Potential Decontamination of Drinking Water Pathogens through k-Carrageenan Integrated Green Bottle Fly Bio-Synthesized Silver Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Silver Nanoparticles
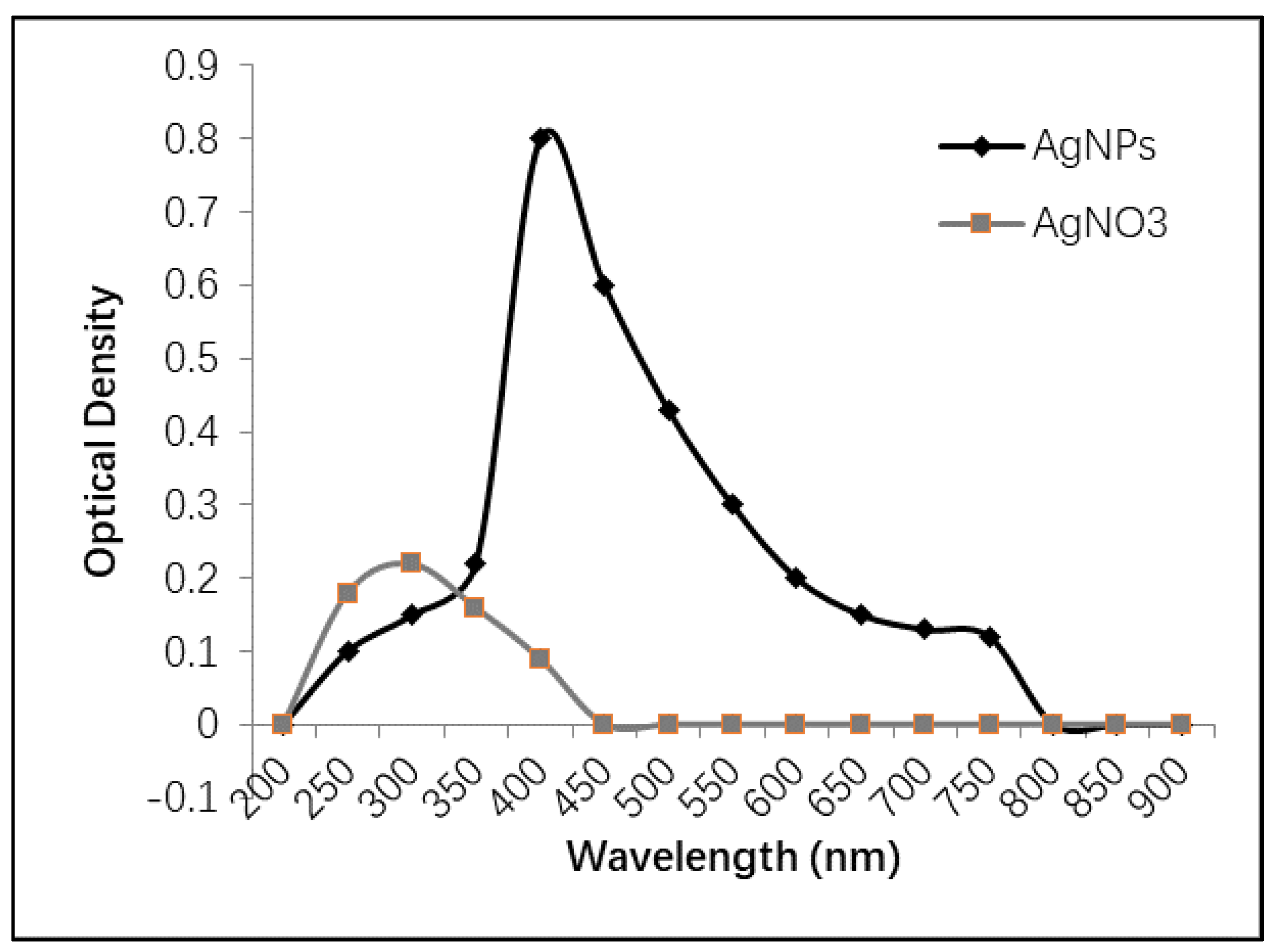
2.1.1. UV-Vis Spectrophotometry
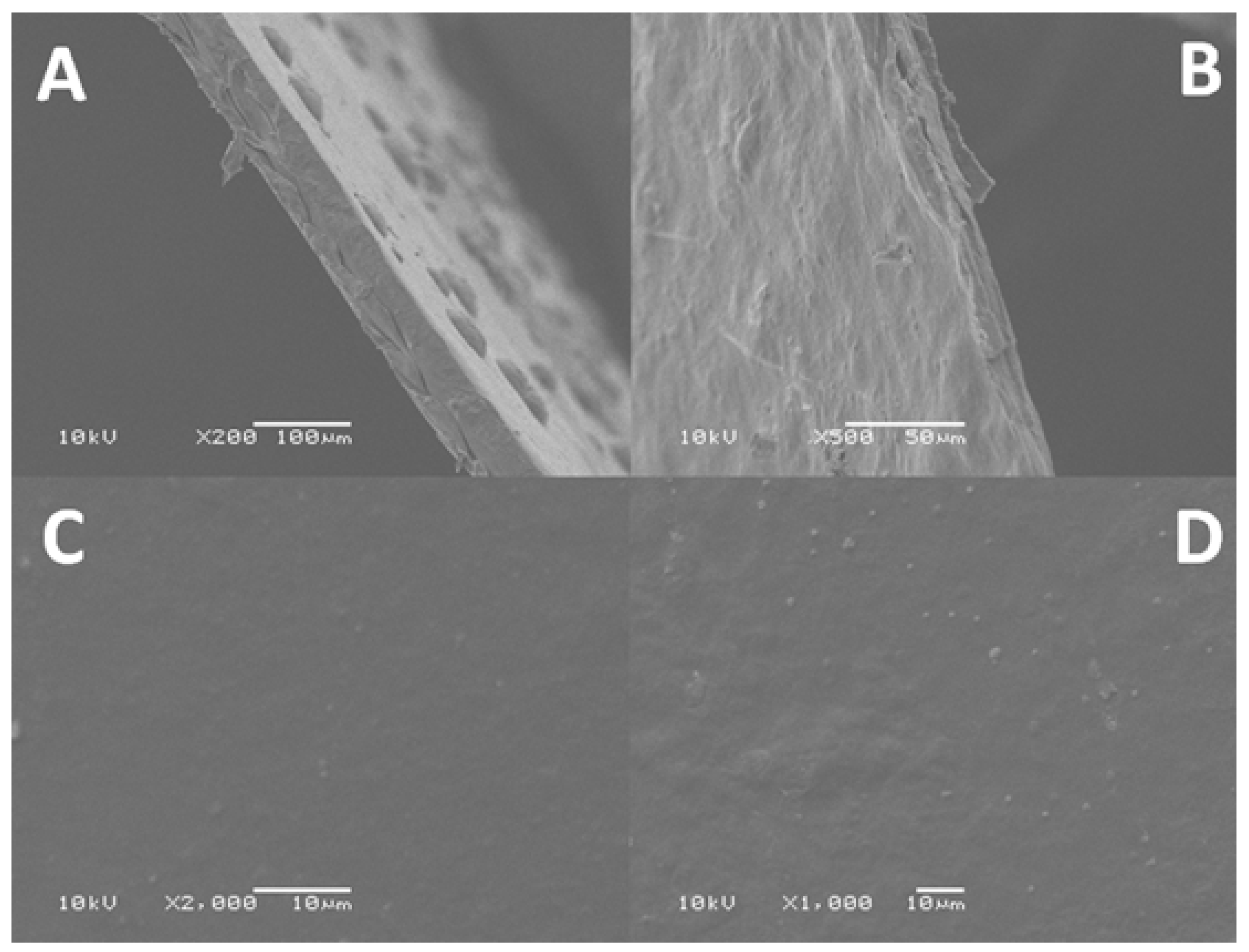
2.1.2. Scanning Electron Microscopy (SEM)
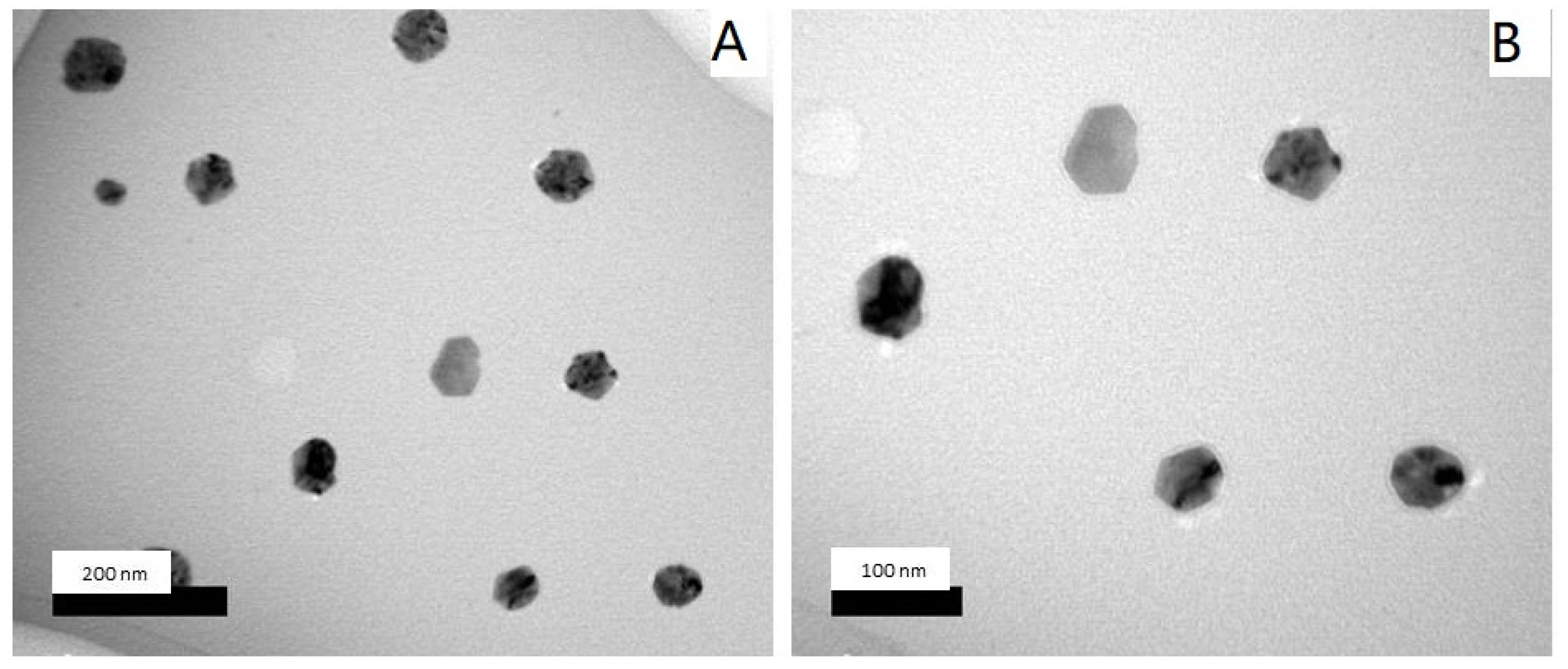
2.1.3. Transmission Electron Microscopy (TEM)
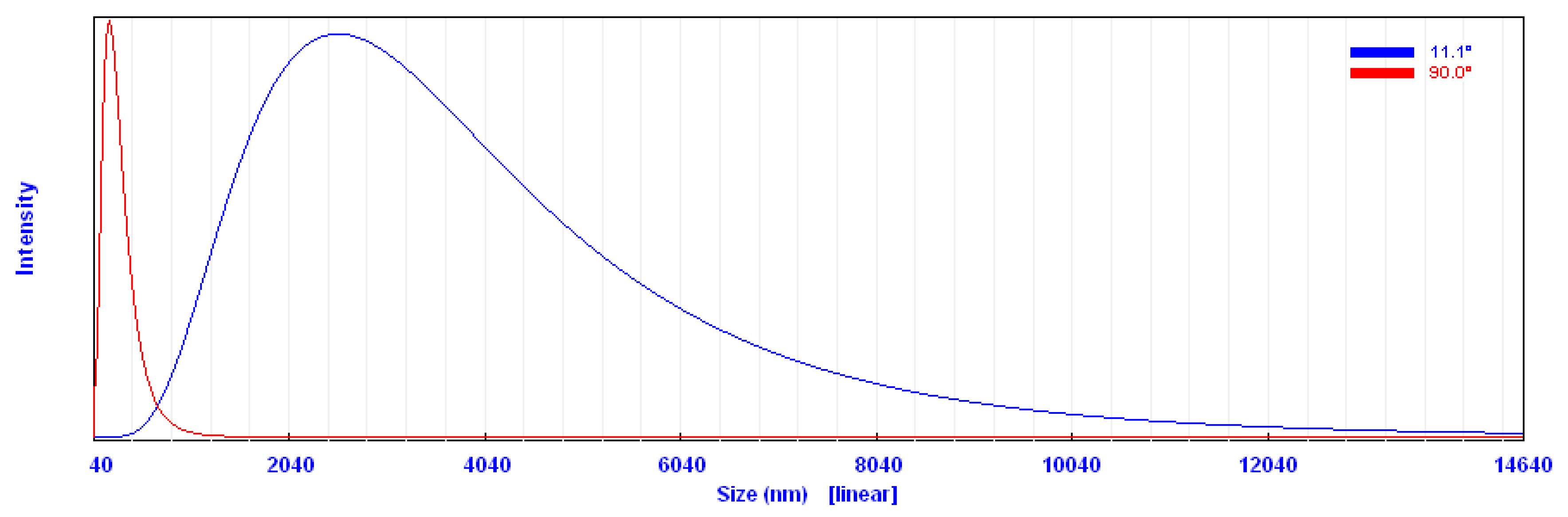
2.1.4. Particle Size Analysis (PSA)
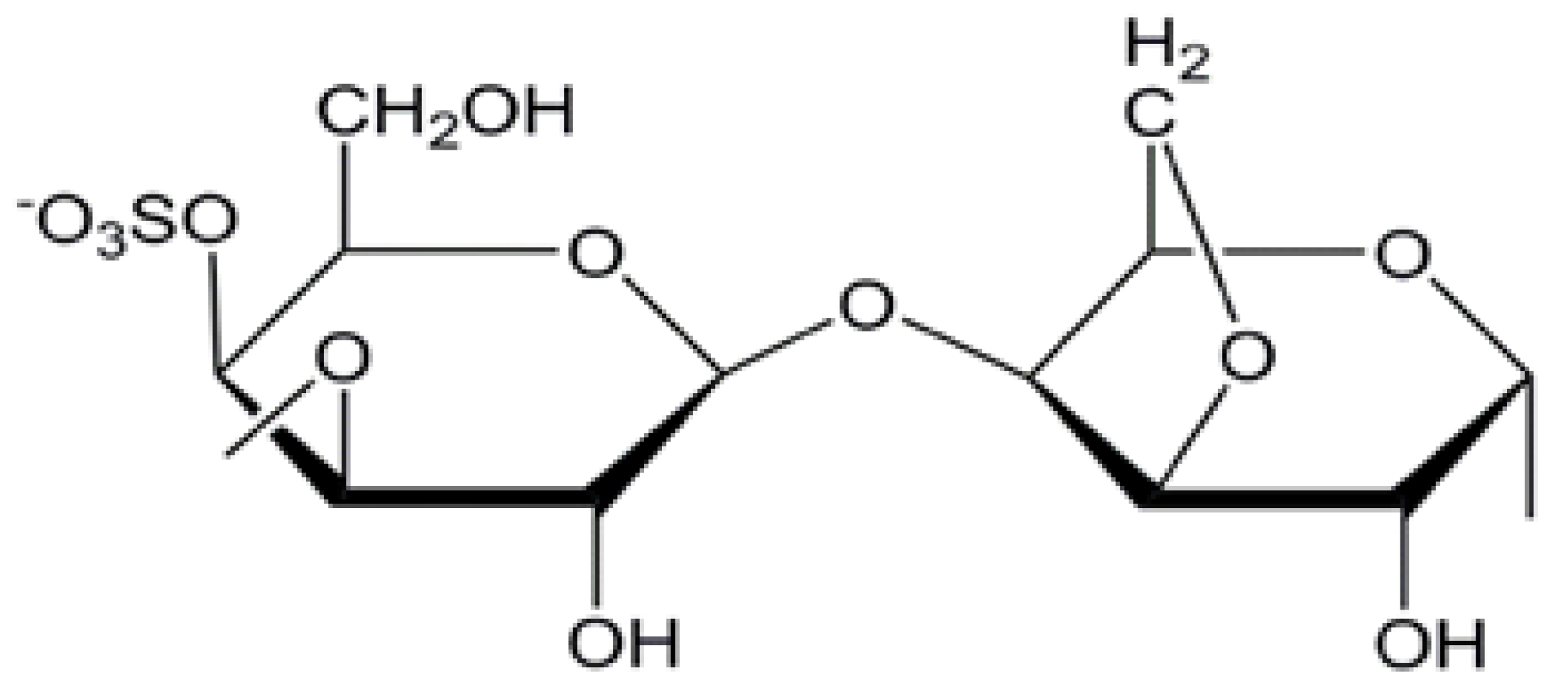
2.2. Characterization of k-Carrageenans/Silver Nanoparticles Film
2.2.1. Mechanical Properties
2.2.2. Fourier Transform Infrared (FT-IR)
2.2.3. Thermogravimetric Analysis
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Inductively Coupled Plasma (ICP)
2.3. Antimicrobial Activity
2.3.1. Antimicrobial Activity of k-Carrageenan/Silver Nanoparticles Film against Real Water Sample
2.3.2. Qualitative Determination of Some Bacterial Pathogens in Water
3. Materials and Methods
3.1. Materials
3.2. Preparation of Insects’ Pupa
3.3. Extraction of the Metabolic Contents of Pupa
3.4. Separation of the Obtained Silver Nanoparticles
3.5. Spectrophotometric Characterization of Silver Nanoparticles
3.6. Preparation of k-Carrageenan Film Amended with Silver Nanoparticles
3.7. Characterization of k-Carrageenan Films with/without Silver Nanoparticles
3.8. Antimicrobial Activity of k-Carrageenan Films with/without Silver Nanoparticles
3.9. Antimicrobial Activity of k-Carrageenan/Silver Nanoparticles against Drinking Water Sample
3.10. Qualitative Determination of Some Bacterial Pathogens in Water
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Liu, L.; Zhang, D.; Yang, D.; Guo, J.; Wei, J. Fabrication of polyaniline/silver nanoparticles/multi-walled carbon nanotubes composites for flexible microelectronic circuits. Synth. Met. 2014, 192, 15–22. [Google Scholar] [CrossRef]
- Santos, V.; Ribeiro, R.; Bosco, A.; Alhadeff, E.; Bojorge, N. Characterization and Evaluation of Silver-Nanoparticle-Incorporated in Composite Graphite Aiming at their Application in Biosensors. Braz. J. Chem. Eng. 2017, 34, 647–657. [Google Scholar] [CrossRef] [Green Version]
- Van der Horst, C.; Silwana, B.; Iwuoha, E.; Somerset, V. Synthesis and characterization of bismuth-silver nanoparticles for electrochemical sensor applications. Anal. Lett. 2015, 48, 1311–1332. [Google Scholar] [CrossRef]
- Szymanski, M.; Porter, R.; Dep, G.V.; Wang, Y.; Haggett, B.G. Silver nanoparticles and magnetic beads with electrochemical measurement as a platform for immunosensing devices. Phys. Chem. Chem. Phys. 2011, 13, 5383–5387. [Google Scholar] [CrossRef] [PubMed]
- Femila, E.; Srimathi, R.; Deivasigamani, C. Removal of malachite green using silver nanoparticles via adsorption and catalytic degradation. Int. J. Pharm. Pharm. Sci. 2014, 6, 579–583. [Google Scholar]
- Ahmadi, F.; Abolghasemi, S.; Parhizgari, N.; Moradpour, F. Effect of silver nanoparticles on common bacteria in hospital surfaces. Jundishapur J. Microbiol. 2013, 6, 209. [Google Scholar] [CrossRef] [Green Version]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Liao, X.-H.; Zhao, X.-N.; Chen, H.-Y. Preparation of silver nanorods by electrochemical methods. Mater. Lett. 2001, 49, 91–95. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, O. One-step green synthesis of gold nanoparticles using black cardamom and effect of pH on its synthesis. Nanoscale Res. Lett. 2015, 10, 353. [Google Scholar] [CrossRef] [Green Version]
- Baltazar-Encarnación, E.; Escárcega-González, C.E.; Vasto-Anzaldo, X.G.; Cantú-Cárdenas, M.E.; Morones-Ramírez, J.R. Silver Nanoparticles Synthesized through Green Methods Using Escherichia coli Top 10 (Ec-Ts) Growth Culture Medium Exhibit Antimicrobial Properties against Nongrowing Bacterial Strains. J. Nanomater. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- de Araújo, I.W.F.; Vanderlei, E.d.S.O.; Rodrigues, J.A.G.; Coura, C.O.; Quinderé, A.L.G.; Fontes, B.P.; de Queiroz, I.N.L.; Jorge, R.J.B.; Bezerra, M.M.; e Silva, A.A.R. Effects of a sulfated polysaccharide isolated from the red seaweed Solieria filiformis on models of nociception and inflammation. Carbohydr. Polym. 2011, 86, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Osorio, F.A.; Molina, P.; Matiacevich, S.; Enrione, J.; Skurtys, O. Characteristics of hydroxy propyl methyl cellulose (HPMC) based edible film developed for blueberry coatings. Procedia Food Sci. 2011, 1, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Skurtys, O.; Acevedo, C.; Pedreschi, F.; Enronoe, J.; Osorio, F.; Aguilera, J. Food Hydrocolloid Edible Films and Coatings; Nova Science Publishers: New York, NY, USA, 2014. [Google Scholar]
- McHugh, D.J. A Guide to the Seaweed Industry; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef] [Green Version]
- DI ROSA, M. Biological properties of carrageenan. J. Pharm. Pharmacol. 1972, 24, 89–102. [Google Scholar] [CrossRef]
- Hambleton, A.; Fabra, M.-J.; Debeaufort, F.; Dury-Brun, C.; Voilley, A. Interface and aroma barrier properties of iota-carrageenan emulsion–based films used for encapsulation of active food compounds. J. Food Eng. 2009, 93, 80–88. [Google Scholar] [CrossRef]
- Hsu, S.; Chung, H.-Y. Comparisons of 13 edible gum-hydrate fat substitutes for low fat Kung-wan (an emulsified meatball). J. Food Eng. 1999, 40, 279–285. [Google Scholar] [CrossRef]
- Varela, P.; Fiszman, S. Hydrocolloids in fried foods. A review. Food Hydrocoll. 2011, 25, 1801–1812. [Google Scholar] [CrossRef]
- Cosenza, V.A.; Navarro, D.A.; Fissore, E.N.; Rojas, A.M.; Stortz, C.A. Chemical and rheological characterization of the carrageenans from Hypnea musciformis (Wulfen) Lamoroux. Carbohydr. Polym. 2014, 102, 780–789. [Google Scholar] [CrossRef]
- Cohen, S.M.; Ito, N. A critical review of the toxicological effects of carrageenan and processed eucheuma seaweed on the gastrointestinal tract. Crit. Rev. Toxicol. 2002, 32, 413–444. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Maheriya, P.M.; Jani, G.K.; Solanki, H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydr. Polym. 2014, 105, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Abu-Saied, M.; Taha, T.H.; El-Deeb, N.M.; Hafez, E.E. Polyvinyl alcohol/Sodium alginate integrated silver nanoparticles as probable solution for decontamination of microbes contaminated water. Int. J. Biol. Macromol. 2018, 107, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Taha, T.H.; Abu-Saied, M.; Elnouby, M.S.; Hashem, M.; Alamri, S.; Mostafa, Y. Designing of pressure-free filtration system integrating polyvinyl alcohol/chitosan-silver nanoparticle membrane for purification of microbe-containing water. Water Supply 2019, 19, 2443–2452. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, B.R.; Won, J.; Kang, Y.S. Anomalous temperature dependence of facilitated propylene transport in silver polymer electrolyte membranes. J. Membr. Sci. 2003, 227, 197–206. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, B.R.; Won, J.; Kang, Y.S. Effect of the polymer matrix on the formation of silver nanoparticles in polymer–silver salt complex membranes. J. Polym. Sci. Part. B: Polym. Phys. 2006, 44, 1168–1178. [Google Scholar] [CrossRef]
- Fouad, H.; Hongjie, L.; Yanmei, D.; Baoting, Y.; El-Shakh, A.; Abbas, G.; Jianchu, M. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilis to control filarial vector Culex pipiens pallens and its antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1369–1378. [Google Scholar] [CrossRef] [Green Version]
- Ramteke, C.; Chakrabarti, T.; Sarangi, B.K.; Pandey, R.-A. Synthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. J. Chem. 2012, 2013, 93–108. [Google Scholar]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Jegadeeswaran, P.; Shivaraj, R.; Venckatesh, R. Green synthesis of silver nanoparticles from extract of Padina tetrastromatica leaf. Dig. J. Nanomater. Biostructures 2012, 7, 991–998. [Google Scholar]
- Hu, S.; Hsieh, Y.-L. Silver nanoparticle synthesis using lignin as reducing and capping agents: A kinetic and mechanistic study. Int. J. Biol. Macromol. 2016, 82, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Bhakya, S.; Muthukrishnan, S.; Sukumaran, M.; Muthukumar, M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl. Nanosci. 2016, 6, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Prathna, T.; Chandrasekaran, N.; Raichur, A.M.; Mukherjee, A. Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf. B: Biointerfaces 2011, 82, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.; Costa, N.; Hilliou, L.; Larotonda, F.; Gonçalves, M.; Sereno, A.; Coelhoso, I. Design of biodegradable composite films for food packaging. Desalination 2006, 199, 331–333. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Jyoti, K.; Baunthiyal, M.; Singh, A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 2016, 9, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.-L.; Xu, F.; Chen, Y.-S.; Jia, C.-Y. Assembly, characterization of Ag nanoparticles in P (AAm-co-NVP)/CS semi-IPN, and swelling of the resulting composite hydrogels. Polym. Bull. 2010, 65, 181–199. [Google Scholar] [CrossRef]
- Wei, Q.-B.; Fu, F.; Zhang, Y.-Q.; Tang, L. Preparation, characterization, and antibacterial properties of pH-responsive P (MMA-co-MAA)/silver nanocomposite hydrogels. J. Polym. Res. 2014, 21, 349. [Google Scholar] [CrossRef]
- Nezami, S.; Sadeghi, M. pH-sensitive free AgNPs composite and nanocomposite beads based on starch as drug delivery systems. Polym. Bull. 2020, 77, 1255–1279. [Google Scholar] [CrossRef]
- Moustafa, M.; Alamri, S.; Elnouby, M.; Tarek, T.; Abu-Saied, M.; Shati, A.; Mohamed, A.-K.; Alrumman, S. Hydrothermal preparation of TiO2-Ag nanoparticles and its antimicrobial performance against human pathogenic microbial cells in water. BioCell 2018, 42, 3. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, Y.; Vlahovic, B. A review on preparation and applications of silver-containing nanofibers. Nanoscale Res. Lett. 2016, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of edible films and coatings from alginates and carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| Sample | Max Force (N) | Max Displacement (mm) | Max Stress (N/mm2) | Max Strain (%) |
|---|---|---|---|---|
| k-carrageenan | 14.37 ± 0.15 | 0.60 ± 0.02 | 17.96 ± 0.17 | 1.98 ± 0.01 |
| k-carrageenan with silver nanoparticles | 17.21 ± 0.15 | 0.66 ± 0.02 | 18.15 ± 0.17 | 2.25 ± 0.01 |
| Pathogenic Microbes | Clear Zones (mm) | ||
|---|---|---|---|
| k-Carrageenan | k-Carrageenan/Pupa Extract | k-Carrageenan/Silver Nanoparticles | |
| Vibrio cholerae | 0 | 0 | 15 |
| Candida albicans | 0 | 0 | 14 |
| Pseudomonas aeruginosa | 0 | 0 | 16 |
| Escherichia coli | 0 | 0 | 12 |
| Klebsiella pneumoniae | 0 | 0 | 16 |
| Bacillus cereus | 0 | 0 | 14 |
| Sample | TPC (CFU/mL) | Coliform (CFU/mL) | E. coli (CFU/mL) |
|---|---|---|---|
| Mix 1 (Control) | 6.39 × 105 | Absent | Absent |
| Mix 1 (Treated) | 5.3 × 104 | Absent | Absent |
| Mix 2 (Control) | 9.17 × 105 | 44 × 105 | 172 × 105 |
| Mix 2 (Treated) | 5.2 × 104 | 1600 | 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Saied, M.A.; Elnouby, M.; Taha, T.; El-shafeey, M.; G. Alshehri, A.; Alamri, S.; Alghamdi, H.; Shati, A.; Alrumman, S.; Al-Kahtani, M.; et al. Potential Decontamination of Drinking Water Pathogens through k-Carrageenan Integrated Green Bottle Fly Bio-Synthesized Silver Nanoparticles. Molecules 2020, 25, 1936. https://doi.org/10.3390/molecules25081936
Abu-Saied MA, Elnouby M, Taha T, El-shafeey M, G. Alshehri A, Alamri S, Alghamdi H, Shati A, Alrumman S, Al-Kahtani M, et al. Potential Decontamination of Drinking Water Pathogens through k-Carrageenan Integrated Green Bottle Fly Bio-Synthesized Silver Nanoparticles. Molecules. 2020; 25(8):1936. https://doi.org/10.3390/molecules25081936
Chicago/Turabian StyleAbu-Saied, M. A., Mohamed Elnouby, Tarek Taha, Muhammad El-shafeey, Ali G. Alshehri, Saad Alamri, Huda Alghamdi, Ali Shati, Sulaiman Alrumman, Mohamed Al-Kahtani, and et al. 2020. "Potential Decontamination of Drinking Water Pathogens through k-Carrageenan Integrated Green Bottle Fly Bio-Synthesized Silver Nanoparticles" Molecules 25, no. 8: 1936. https://doi.org/10.3390/molecules25081936






