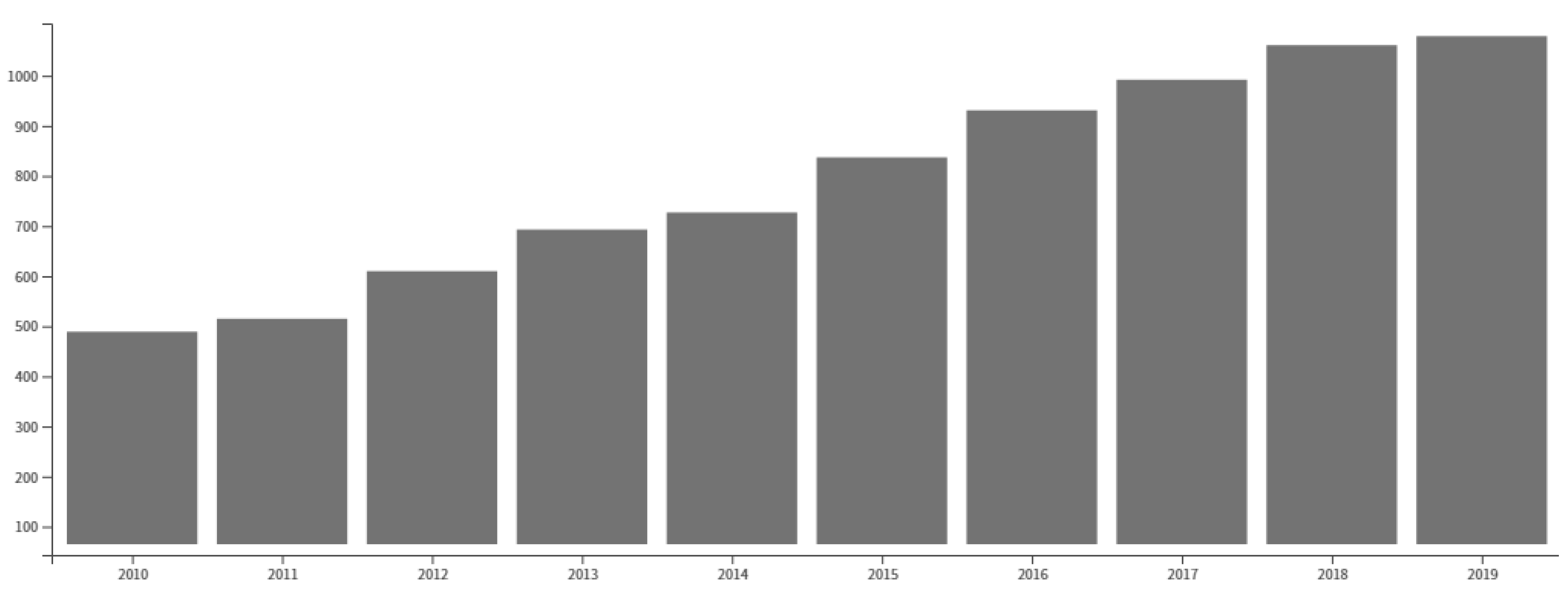
Recent Progress of Cu-Catalyzed Azide-Alkyne Cycloaddition Reactions (CuAAC) in Sustainable Solvents: Glycerol, Deep Eutectic Solvents, and Aqueous Media
Abstract
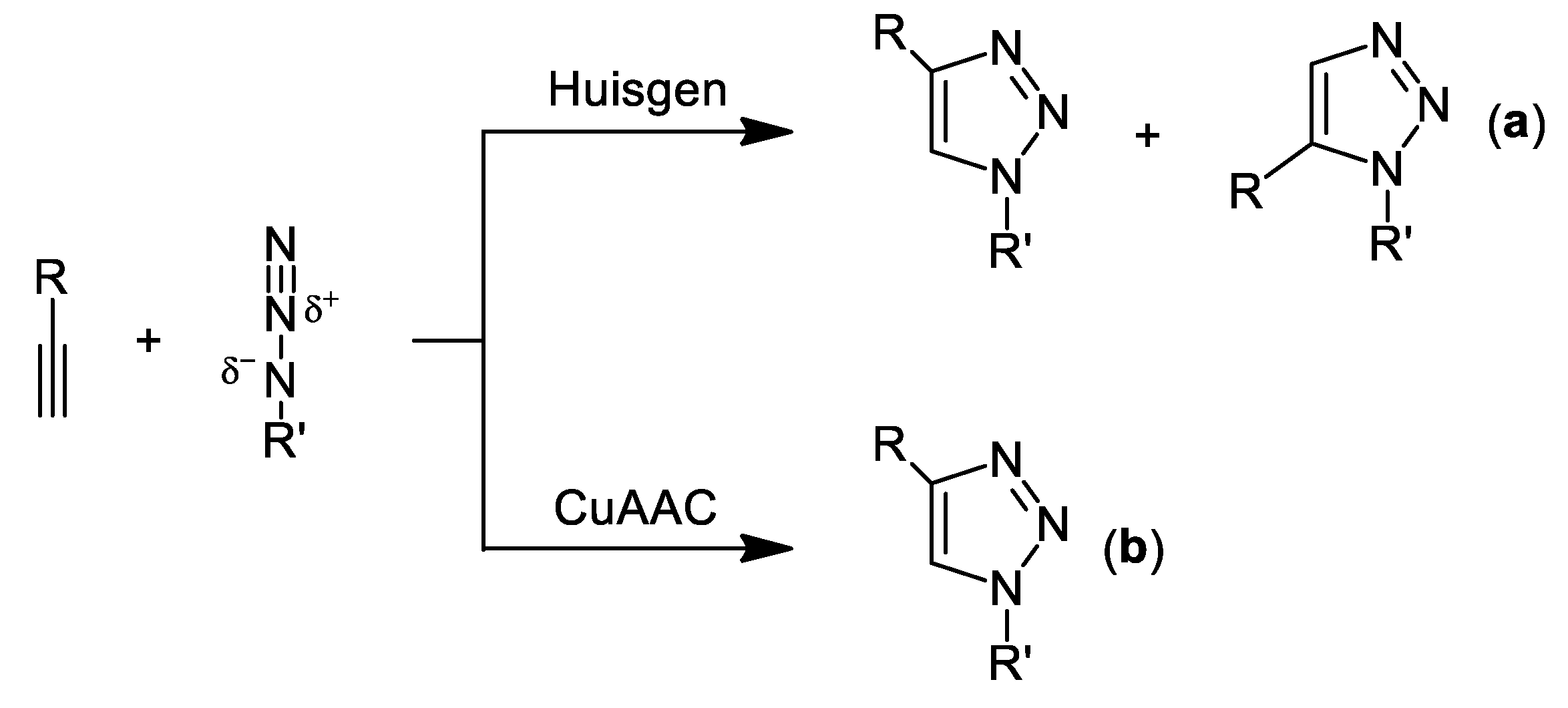
:1. Introduction
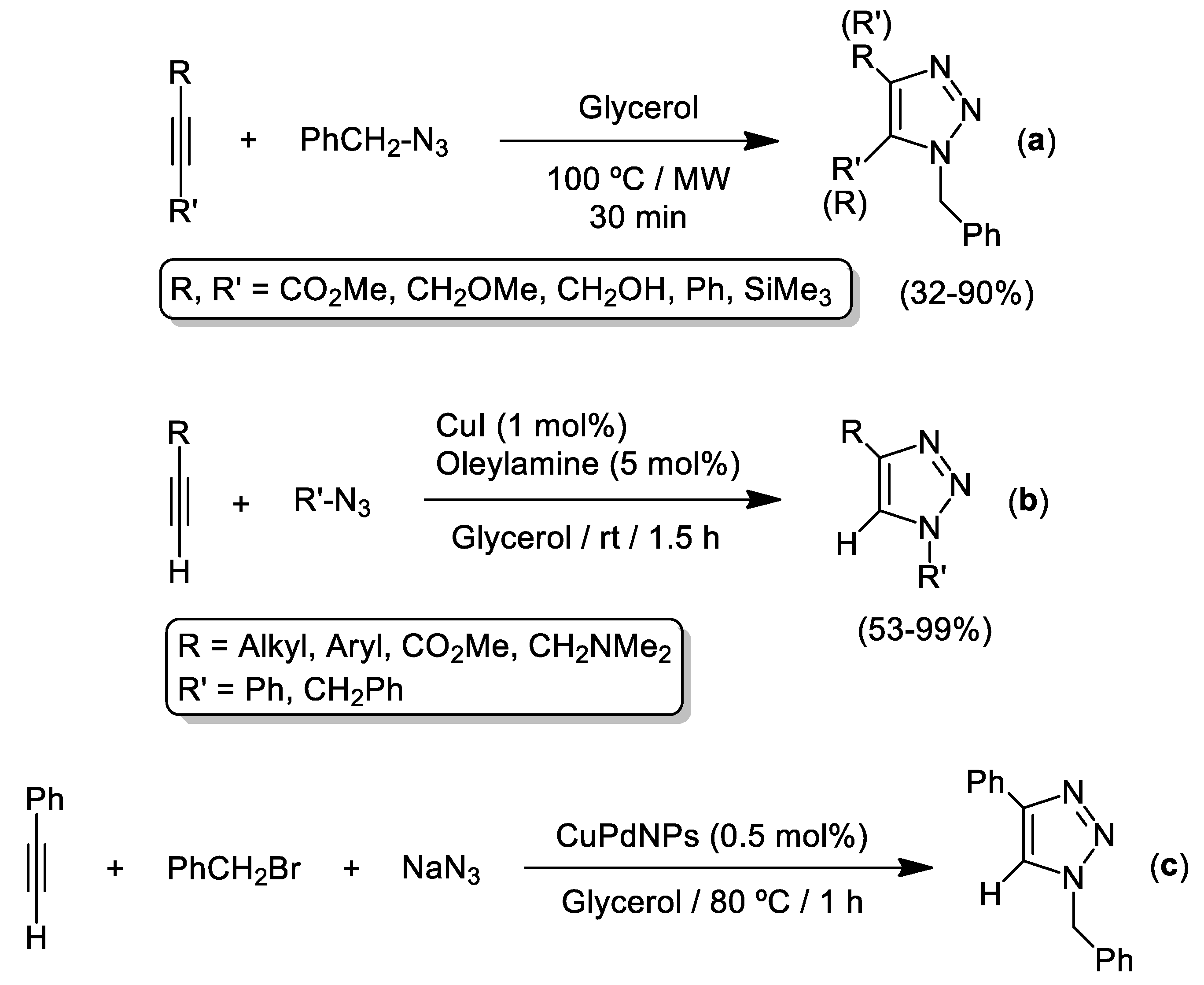
2. Cu-Catalyzed 1,3-Dipolar Cycloaddition of Azides and Alkynes (CuAAC) in Glycerol (Gly)
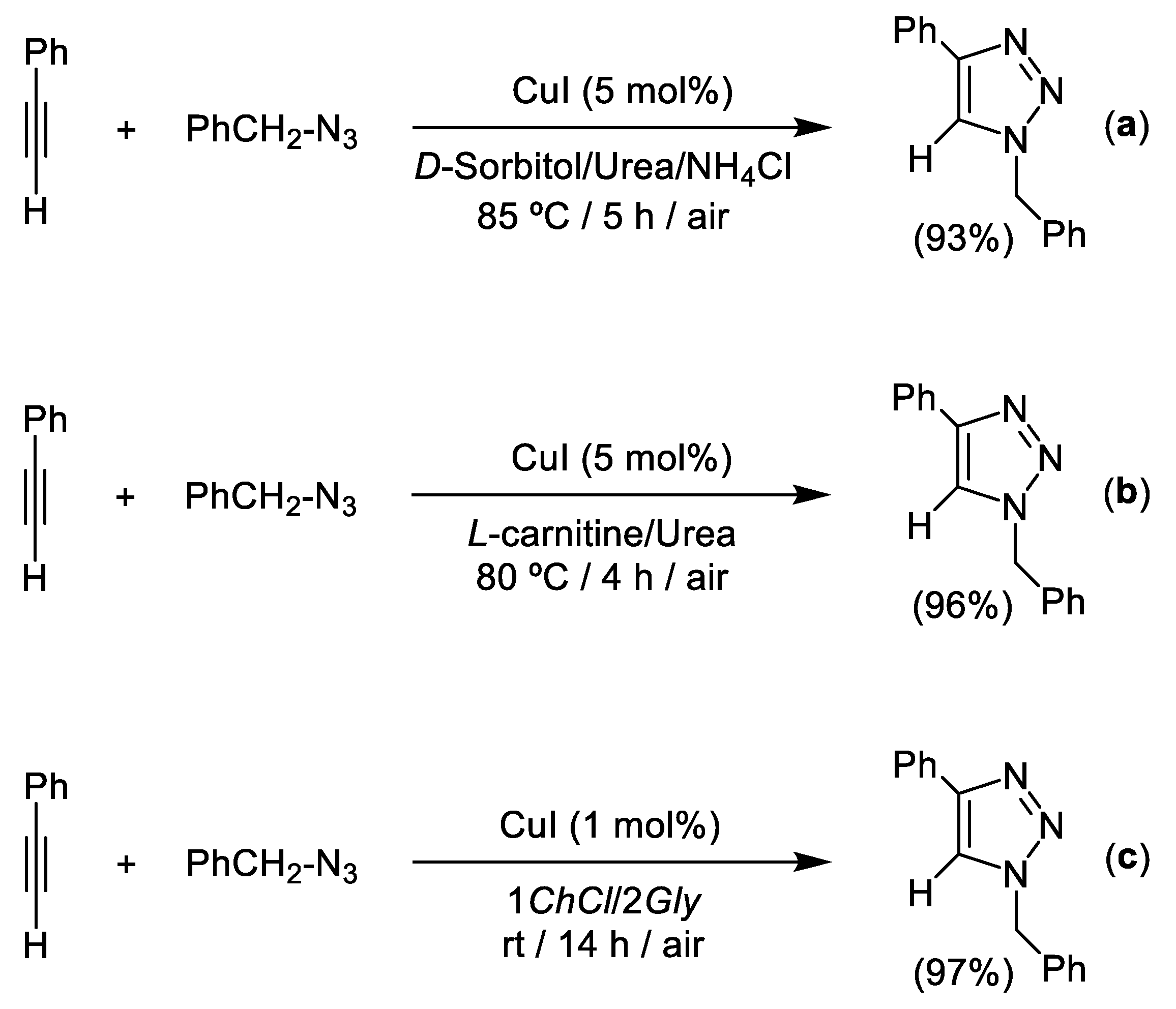
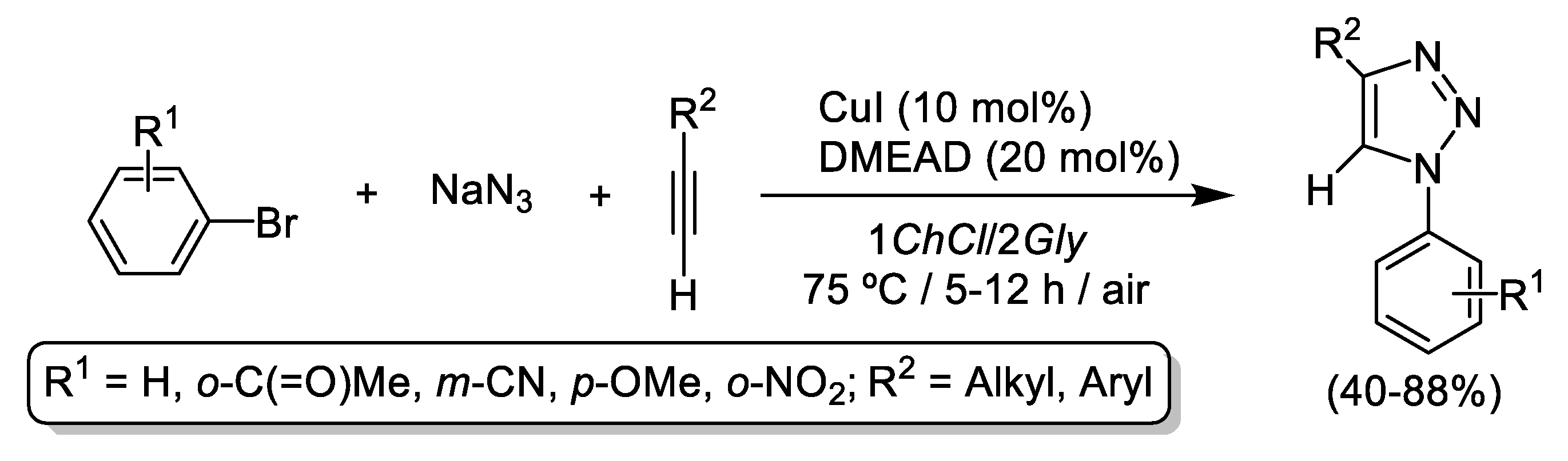
3. Cu-Catalyzed 1,3-Dipolar Cycloaddition of Azides and Alkynes (CuAAC) in Deep Eutectic Solvents (DESs)
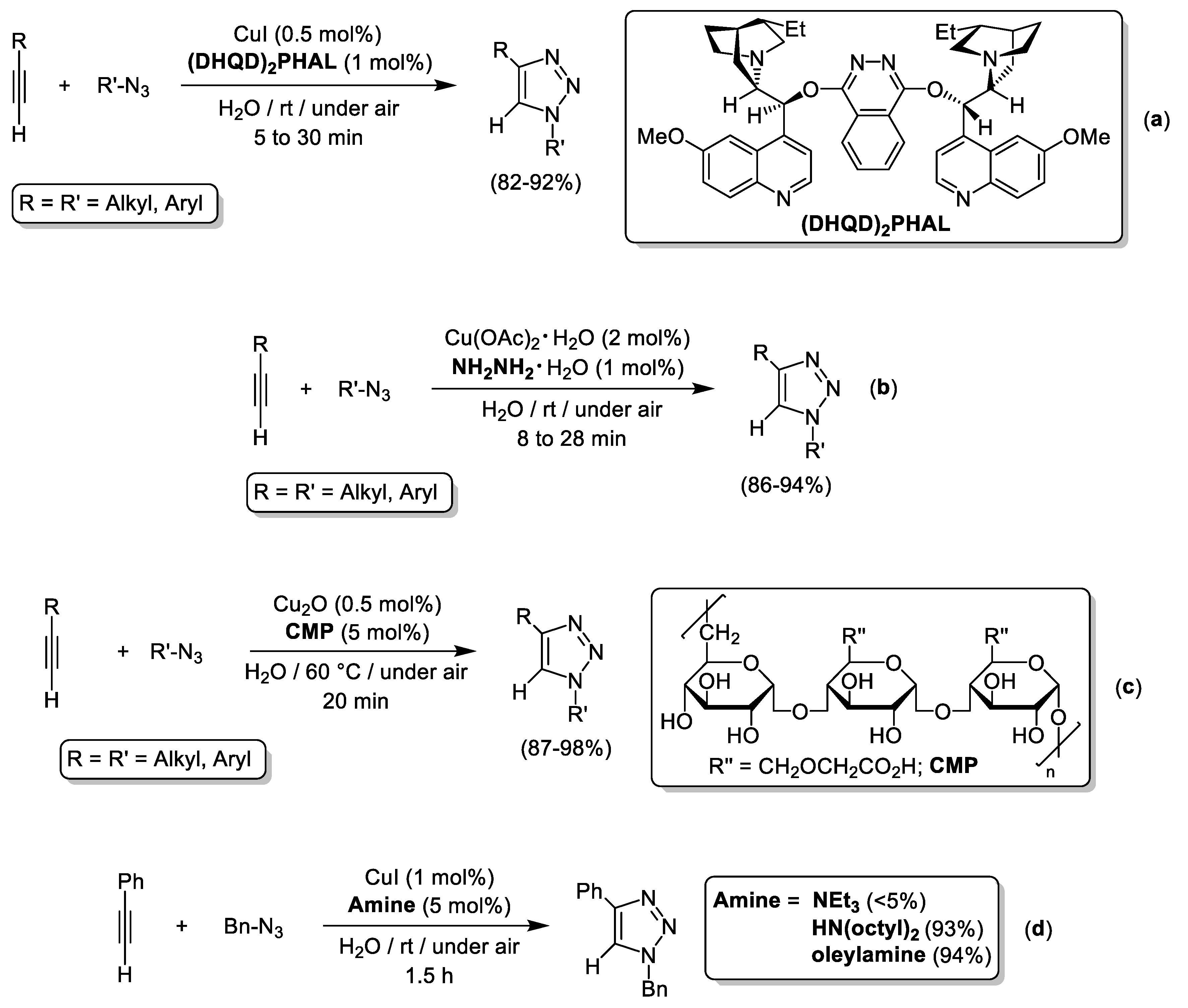
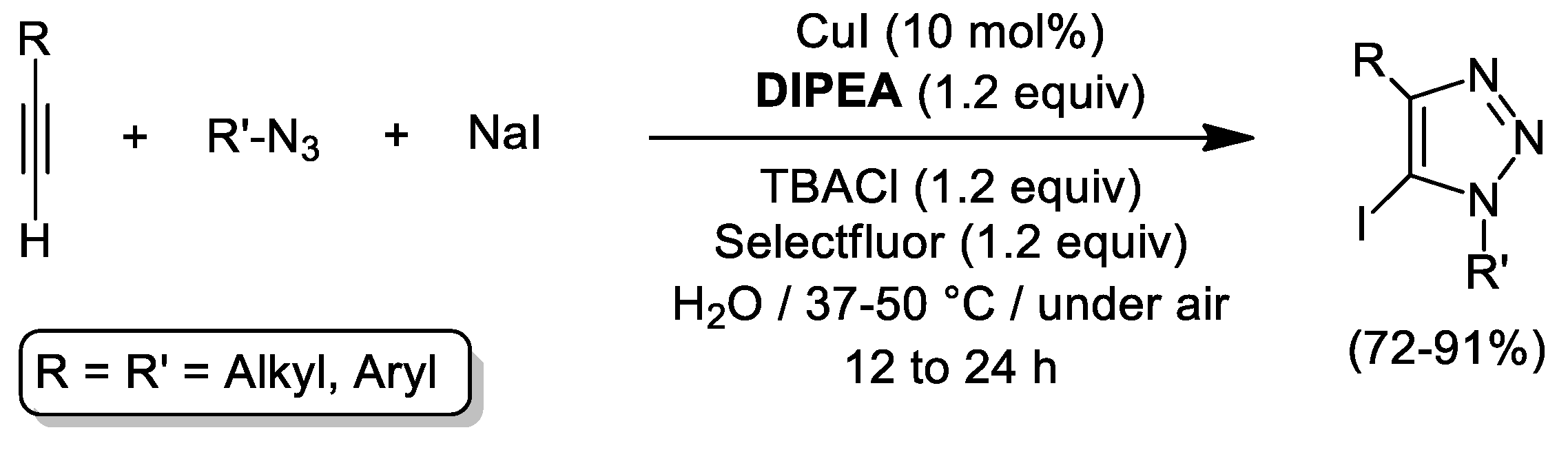
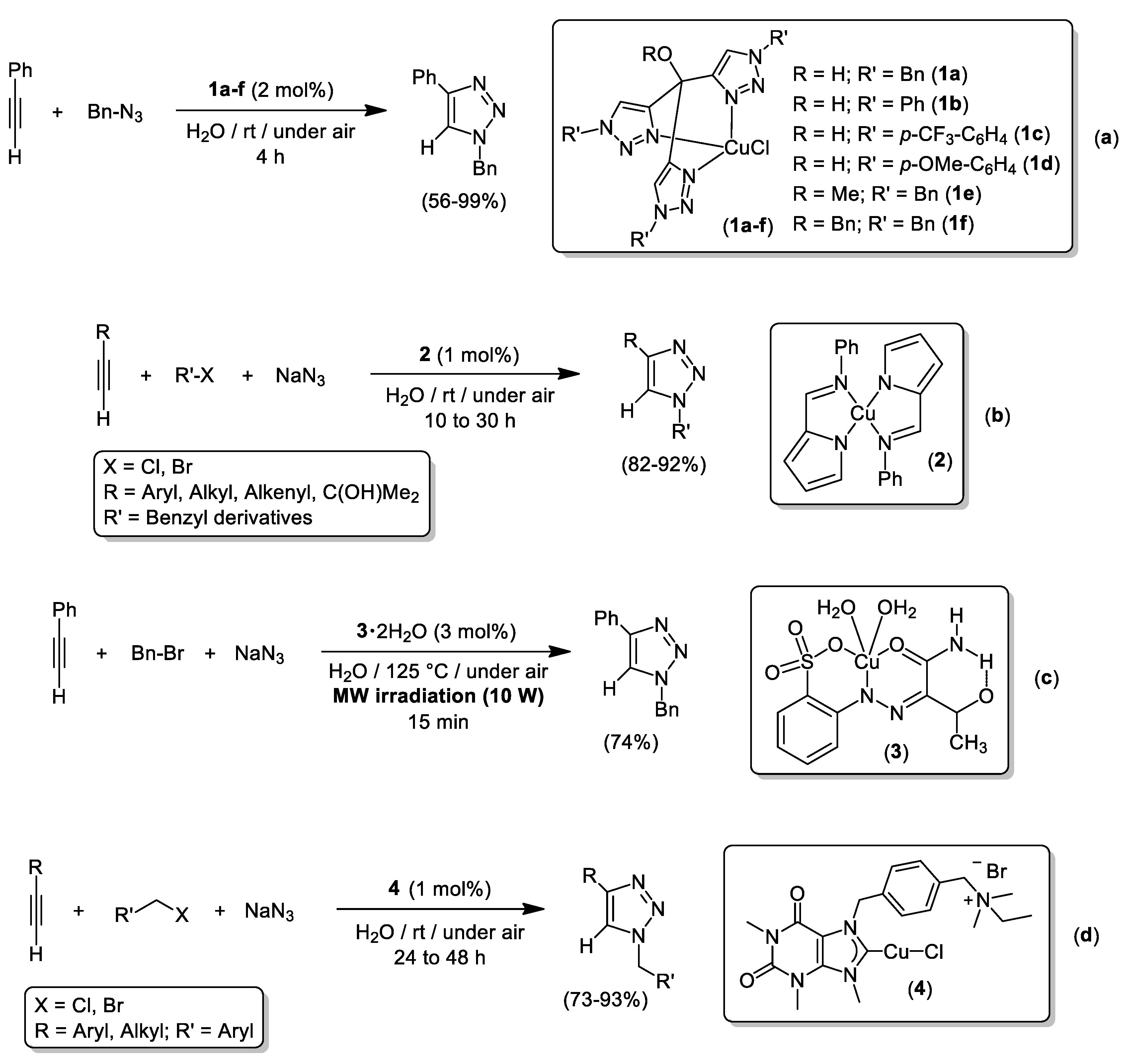
4. Cu-Catalyzed 1,3-Dipolar Cycloaddition of Azides and Alkynes (CuAAC) in Water
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Anilkumar, G.; Saranya, S. (Eds.) Copper Catalysis in Organic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020. [Google Scholar]
- Alexakis, A.; Krause, N.; Woodward, S. (Eds.) Copper Catalyzed Asymmetric Synthesis; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2014. [Google Scholar]
- Evano, G.; Blanchard, N. (Eds.) Copper-Mediated Cross-Coupling Reactions; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Lipshutz, B.; Yoshinori, Y. Introduction: Coinage Metals in Organic Synthesis. Chem. Rev. 2008, 108, 2793–2795. [Google Scholar] [CrossRef]
- Festa, R.A.; Thiele, D.J. Copper: An Essential Metal in Biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [Green Version]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Huisgen, R.; Pawda, A. (Eds.) 1,3-Dipolar Cycloaddition Chemistry; John Wiley & Sons, Ltd.: New York, NY, USA, 1984; Volume 1. [Google Scholar]
- Baskin, J.M.; Bertozzi, C.R. Copper-free Click Chemistry: Bioorthogonal Reagents for Tagging Azides. Aldrichim. Acta 2010, 43, 15–23. [Google Scholar]
- McNulty, J.; Keskar, K.; Vemula, R. The First Well-Defined Silver(I)-Complex-Catalyzed Cycloaddition of Azides onto Terminal Alkynes at Room Temperature. Chem. Eur. J. 2011, 17, 14727–14730. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xue, P.; Sun, H.H.Y.; Williams, I.D.; Sharpless, K.B.; Fokin, V.V.; Jia, G. Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides. J. Am. Chem. Soc. 2005, 127, 15998–15999. [Google Scholar] [CrossRef]
- Ding, S.; Jia, G.; Sun, J. Iridium-catalyzed intermolecular azide-alkyne cycloaddition of internal thioalkynes under mild conditions. Angew. Chem. Int. Ed. 2014, 53, 1877–1880. [Google Scholar] [CrossRef]
- Rao, H.S.P.; Chakibanda, G. Raney Ni catalyzed azide-alkyne cycloaddition reaction. RSC Adv. 2014, 4, 46040–46048. [Google Scholar] [CrossRef]
- Chandrasekaran, S. (Ed.) Click Reactions in Organic Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016. [Google Scholar]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Scott, J.L.; Sneddon, H.F. Green Solvents. In Green Techniques for Organic Synthesis and Medicinal Chemistry; Zhang, W., Cue, B.W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Anastas, P.T. Handbook of Green Chemistry, Volume 5: Reactions in Water; Li, C.-J., Ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Ravichandiran, P.; Gu, Y. Glycerol as Eco-Efficient Solvent for Organic Transformations, in Bio-Based Solvents; Jérôme, F., Luque, R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Vaccaro, L.; Santoro, S.; Curini, M.; Lanari, D. The emerging use of γ-valerolactone as a green solvent. Chem. Today 2017, 35, 46–48. [Google Scholar]
- Pace, V.; Hoyos, P.; Castoldi, L.; Domínguez de María, P.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A Biomass-Derived Solvent with Broad Application in Organic Chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tan, J.-N.; Gu, Y. Lactic acid as an invaluable bio-based solvent for organic reactions. Green Chem. 2012, 14, 3304–3317. [Google Scholar] [CrossRef]
- García-Álvarez, J. Deep Eutectic Solvents and Their Applications as New Green and Biorenewable Reaction Media. In Handbook of Solvents, Volume 2: Use, Health, and Environment, 3rd ed.; Wypych, G., Ed.; ChemTec Publishing: Toronto, ON, Canada, 2019. [Google Scholar]
- Horváth, I.T. Solvents from nature. Green Chem. 2008, 10, 1024–1028. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Dapsens, P.Y.; Mondelli, C.; Pérez-Ramírez, J. Biobased Chemicals from Conception toward Industrial Reality: Lessons Learned and To Be Learned. ACS Catal. 2012, 2, 1487–1499. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Jérôme, F. Glycerol as a sustainable solvent for green chemistry. Green Chem. 2010, 12, 1127–1138. [Google Scholar] [CrossRef]
- Vidal, C.; García-Álvarez, J. Glycerol: A biorenewable solvent for base-free Cu(I)-catalyzed 1,3-dipolar cycloaddition of azides with terminal and 1-iodoalkynes. Highly efficient transformations and catalyst recycling. Green Chem. 2014, 16, 3515–3521. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, M.; Hull-Crew, C.; Outlaw, A.; Stewart, K.; Taylor, L.; George, L.; Duensing, A.; Tracey, B.; Schoffstall, A. Green Methodologies for Copper(I)-Catalyzed Azide-Alkyne Cycloadditions: A Comparative Study. Molecules 2019, 24, 973. [Google Scholar] [CrossRef] [Green Version]
- Pasupuleti, B.G.; Bez, G. CuI/L-proline catalyzed click reaction in glycerol for the synthesis of 1,2,3-triazoles. Tetrahedron Lett. 2019, 60, 142–146. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, M.; Gras, E.; Pericàs, M.A.; Gómez, M. Metal-Free Intermolecular Azide-Alkyne Cycloaddition Promoted by Glycerol. Chem. Eur. J. 2015, 21, 18706–18710. [Google Scholar] [CrossRef] [PubMed]
- Nandre, K.P.; Salunke, J.K.; Nandre, J.P.; Patil, V.S.; Borse, A.U.; Bhosale, S.V. Glycerol mediated synthesis of 5-substituted 1H-tetrazole under catalyst free condition. Chin. Chem. Lett. 2012, 23, 161–164. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, M.; Llanes, P.; Pradel, C.; Pericàs, M.A.; Gómez, M. Key non-metal ingredients for Cu-catalyzed’ “Click” reactions in glycerol: Nanoparticles as efficient forwarders. Chem. Eur. J. 2016, 22, 18247–18253. [Google Scholar] [CrossRef] [PubMed]
- Dang-Bao, T.; Pradel, C.; Favier, I.; Gómez, M. Bimetallic Nanocatalysts in Glycerol for Applications in Controlled Synthesis. A Structure-Reactivity Relationship Study. ACS Appl. Nano Mater. 2019, 2, 1033–1044. [Google Scholar] [CrossRef]
- Sreedhar, B.; Reddy, P.S.; Kumar, N.S. Cu(I)-catalyzed one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles via nucleophilic displacement and 1,3-dipolar cycloaddition. Tetrahedron Lett. 2006, 47, 3055–3058. [Google Scholar] [CrossRef]
- Kumaraswamy, G.; Ankamma, K.; Pichaiah, A. Tandem Epoxide or Aziridine Ring Opening by Azide/Copper Catalyzed [3+2] Cycloaddition: Efficient Synthesis of 1,2,3-Triazolo β-Hydroxy or β-Tosylamino Functionality Motif. J. Org. Chem. 2007, 72, 9822–9825. [Google Scholar] [CrossRef]
- Damodiran, M.; Muralidharan, D.; Perumal, P.T. Regioselective synthesis and biological evaluation of bis(indolyl)methane derivatized 1,4-disubstituted 1,2,3-bistriazoles as anti-infective agents. Bioorg. Med. Chem. Lett. 2009, 19, 3611–3614. [Google Scholar] [CrossRef]
- Kumar, D.P.; Reddy, V.B. An Efficient, One-Pot, Regioselective Synthesis of 1,4-Diaryl-1H-1,2,3-triazoles Using Click Chemistry. Synthesis 2010, 1687–1691. [Google Scholar] [CrossRef]
- Pericherla, K.; Khedar, P.; Khungar, B.; Kumar, A. Click chemistry inspired structural modification of azole antifungal agents to synthesize novel ‘drug like’ molecules. Tetrahedron Lett. 2012, 53, 6761–6764. [Google Scholar] [CrossRef]
- Nagesh, H.N.; Suresh, A.; Reddy, M.N.; Suresh, N.; Subbalakshmia, J.; Sekhar, K.V.G.C. Multicomponent cascade reaction: Dual role of copper in thesynthesis of 1,2,3-triazole tethered benzimidazo[1,2-a]quinoline and their photophysical studies. RSC Adv. 2016, 6, 15884–15894. [Google Scholar] [CrossRef]
- Sindhu, J.; Singh, H.; Khurana, J.M.; Bhardwaj, J.K.; Saraf, P.; Sharma, C. Synthesis and biological evaluation of some functionalized 1H-1,2,3-triazole tethered pyrazolo[3,4-b]pyridin-6(7H)-ones as antimicrobial and apoptosis inducing agents. Med. Chem. Res. 2016, 25, 1813–1830. [Google Scholar] [CrossRef]
- Fu, F.; Martínez, A.; Wang, C.; Ciganda, R.; Yate, L.; Escobar, A.; Moya, S.; Fouquet, E.; Ruiz, J.; Astruc, D. Exposure to air boosts CuAAC reactions catalyzed by PEG-stabilized Cu nanoparticles. Chem. Commun. 2017, 53, 5384–5387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Spear, S.K.; Huddleston, J.G.; Rogers, R.D. Polyethylene glycol and solutions of polyethylene glycol as green reaction media. Green Chem. 2005, 7, 64–82. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. Liquid structure of the choline chloride-urea deep eutectic solvent (reline) from neutron diffraction and atomistic modelling. Green Chem. 2016, 18, 2736–2744. [Google Scholar] [CrossRef] [Green Version]
- Blusztajn, J.K. Choline, a vital amine. Science 1998, 284, 794–795. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S.; D’Agostino, C.; Gladden, L.F.; Mantle, M.D. Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 612–632. [Google Scholar] [CrossRef] [Green Version]
- García-Álvarez, J. Deep Eutectic Mixtures: Promising sustainable solvents for metal-catalysed and metal-mediated organic reactions. Eur. J. Inorg. Chem. 2015, 5147–5157. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illgen, F.; König, B. Organic reactions in low melting mixtures based on carbohydrates and l-carnitine-a comparison. Green Chem. 2009, 11, 848–854. [Google Scholar] [CrossRef]
- Kafle, A.; Handy, S.T. A one-pot, copper-catalyzed azidation/click reaction of aryl and heteroaryl bromides in an environmentally friendly deep eutectic solvent. Tetrahedron 2017, 73, 7024–7029. [Google Scholar] [CrossRef]
- Xiong, X.; Yi, C.; Liao, X.; Lai, S. A practical multigram-scale method for the green synthesis of 5-substituted-1H-tetrazoles in deep eutectic solvent. Tetrahedron Lett. 2019, 60, 402–406. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef]
- Kappe, C.O.; Van der Eycken, E. Click chemistry under non-classical reaction conditions. Chem. Soc. Rev. 2010, 39, 1280–1290. [Google Scholar] [CrossRef]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(i) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Alonso, F.; Moglie, Y.; Radivoy, G. Copper Nanoparticles in Click Chemistry. Acc. Chem. Res. 2015, 48, 2516–2528. [Google Scholar] [CrossRef] [Green Version]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-Catalysed Azide–Alkyne Cycloadditions (CuAAC): An Update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Müller, T.J.J. Multicomponent Syntheses based upon Copper-Catalyzed Alkyne-Azide Cycloaddition. Adv. Synth. Catal. 2015, 357, 617–666. [Google Scholar] [CrossRef]
- Wang, K.; Bi, X.; Xing, S.; Liao, P.; Fang, Z.; Meng, X.; Zhang, Q.; Liu, Q.; Ji, Y. Cu2O acting as a robust catalyst in CuAAC reactions: Water is the required medium. Green Chem. 2011, 13, 562–565. [Google Scholar] [CrossRef]
- Jiang, Y.; Kong, D.; Zhao, J.; Zhang, W.; Xu, W.; Li, W.; Xu, G. A simple, efficient thermally promoted protocol for Huisgen-click reaction catalyzed by CuSO4-5H2O in water. Tetrahedron Lett. 2014, 55, 2410–2414. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Li, X.; Sun, Y.; Zhao, Y.; Jia, S.; Guo, N.; Xu, G.; Zhang, W. Copper(II) Acetylacetonate: An Efficient Catalyst for Huisgen-Click Reaction for Synthesis of 1,2,3-Triazoles in Water. Chin. J. Chem. 2017, 35, 1239–1245. [Google Scholar] [CrossRef]
- Castro-Godoy, W.D.; Heredia, A.A.; Schmidt, L.C.; Argüello, J.E. A straightforward and sustainable synthesis of 1,4-disubstituted 1,2,3-triazoles via visible-light promoted copper-catalyzed azide–alkyne cycloaddition (CuAAC). RSC Adv. 2017, 7, 33967–33973. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, X.; Jiang, Y.; Chen, S.; Qu, L.; Qu, Z.; Yuan, J.; Shi, H. Highly Efficient Ultrasonic-Assisted CuCl-Catalyzed 1,3-Dipolar Cycloaddition Reactions in Water: Synthesis of Coumarin Derivatives Linked with 1,2,3-Triazole Moiety. J. Heterocycl. Chem. 2016, 53, 1402–1411. [Google Scholar] [CrossRef]
- Yan, Z.-Y.; Zhao, Y.-B.; Fan, M.-J.; Liu, W.-M.; Liang, Y.-M. General synthesis of (1-substituted-1H-1,2,3-triazol-4-ylmethyl)-dialkylamines via a copper(I)-catalyzed three-component reaction in water. Tetrahedron 2005, 61, 9331–9337. [Google Scholar] [CrossRef]
- Asano, K.; Matsubara, S. Effects of a Flexible Alkyl Chain on a Ligand for CuAAC Reaction. Org. Lett. 2010, 12, 4988–4991. [Google Scholar] [CrossRef]
- Wang, F.; Fu, H.; Jiang, Y.Y.; Zhao, Y.F. Copper-Catalyzed Cycloaddition of Sulfonyl Azides with Alkynes to Synthesize N-Sulfonyltriazoles on Water at Room Temperature. Adv. Synth. Catal. 2008, 350, 1830–1834. [Google Scholar] [CrossRef]
- Wang, F.; Fu, H.; Jiang, Y.Y.; Zhao, Y.F. Quick and highly efficient copper-catalyzed cycloaddition of aliphatic and aryl azides with terminal alkynes “on water”. Green Chem. 2008, 10, 452–456. [Google Scholar] [CrossRef]
- Ali, A.A.; Chetia, M.; Saikia, P.J.; Sarma, D. (DHQD)2PHAL ligand-accelerated Cu-catalyzed azide–alkyne cycloaddition reactions in water at room temperature. RSC Adv. 2014, 4, 64388–64392. [Google Scholar] [CrossRef]
- Jiang, Y.; Kong, D.; Zhao, J.; Qi, Q.; Li, W.; Xu, Q. Cu(OAc)2·H2O/NH2NH2·H2O: An efficient catalyst system that in situ generates Cu2O nanoparticles and HOAc for Huisgen click reactions. RSC Adv. 2014, 4, 1010–1014. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, B.; Jiang, Y.; Hu, Z. Carboxymethylpullulans Promoted Cu2O-Catalyzed Huisgen-Click Reaction. RSC Adv. 2015, 5, 12043–12047. [Google Scholar] [CrossRef]
- Hein, J.E.; Tripp, J.C.; Krasnova, L.B.; Sharpless, K.B.; Fokin, V.V. Copper(I)-Catalyzed Cycloaddition of Organic Azides and 1-Iodoalkynes. Angew. Chem. Int. Ed. 2009, 48, 8018–8021. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ding, S.; Yang, Y.; Zhu, A.; Fan, X.; Cui, M.; Chen, C.; Zhang, G. Multicomponent Aqueous Synthesis of Iodo-1,2,3-triazoles: Single-Step Models for Dual Modification of Free Peptide and Radioactive Iodo Labeling. Chem. Eur. J. 2017, 23, 1166–1172. [Google Scholar] [CrossRef]
- Lal, S.; McNally, J.; White, A.J.P.; Díez-González, S. Novel Phosphinite and Phosphonite Copper(I) Complexes: Efficient Catalysts for Click Azide-Alkyne Cycloaddition Reactions. Organometallics 2011, 30, 6225–6232. [Google Scholar] [CrossRef]
- Lal, S.; Díez-González, S. [CuBr(PPh3)3] for Azide-Alkyne Cycloaddition Reactions under Strict Click Conditions. J. Org. Chem. 2011, 76, 2367–2373. [Google Scholar] [CrossRef]
- Díez-González, S.; Nolan, S.P. [(NHC)2Cu]X Complexes as Efficient Catalysts for Azide–Alkyne Click Chemistry at Low Catalyst Loadings. Angew. Chem. Int. Ed. 2008, 47, 8881–8884. [Google Scholar] [CrossRef] [Green Version]
- Gaulier, C.; Hospital, A.; Legeret, B.; Delmas, A.F.; Aucagne, V.; Cisnettia, F.; Gautier, A. A water soluble CuI–NHC for CuAAC ligation of unprotected peptides under open air conditions. Chem. Commun. 2012, 48, 4005–4007. [Google Scholar] [CrossRef]
- Díez-González, S.; Correa, A.; Cavallo, L.; Nolan, S.P. (NHC)Copper(I)-Catalyzed [3+2] Cycloaddition of Azides and Mono- or Disubstituted Alkynes. Chem. Eur. J. 2006, 12, 7558–7564. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, J.; Díez, J.; Gimeno, J. A highly efficient copper(i) catalyst for the 1,3-dipolar cycloaddition of azides with terminal and 1-iodoalkynes in water: Regioselective synthesis of 1,4-disubstituted and 1,4,5-trisubstituted 1,2,3-triazoles. Green Chem. 2010, 12, 2127–2130. [Google Scholar] [CrossRef]
- García-Álvarez, J.; Díez, J.; Gimeno, J.; Suárez, F.J.; Vincent, C. (Iminophosphorane)copper(I) Complexes as Highly Efficient Catalysts for 1,3‐Dipolar Cycloaddition of Azides with Terminal and 1‐Iodoalkynes in Water: One‐Pot Multi‐Component Reaction from Alkynes and in situ Generated Azides . J. Inorg. Chem. 2012, 35, 5854–5863. [Google Scholar] [CrossRef]
- Özçubukçu, S.; Ozkal, E.; Jimeno, C.; Pericàs, M.A. A Highly Active Catalyst for Huisgen 1,3-Dipolar Cycloadditions Based on the Tris(triazolyl)methanol-Cu(I) Structure. Org. Lett. 2009, 11, 4682–4683. [Google Scholar] [CrossRef]
- Ozkal, E.; Llanes, P.; Bravo, F.; Ferrali, A.; Pericàs, M.A. Fine-Tunable Tris(triazolyl)methane Ligands for Copper(I)-Catalyzed Azide–Alkyne Cycloaddition Reactions. Adv. Synth. Catal. 2014, 356, 857–869. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Liu, P.; Xie, J.; Dai, B. 2-Pyrrolecarbaldiminato–Cu(II) complex catalyzed three-component 1,3-dipolar cycloaddition for 1,4-disubstituted 1,2,3-triazoles synthesis in water at room temperature. RSC Adv. 2015, 5, 6661–6665. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; Guedes da Silva, M.F.C.; Mahmudov, K.T.; Pombeiro, A.J.L. Arylhydrazone ligands as Cu-protectors and -catalysis promoters in the azide–alkyne cycloaddition reaction. Dalton Trans. 2019, 48, 1774–1785. [Google Scholar] [CrossRef]
- Szadkowska, A.; Staszko, S.; Zaorska, E.; Pawłowski, R. A theophylline based copper N-heterocyclic carbene complex: Synthesis and activity studies in green media. RSC Adv. 2016, 6, 44248–44253. [Google Scholar] [CrossRef]
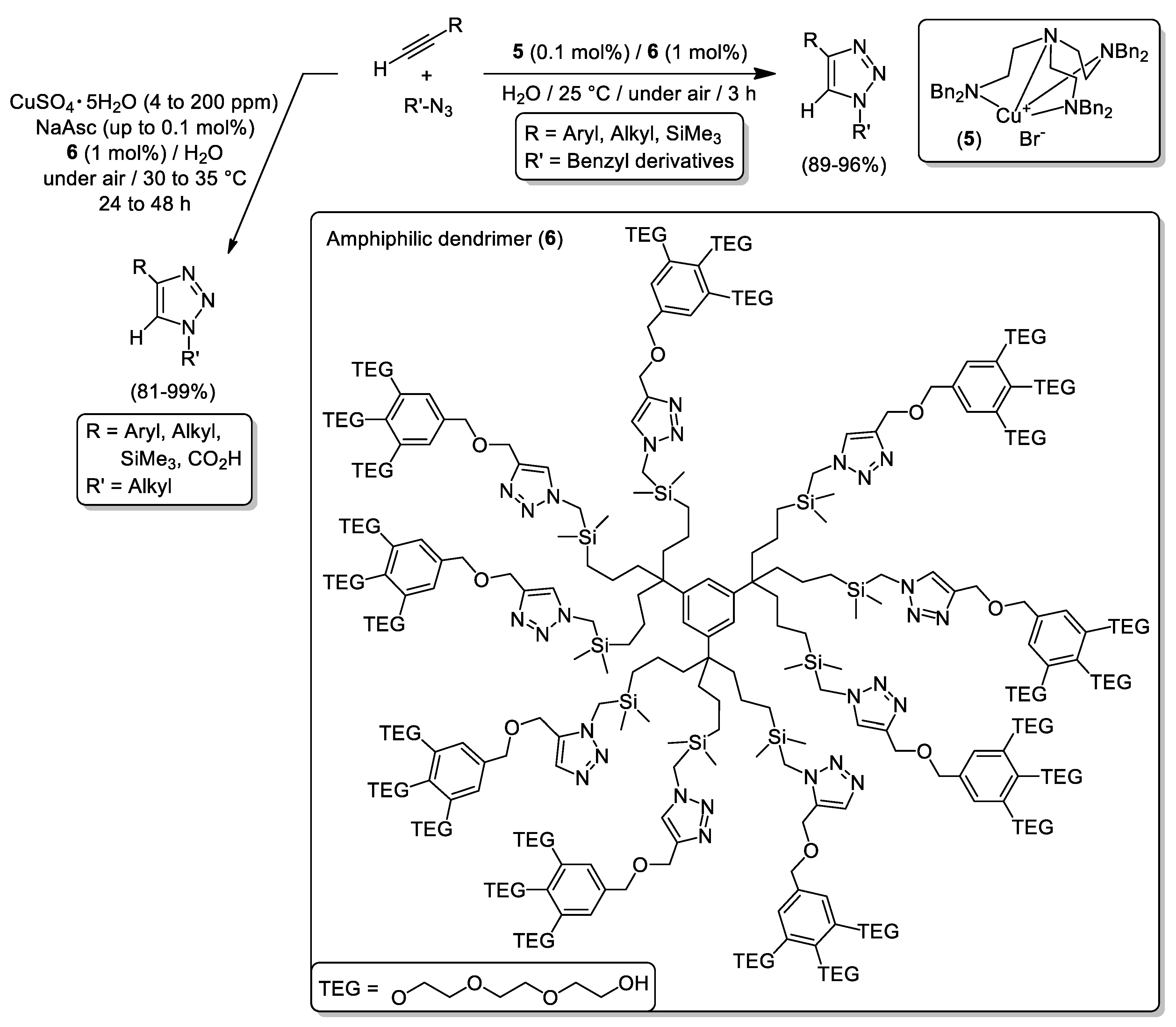
- Deraedt, C.; Pinaud, N.; Astruc, D. Recyclable Catalytic Dendrimer Nanoreactor for Part-Per-Million CuI Catalysis of “Click” Chemistry in Water. J. Am. Chem. Soc. 2014, 136, 12092–12098. [Google Scholar] [CrossRef]
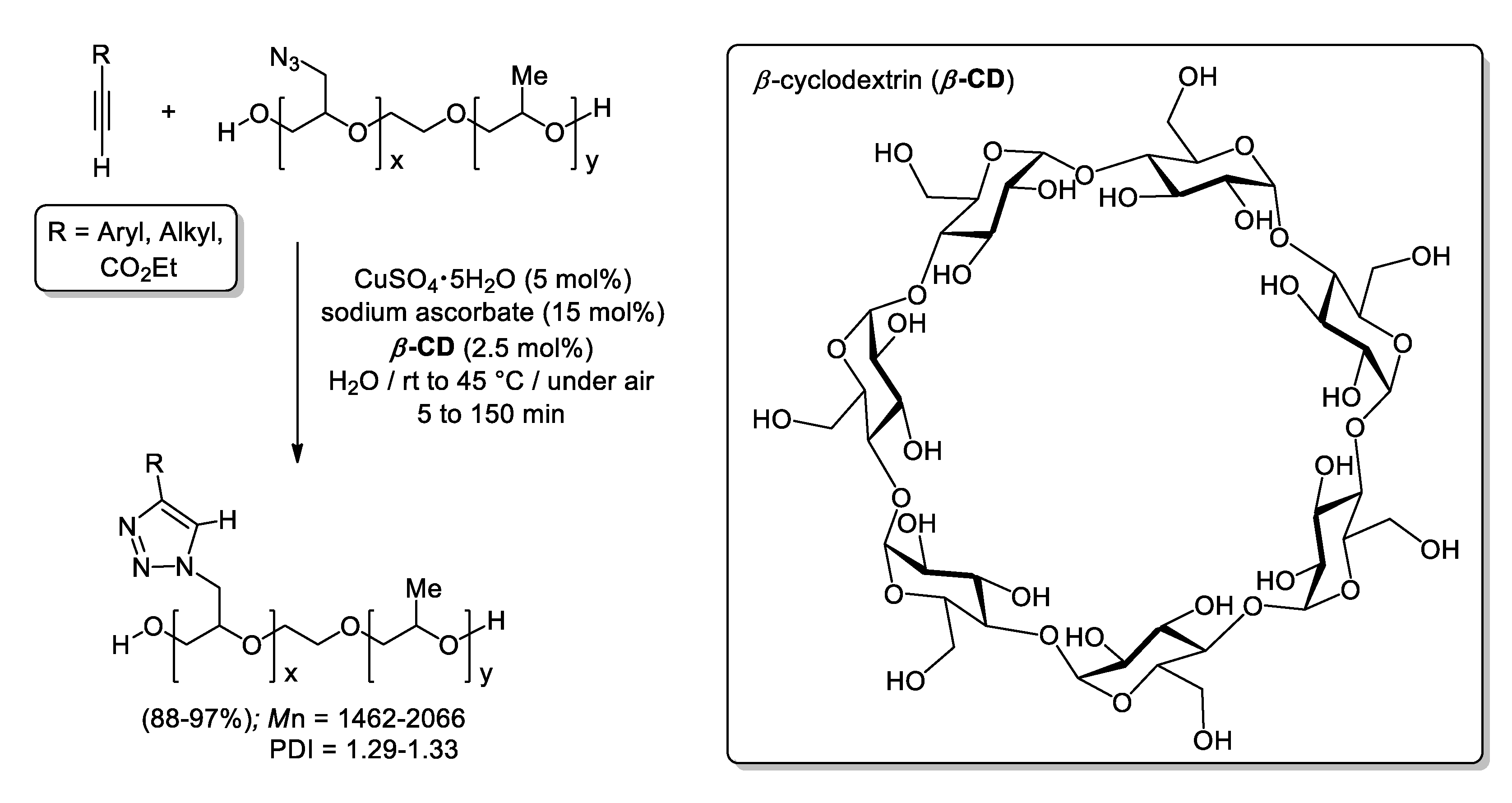
- Shin, J.-A.; Lim, Y.-G. Facile Synthesis of Glycidyl Triazolyl Polymers in Water Using β-Cyclodextrin. Bull. Korean Chem. Soc. 2015, 36, 2367–2370. [Google Scholar] [CrossRef]
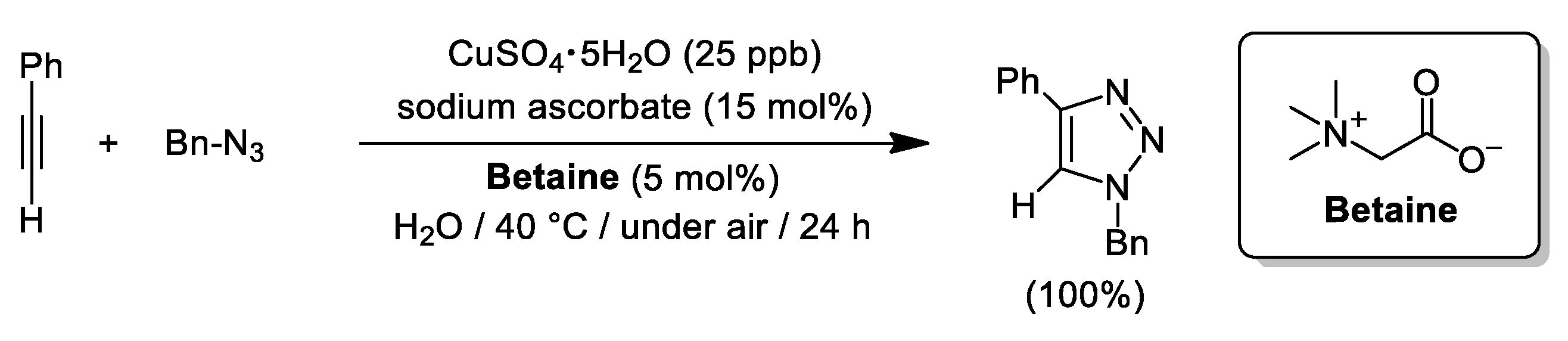
- Shin, J.-A.; Oh, S.-J.; Lee, H.-Y.; Lim, Y.-G. An efficient Cu-catalyzed azide–alkyne cycloaddition (CuAAC) reaction in aqueous medium with a zwitterionic ligand, betaine. Catal. Sci. Technol. 2017, 7, 2450–2456. [Google Scholar] [CrossRef]
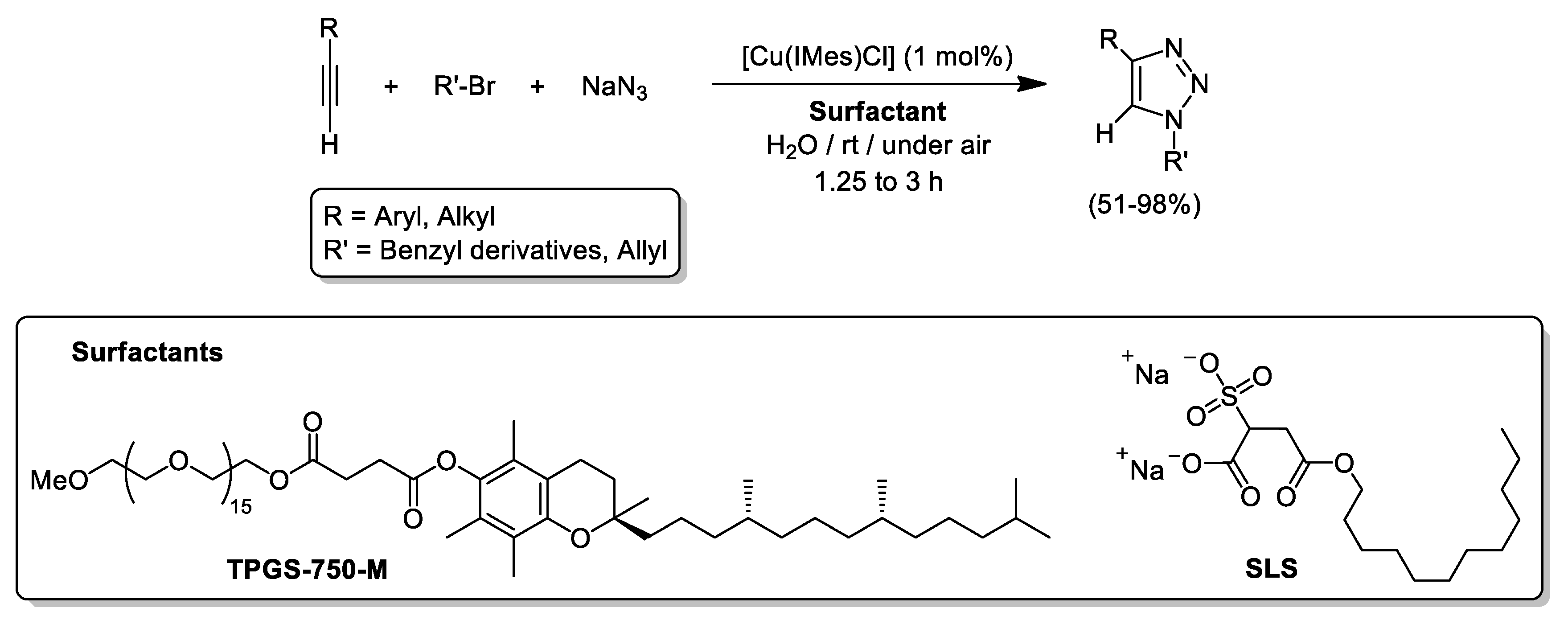
- Tasca, E.; La Sorella, G.; Sperni, L.; Strukul, G.; Scarso, A. Micellar promoted multi-component synthesis of 1,2,3-triazoles in water at room temperature. Green Chem. 2015, 17, 1414–1422. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebra, N.; García-Álvarez, J. Recent Progress of Cu-Catalyzed Azide-Alkyne Cycloaddition Reactions (CuAAC) in Sustainable Solvents: Glycerol, Deep Eutectic Solvents, and Aqueous Media. Molecules 2020, 25, 2015. https://doi.org/10.3390/molecules25092015
Nebra N, García-Álvarez J. Recent Progress of Cu-Catalyzed Azide-Alkyne Cycloaddition Reactions (CuAAC) in Sustainable Solvents: Glycerol, Deep Eutectic Solvents, and Aqueous Media. Molecules. 2020; 25(9):2015. https://doi.org/10.3390/molecules25092015
Chicago/Turabian StyleNebra, Noel, and Joaquín García-Álvarez. 2020. "Recent Progress of Cu-Catalyzed Azide-Alkyne Cycloaddition Reactions (CuAAC) in Sustainable Solvents: Glycerol, Deep Eutectic Solvents, and Aqueous Media" Molecules 25, no. 9: 2015. https://doi.org/10.3390/molecules25092015






