. Department of Agriculture’s (USDA’s). FoodData Central. USDA National Nutrient Database for Standard Reference, Iron, Release 28 October 22, 2015 11:13 EDT. Available online: https://fdc.nal.usda.gov (accessed on 14 April 2020).
- The U.S. Department of Agriculture’s (USDA’s). FoodData Central. Vitamin D. 2019. Available online: https://fdc.nal.usda.gov (accessed on 14 April 2020).

| Creatine Natural and Synthesized Sources | Major Sources | References |
|---|---|---|
| Creatine dietary sources | Red meat | Post et al. [46] Kreider et al. [47] |
| Dairy products | ||
| Seafood | ||
| Creatine supplements | Creatine monohydrate | Inácio [48] Buford et al. [49] |
| Creatine nitrate | Galvan et al. [44] | |
| Creatine hydrochloride | Gufford et al. [50] | |
| Creatine ethyl ester | Gufford et al. [51] | |
| Buffered creatine | Jagim et al. [52] | |
| Liquid creatine | Gill et al. [53] | |
| Creatine magnesium chelate | Selsby et al. [54] |
| Compounds | Healthy Humans/Animal Models | Type 2 Diabetes Mellitus – Humans | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight-Gain | Weight-Loss | (↑) SSAO Upregulation | (↓) SSAO Inhibition | Water-Retention | Increased Urinary-Excretion | Ergogenic Effect | Reducing Insulin Sensitivity | Improving Glucose Tolerance | Anti-Hyperglycemic Effect | Impairing Kidney Function | |
| Creatine | Yes [105] human | No [105] human | Yes [21] animal (healthy) | No [21] animal (healthy) | Yes [105] human | No [105] human | Yes [106] human (healthy) | No [70] human (healthy) | Yes [70] human (healthy) | Yes [64] | No [61] |
| Caffeine | No [107] human | Yes [107] human | No [68] animal | Yes [68] animal | No [108] human | Yes [108] human | Yes [109] human | Yes [110] animal | No effect on healthy animals Yes (in diabetic animals) [111] | Yes [82] No [86] Yes [105] | No [84] |
| Title of Study | Study Object | Used Doses | Summary | References |
|---|---|---|---|---|
| Caffeine is ergogenic after supplementation of oral creatine monohydrate | Humans | Creatine - 0.3 g/kg/day Caffeine - 5 mg/kg/day | Caffeine ingestion has an ergogenic effect in trained males after 6 days of creatine loading administration and caffeine abstinence | Doherty et al. [96] |
| Effect of caffeine ingestion after creatine supplementation on intermittent high-intensity sprint performance | Humans | Creatine - 0.3 g/kg/day Caffeine - 6 mg/kg/day | Caffeine ingestion after creatine loading for 5 days increased the strength of physically active men as compared to the control group | Lee et al. [97] |
| Effects of coffee and caffeine anhydrous intake during creatine loading | Humans | Creatine - 5 g 4 times a day Caffeine - 300 mg 4 times a day | These findings suggest that neither creatine alone, nor in combination with caffeine or coffee, significantly affected performance compared to placebo. | Trexler et al. [99] |
| The effects of a high dosage of creatine and caffeine supplementation on the lean body mass composition of rats submitted to vertical jumping training | Rats | Creatine - 0.430 g/kg/day (loading), 0.143 g/kg (maintenance) Caffeine −15 mg/kg/day | Creatine and caffeine combination did not influence on lean body mass in sedentary and exercised rats while caffeine administration reduced fat | Franco et al. [102] |
| Promising Agents to Combine with Creatine | Major Dietary Sources | References |
|---|---|---|
| Caffeine | Coffee | [162] |
| Tea | ||
| Cocoa | ||
| Chocolates | ||
| Histidine | Meat and meat products | [163] |
| Grain products | ||
| Dairy products | ||
| Vegetables | ||
| Seafood | ||
| Egg | ||
| Beans | ||
| Nuts | ||
| Zn | Meat | [164] |
| Legumes | ||
| Poultry | ||
| Dairy products | ||
| Nuts | ||
| Seafood | ||
| Cu | Legumes | [165] |
| Mushrooms | ||
| Chocolate | ||
| Nuts | ||
| Beef | ||
| Seafood | ||
| Fe | Liver | [166] |
| Beef, pork, lamb | ||
| Beans | ||
| Cereals | ||
| Seafood | ||
| Nuts | ||
| Peas | ||
| Vitamin D | Fish | [167] |
| Mushrooms | ||
| Egg | ||
| Liver | ||
| Beef | ||
| Chicken breast | ||
| Dairy products | ||
| Soybeans |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papukashvili, D.; Rcheulishvili, N.; Deng, Y. Beneficial Impact of Semicarbazide-Sensitive Amine Oxidase Inhibition on the Potential Cytotoxicity of Creatine Supplementation in Type 2 Diabetes Mellitus. Molecules 2020, 25, 2029. https://doi.org/10.3390/molecules25092029
Papukashvili D, Rcheulishvili N, Deng Y. Beneficial Impact of Semicarbazide-Sensitive Amine Oxidase Inhibition on the Potential Cytotoxicity of Creatine Supplementation in Type 2 Diabetes Mellitus. Molecules. 2020; 25(9):2029. https://doi.org/10.3390/molecules25092029
Chicago/Turabian StylePapukashvili, Dimitri, Nino Rcheulishvili, and Yulin Deng. 2020. "Beneficial Impact of Semicarbazide-Sensitive Amine Oxidase Inhibition on the Potential Cytotoxicity of Creatine Supplementation in Type 2 Diabetes Mellitus" Molecules 25, no. 9: 2029. https://doi.org/10.3390/molecules25092029





