Rapid Raman Spectroscopic Analysis of Stress Induced Degradation of the Pharmaceutical Drug Tetracycline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Optical Spectroscopy
2.3. Data Preprocessing and Quantification
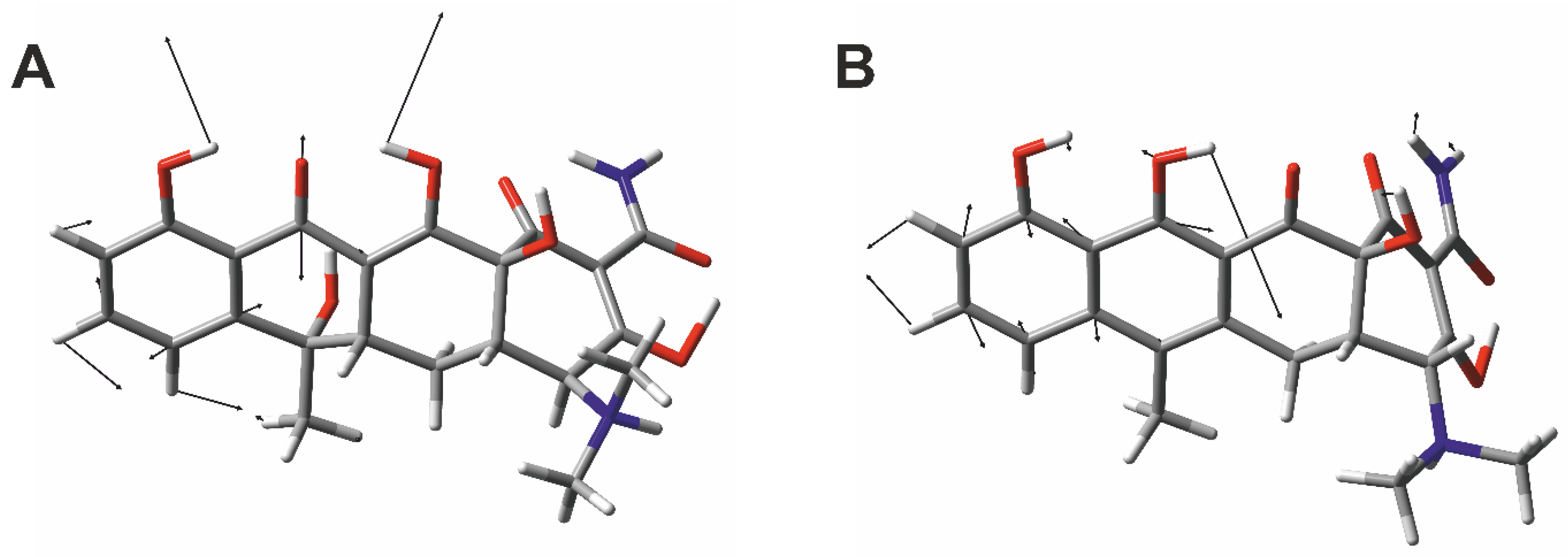
2.4. Density Functional Theory Calculation for Vibrational Assignment of Raman Marker Bands
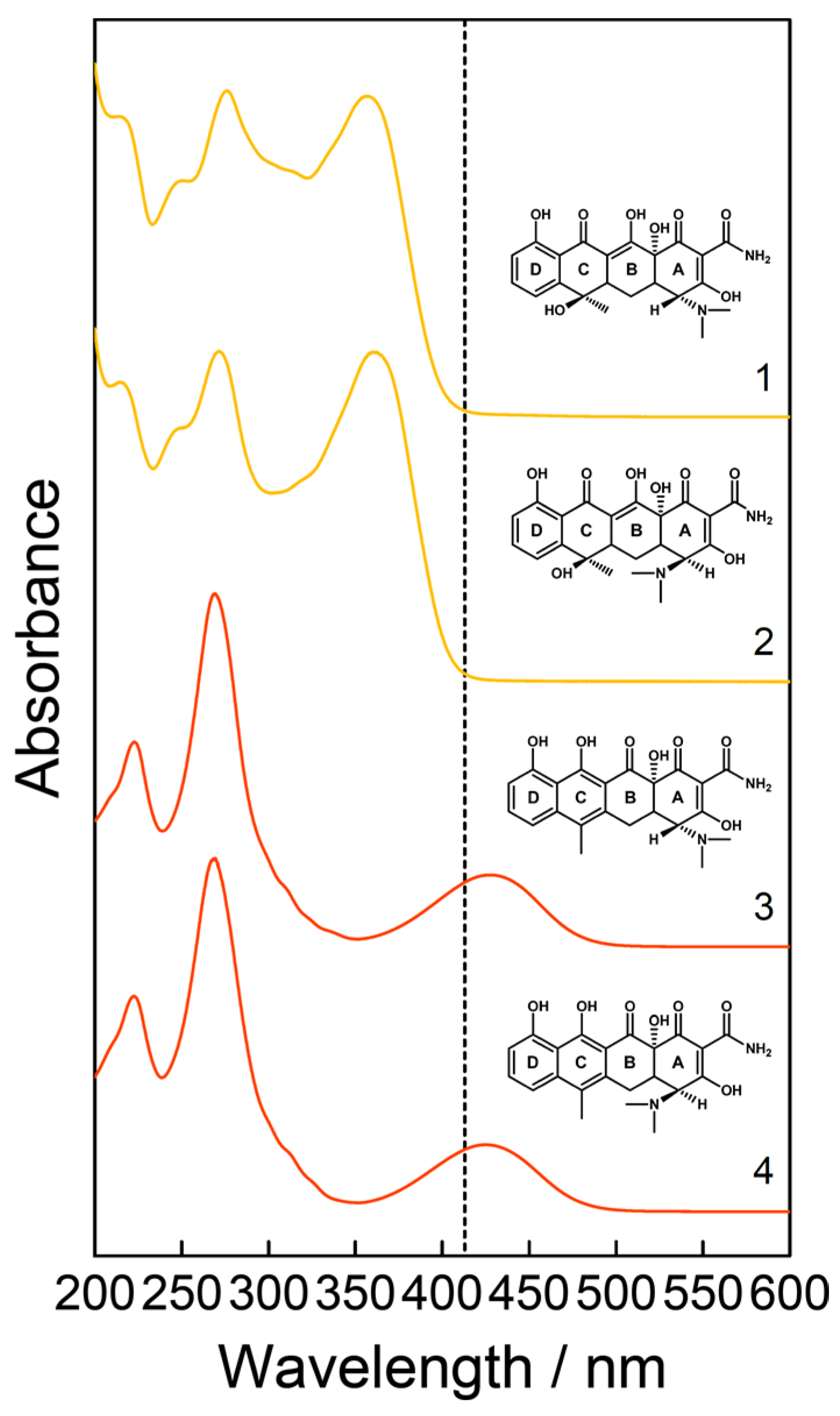
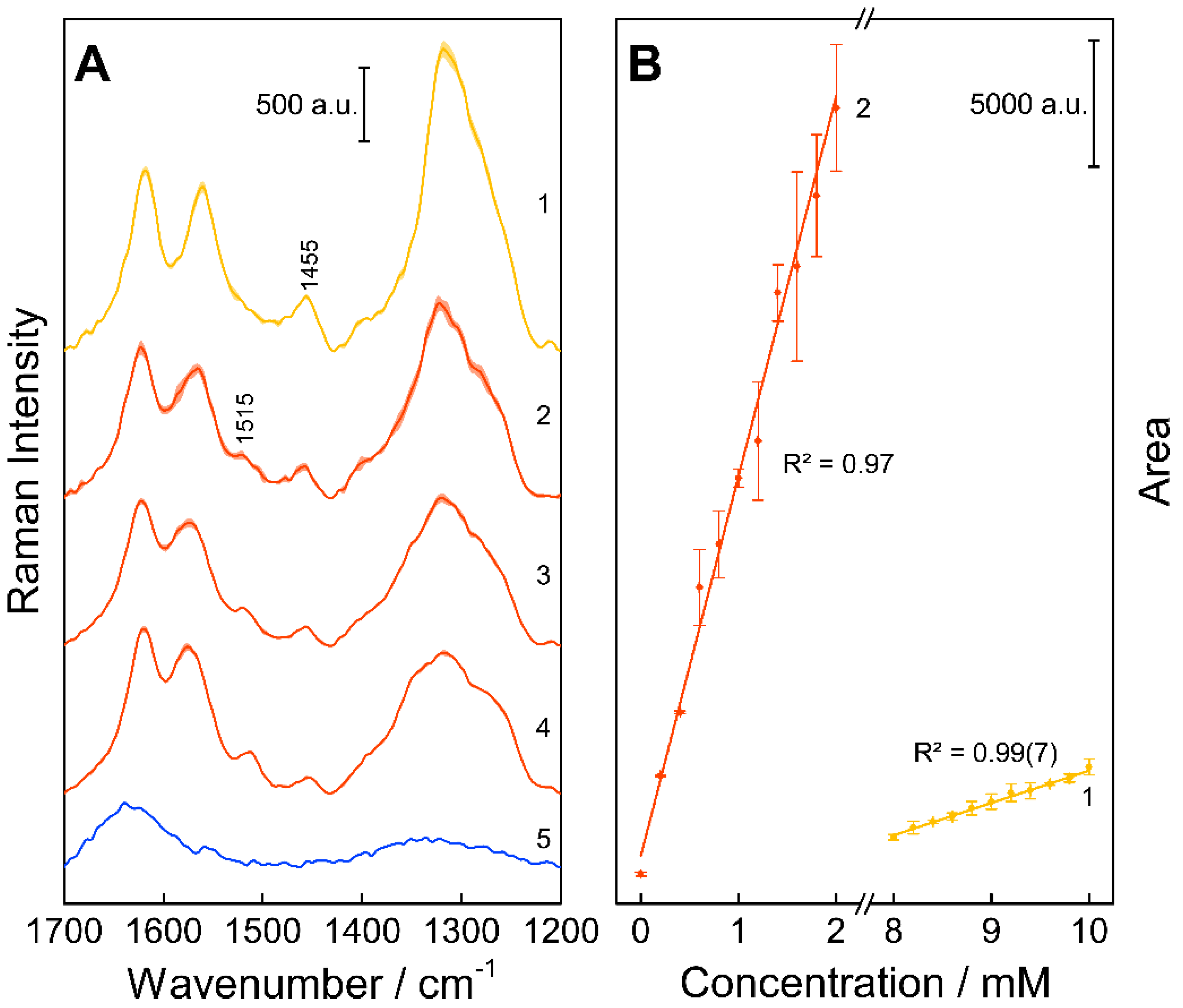
3. Results and Discussion
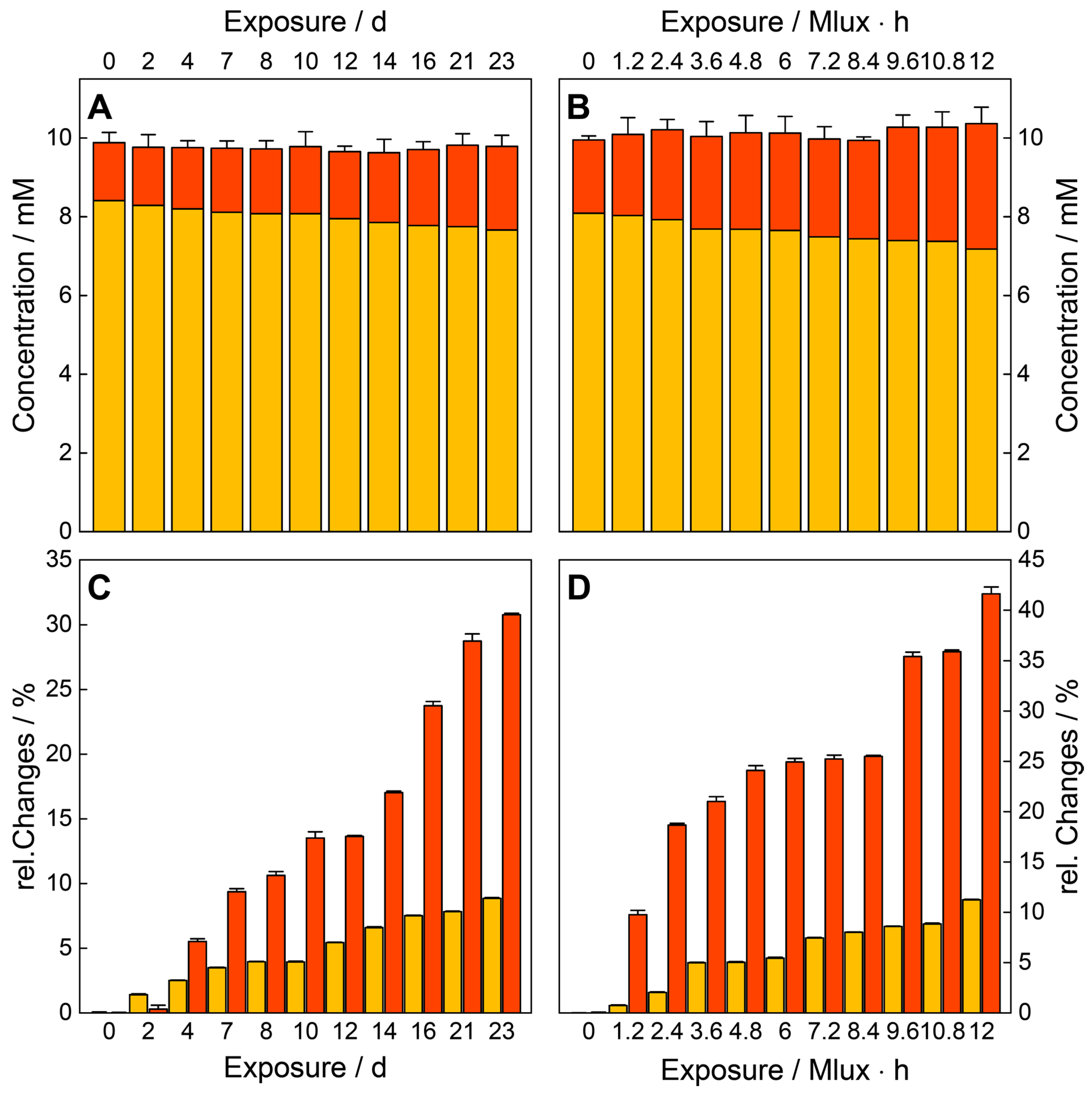
Resonance Raman Spectroscopy for Quantification of Concentration Changes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sarmah, A.; Meyer, M.; Boxall, A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Duggar, B. Aureomycin: A product of the continuing search for new antibiotics. Ann. N. Y. Acad. Sci. 1948, 51, 177–181. [Google Scholar] [CrossRef]
- Chopra, I.; Howe, T.; Linton, A.; Linton, K.; Richmond, M.; Speller, D. The tetracyclines: Prospects at the beginning of the 1980s. J. Antimicrob. Chemother. 1981, 8, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.J.; Okeke, I.N.; Laxminarayan, R.; Perencevich, E.N.; Weisenberg, S. Non-prescription antimicrobial use worldwide: A systematic review. Lancet Infect. Dis. 2011, 11, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Chopra, I.; Hawkey, P.; Hinton, M. Tetracyclines, molecular and clinical aspects. J. Antimicrob. Chemother. 1992, 29, 245–277. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Kehrenberg, C.; Walsh, T. Use of antimicrobial agents in veterinary medicine and food animal production. Int. J. Antimicrob. Agents 2001, 17, 431–437. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050795s005lbl.pdf (accessed on 13 September 2019).
- Food and Drug Administration (FDA). Available online: https://www.drugs.com/cons/tetracycline-class-oral-parenteral.html (accessed on 13 September 2019).
- Hoener, B.A.; Sokoloski, T.; Mitscher, L.; Malspeis, L. Kinetics of dehydration of epitetracycline in solution. J. Pharm. Sci. 1974, 63, 1901–1904. [Google Scholar] [CrossRef]
- Schlecht, K.; Frank, C. Dehydration of tetracycline. J. Pharm. Sci. 1975, 64, 352–354. [Google Scholar] [CrossRef]
- Yuen, P.; Sokoloski, T. Kinetics of concomitant degradation of tetracycline to epitetracycline, anhydrotetracycline, and epianhydrotetracycline in acid phosphate solution. J. Pharm. Sci. 1977, 66, 1648–1650. [Google Scholar] [CrossRef]
- Dihuidi, K.; Roets, E.; Hoogmartens, J.; Vanderhaeghe, H. Influence of temperature on the stability of solid tetracycline hydrochloride, measured by high-performance liquid chromatography. J. Chromatogr. A 1982, 246, 350–355. [Google Scholar] [CrossRef]
- Benitz, K.-F.; Diermeier, H. Renal toxicity of tetracycline degradation products. Proc. Soc. Exp. Biol. Med. 1964, 115, 930–935. [Google Scholar] [CrossRef]
- Schnappinger, D.; Hillen, W. Tetracyclines: Antibiotic action, uptake, and resistance mechanisms. Arch. Microbiol. 1996, 165, 359–369. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Sengeløv, G.; Tjørnelund, J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef]
- Alsante, K.; Martin, L.; Baertschi, S. A stress testing benchmarking study. Pharm. Technol. 2003, 27, 60–73. [Google Scholar]
- Alsante, K.; Ando, A.; Brown, R.; Ensing, J.; Hatajik, T.; Kong, W.; Tsuda, Y. The role of degradant profiling in active pharmaceutical ingredients and drug products. Adv. Drug Deliv. Rev. 2007, 59, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singla, D.; Sakhuja, N. Stability testing of pharmaceutical products. J. Appl. Pharm. Sci. 2012, 2, 129–138. [Google Scholar]
- Kelesidis, T.; Kelesidis, I.; Rafailidis, P.; Falagas, M. Counterfeit or substandard antimicrobial drugs: A review of the scientific drugs: A review of the scientific evidence. Journal of Antimicrobial Chemotherapy 60, 214-236. J. Antimicrob. Chemother. 2007, 60, 214–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckley, G.; Gostin, L. Countering the Problem of Falsified and Substandard Drugs; The National Academic Press: Washington, DC, USA, 2013. [Google Scholar]
- Frosch, T.; Wyrwich, E.; Yan, D.; Domes, C.; Domes, R.; Popp, J.; Frosch, T. Counterfeit and Substandard Test of the Antimalarial Tablet Riamet® by Means of Raman Hyperspectral Multicomponent Analysis. Molecules 2019, 24, 3229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okeke, I.; Lamikanra, A. Quality and bioavailability of tetracycline capsules in a Nigerian semi-urban community. Int. J. Antimicrob. Agents 1995, 5, 245–250. [Google Scholar] [CrossRef]
- Nalwade, S.U.; Reddy, V.R.; Rao, D.D.; kumar Morisetti, N. A validated stability indicating ultra performance liquid chromatographic method for determination of impurities in Esomeprazole magnesium gastro resistant tablets. J. Pharm. Biomed. Anal. 2012, 57, 109–114. [Google Scholar] [CrossRef]
- Elzayat, E.; Ibrahim, M.; Abdel-Rahman, A.; Ahmed, S.; Alanazi, F.; Habib, W. A validated stability-indicating UPLC method for determination of diclofenac sodium in its pure form and matrix formulations. Arabian J. Chem. 2017, 10, 3245–3254. [Google Scholar] [CrossRef]
- Dong, X.; Ding, L.; Cao, X.; Jiang, L.; Zhong, S. A sensitive LC-MS/MS method for the simultaneous determination of amoxicillin and ambroxol in human plasma with segmental monitoring. Biomed. Chromatogr. 2013, 27, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Nebsen, M.; Elzanfaly, E. Stability-indicating method and LC–MS-MS characterization of forced degradation products of sofosbuvir. J. Chromatogr. Sci. 2016, 54, 1631–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, R.; Singh, S. Identification and characterization of a photolytic degradation product of telmisartan using LC–MS/TOF, LC–MSn, LC–NMR and on-line H/D exchange mass studies. J. Pharm. Biomed. Anal. 2010, 53, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Frosch, T.; Schmitt, M.; Schenzel, K.; Faber, J.H.; Bringmann, G.; Kiefer, W.; Popp, J. In vivo localization and identification of the antiplasmodial alkaloid dioncophylline A in the tropical liana Triphyophyllum peltatum by a combination of fluorescence, near infrared Fourier transform Raman microscopy, and density functional theory calculations. Biopolymers 2006, 82, 295–300. [Google Scholar]
- Frosch, T.; Popp, J. Structural analysis of the antimalarial drug halofantrine by means of Raman spectroscopy and density functional theory calculations. J. Biomed. Opt. 2010. [Google Scholar] [CrossRef]
- Paudel, A.; Raijada, D.; Rantanen, J. Raman spectroscopy in pharmaceutical product design. Adv. Drug Deliv. Rev. 2015, 89, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Cailletaud, J.; De Bleye, C.; Dumont, E.; Sacré, P.-Y.; Netchacovitch, L.; Gut, Y.; Boiret, M.; Ginot, Y.-M.; Hubert, P.; Ziemons, E. Critical review of surface-enhanced Raman spectroscopy applications in the pharmaceutical field. J. Pharm. Biomed. Anal. 2018, 147, 458–472. [Google Scholar] [CrossRef]
- Yan, D.; Popp, J.; Pletz, M.W.; Frosch, T. Highly Sensitive Broadband Raman Sensing of Antibiotics in Step-Index Hollow-Core Photonic Crystal Fibers. Acs Photonics 2017, 4, 138–145. [Google Scholar] [CrossRef]
- Yan, D.; Frosch, T.; Kobelke, J.; Bierlich, J.; Popp, J.; Pletz, M.W.; Frosch, T. Fiber-Enhanced Raman Sensing of Cefuroxime in Human Urine. Anal. Chem. 2018, 90, 13243–13248. [Google Scholar] [CrossRef]
- Yan, D.; Popp, J.; Pletz, M.W.; Frosch, T. Fiber enhanced Raman sensing of levofloxacin by PCF bandgap-shifting into the visible range. Anal. Methods 2018, 10, 586–592. [Google Scholar] [CrossRef]
- Wolf, S.; Frosch, T.; Popp, J.; Pletz, M.W.; Frosch, T. Highly Sensitive Detection of the Antibiotic Ciprofloxacin by Means of Fiber Enhanced Raman Spectroscopy. Molecules 2019, 24, 4512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frosch, T.; Knebl, A.; Frosch, T. Recent advances in nano-photonic techniques for pharmaceutical drug monitoring with emphasis on Raman spectroscopy. Nanophotonics 2020, 9, 19–37. [Google Scholar] [CrossRef]
- Frosch, T.; Schmitt, M.; Bringmann, G.; Kiefer, W.; Popp, J. Structural analysis of the anti-malaria active agent chloroquine under physiological conditions. J. Phys. Chem. B 2007, 111, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Frosch, T.; Meyer, T.; Schmitt, M.; Popp, J. Device for Raman difference spectroscopy. Anal. Chem. 2007, 79, 6159–6166. [Google Scholar] [CrossRef]
- Frosch, T.; Schmitt, M.; Popp, J. Raman spectroscopic investigation of the antimalarial agent mefloquine. Anal. Bioanal. Chem. 2007, 387, 1749–1757. [Google Scholar] [CrossRef]
- Long, D.A. The Raman Effect: A Unified Treatment of the Theory of Raman Scattering by Molecules; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Frosch, T.; Wyrwich, E.; Yan, D.; Popp, J.; Frosch, T. Fiber-Array-Based Raman Hyperspectral Imaging for Simultaneous, Chemically-Selective Monitoring of Particle Size and Shape of Active Ingredients in Analgesic Tablets. Molecules 2019, 24, 4381. [Google Scholar] [CrossRef] [Green Version]
- Frosch, T.; Popp, J. Relationship between molecular structure and Raman spectra of quinolines. J. Mol. Struct. 2009, 924–926, 301–308. [Google Scholar] [CrossRef]
- Keiner, R.; Herrmann, M.; Kusel, K.; Popp, J.; Frosch, T. Rapid monitoring of intermediate states and mass balance of nitrogen during denitrification by means of cavity enhanced Raman multi-gas sensing. Anal. Chim. Acta 2015, 864, 39–47. [Google Scholar] [CrossRef]
- Jochum, T.; Michalzik, B.; Bachmann, A.; Popp, J.; Frosch, T. Microbial respiration and natural attenuation of benzene contaminated soils investigated by cavity enhanced Raman multi-gas spectroscopy. Analyst 2015, 140, 3143–3149. [Google Scholar] [CrossRef] [Green Version]
- Jochum, T.; von Fischer, J.C.; Trumbore, S.; Popp, J.; Frosch, T. Multigas Leakage Correction in Static Environmental Chambers Using Sulfur Hexafluoride and Raman Spectroscopy. Anal. Chem. 2015, 87, 11137–11142. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Popp, J.; Frosch, T. Analysis of Fiber-Enhanced Raman Gas Sensing Based on Raman Chemical Imaging. Anal. Chem. 2017, 89, 12269–12275. [Google Scholar] [CrossRef]
- Knebl, A.; Yan, D.; Popp, J.; Frosch, T. Fiber enhanced Raman gas spectroscopy. Trac-Trend Anal. Chem. 2018, 103, 230–238. [Google Scholar] [CrossRef]
- Fernández, R.; Dassie, S. Electroanalytical procedure to resolve a sample solution containing tetracycline and its toxic degraded product: Anhydrotetracycline. J. Electroanal. Chem. 2008, 624, 121–128. [Google Scholar] [CrossRef]
- Yan, D.; Domes, C.; Domes, R.; Frosch, T.; Popp, J.; Pletz, M.W.; Frosch, T. Fiber enhanced Raman spectroscopic analysis as a novel method for diagnosis and monitoring of diseases related to hyperbilirubinemia and hyperbiliverdinemia. Analyst 2016, 141, 6104–6115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domes, R.; Domes, C.; Albert, C.R.; Bringmann, G.; Popp, J.; Frosch, T. Vibrational spectroscopic characterization of arylisoquinolines by means of Raman spectroscopy and density functional theory calculations. Phys. Chem. Chem. Phys. 2017, 19, 29918–29926. [Google Scholar] [CrossRef]
- Domes, C.; Domes, R.; Popp, J.; Pletz, M.W.; Frosch, T. Ultrasensitive Detection of Antiseptic Antibiotics in Aqueous Media and Human Urine Using Deep UV Resonance Raman Spectroscopy. Anal. Chem. 2017, 89, 9997–10003. [Google Scholar] [CrossRef]
- Sieburg, A.; Knebl, A.; Jacob, J.M.; Frosch, T. Characterization of fuel gases with fiber-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2019, 411, 7399–7408. [Google Scholar] [CrossRef]
- Knebl, A.; Domes, R.; Yan, D.; Popp, J.; Trumbore, S.; Frosch, T. Fiber-Enhanced Raman Gas Spectroscopy for (18)O-(13)C-Labeling Experiments. Anal. Chem. 2019, 91, 7562–7569. [Google Scholar] [CrossRef]
- Keiner, R.; Gruselle, M.C.; Michalzik, B.; Popp, J.; Frosch, T. Raman spectroscopic investigation of 13CO 2 labeling and leaf dark respiration of Fagus sylvatica L. (European beech). Anal. Bioanal. Chem. 2015, 407, 1813–1817. [Google Scholar] [CrossRef]
- Hanf, S.; Fischer, S.; Hartmann, H.; Keiner, R.; Trumbore, S.; Popp, J.; Frosch, T. Online investigation of respiratory quotients in Pinus sylvestris and Picea abies during drought and shading by means of cavity-enhanced Raman multi-gas spectrometry. Analyst 2015, 140, 4473–4481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogozi, T.; Popp, J.; Frosch, T. Fiber-enhanced Raman multi-gas spectroscopy: What is the potential of its application to breath analysis? Future Sci. Bioanal. 2015, 7, 281–284. [Google Scholar] [CrossRef]
- Jochum, T.; Rahal, L.; Suckert, R.J.; Popp, J.; Frosch, T. All-in-one: A versatile gas sensor based on fiber enhanced Raman spectroscopy for monitoring postharvest fruit conservation and ripening. Analyst 2016, 141, 2023–2029. [Google Scholar] [CrossRef] [Green Version]
- Sieburg, A.; Jochum, T.; Trumbore, S.E.; Popp, J.; Frosch, T. Onsite cavity enhanced Raman spectrometry for the investigation of gas exchange processes in the Earth’s critical zone. Analyst 2017, 142, 3360–3369. [Google Scholar] [CrossRef] [PubMed]
- Jochum, T.; Fastnacht, A.; Trumbore, S.E.; Popp, J.; Frosch, T. Direct Raman Spectroscopic Measurements of Biological Nitrogen Fixation under Natural Conditions: An Analytical Approach for Studying Nitrogenase Activity. Anal. Chem. 2017, 89, 1117–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieburg, A.; Schneider, S.; Yan, D.; Popp, J.; Frosch, T. Monitoring of gas composition in a laboratory biogas plant using cavity enhanced Raman spectroscopy. Analyst 2018, 143, 1358–1366. [Google Scholar] [CrossRef]
- Anderson, G.; Scott, M. Determination of product shelf life and activation energy for five drugs of abuse. Clin. Chem. 1991, 37, 398–402. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Guideline for the photostability testing of new drug substances and new drug products. Fed. Regist. 62 1997, 95, 27115–27122. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: https://www.R-project.org/ (accessed on 10 April 2020).
- Signal Developers. Signal: Signal Processing. 2014. Available online: http://r-forge.r-project.org/projects/signal/ (accessed on 10 April 2020).
- Liland, K.H. EMSC: Extended Multiplicative Signal Correction. R Package Version 0.9.0. 2017. Available online: https://CRAN.R-project.org/package=EMSC (accessed on 10 April 2020).
- Liland, K.H.; Almøy, T.; Mevik, B.H. Optimal choice of baseline correction for multivariate calibration of spectra. Applied spectroscopy. 2010, 64, 1007–1016. [Google Scholar] [CrossRef]
- Elzhov, T.V.; . Mullen, K.M.; Spiess, A.-K.; Bolker, B. minpack.lm: R Interface to the Levenberg-Marquardt Nonlinear Least-Squares Algorithm Found in MINPACK, Plus Support for Bounds. R Package Version 1.2-1. 2016; Available online: https://CRAN.R-project.org/package=minpack.lm (accessed on 10 April 2020).
- Dörfer, T.; Bocklitz, T.; Tarcea, N.; Schmitt, M.; Popp, J. Checking and improving calibration of Raman spectra using chemometric approaches. Z. für Physikalische Chem. 2011, 225, 753–764. [Google Scholar]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision A. 02; Gaussian Inc.: Wallingford, CT, USA, 2009; pp. 5648–5652. [Google Scholar]
- Becke, A. Density-functional thermochemistry. II. The effect of the Perdew–Wang generalized-gradient correlation correction. J. Chem. Phys. 1992, 97, 9173–9177. [Google Scholar] [CrossRef]
- Stephens, P.; Devlin, F.; Chabalowski, C.; Frisch, M. Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunning, T. A road map for the calculation of molecular binding energies. J. Phys. Chem. A 2000, 104, 9062–9080. [Google Scholar] [CrossRef]
- Polavarapu, P. Ab initio vibrational Raman and Raman optical activity spectra. J. Phys. Chem. 1990, 94, 8106–8112. [Google Scholar] [CrossRef]
- Sharma, K.; Mullangi, R. A concise review of HPLC, LC-MS and LC-MS/MS methods for determination of azithromycin in various biological matrices. Biomed. Chromatogr. 2013, 27, 1243–1258. [Google Scholar] [CrossRef]
- Li, R.; Zhang, H.; Chen, Q.-W.; Yan, N.; Wang, H. Improved surface-enhanced Raman scattering on micro-scale Au hollow spheres: Synthesis and application in detecting tetracycline. Analyst 2011, 136, 2527–2532. [Google Scholar] [CrossRef]
- Jin, D.; Bai, Y.; Chen, H.; Liu, S.; Chen, N.; Huang, J.; Huang, S.; Chen, Z. SERS detection of expired tetracycline hydrochloride with an optical fiber nano-probe. Anal. Methods 2015, 7, 1307–1312. [Google Scholar] [CrossRef]
- Filgueiras, A.; Paschoal, D.; Dos Santos, H.; Sant’Ana, A. Adsorption study of antibiotics on silver nanoparticle surfaces by surface-enhanced Raman scattering spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 979–985. [Google Scholar] [CrossRef]
- Dhakal, S.; Chao, K.; Huang, Q.; Kim, M.; Schmidt, W.; Qin, J.; Broadhurst, C. A simple surface-enhanced Raman spectroscopic method for on-site screening of tetracycline residue in whole milk. Sensors 2018, 18, 424. [Google Scholar] [CrossRef] [Green Version]
- Leypold, F.C.; Reiher, M.; Brehm, G.; Schmitt, M.O.; Schneider, S.; Matousek, P.; Towrie, M. Tetracycline and derivatives—assignment of IR and Raman spectra via DFT calculations. Phys. Chem. Chem. Phys. 2003, 5, 1149–1157. [Google Scholar] [CrossRef]
- Chen, X.; Liang, W.; Yang, C.; Lin, W.; Bi, M. Simultaneous quantitative detection of tetracyclines derivatives by raman spectroscopy. In Proceedings of the Virtual Environments Human-Computer Interfaces and Measurement Systems (VECIMS), Tianjin, China, 2–4 July 2012; IEEE: New York, NY, USA, 2012; pp. 111–114. [Google Scholar]
- Chen, Y.; Hu, C.; Qu, J.; Yang, M. Photodegradation of tetracycline and formation of reactive oxygen species in aqueous tetracycline solution under simulated sunlight irradiation. J. Photochem. Photobiol. A Chem. 2008, 197, 81–87. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Wang, Z.; Tao, T.; Hu, C. Photoproducts of tetracycline and oxytetracycline involving self-sensitized oxidation in aqueous solutions: Effects of Ca2+ and Mg2+. J. Environ. Sci. 2011, 23, 1634–1639. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domes, C.; Frosch, T.; Popp, J.; Frosch, T. Rapid Raman Spectroscopic Analysis of Stress Induced Degradation of the Pharmaceutical Drug Tetracycline. Molecules 2020, 25, 1866. https://doi.org/10.3390/molecules25081866
Domes C, Frosch T, Popp J, Frosch T. Rapid Raman Spectroscopic Analysis of Stress Induced Degradation of the Pharmaceutical Drug Tetracycline. Molecules. 2020; 25(8):1866. https://doi.org/10.3390/molecules25081866
Chicago/Turabian StyleDomes, Christian, Timea Frosch, Juergen Popp, and Torsten Frosch. 2020. "Rapid Raman Spectroscopic Analysis of Stress Induced Degradation of the Pharmaceutical Drug Tetracycline" Molecules 25, no. 8: 1866. https://doi.org/10.3390/molecules25081866