Full Parametric Study of the Influence of Ionomer Content, Catalyst Loading and Catalyst Type on Oxygen and Ion Transport in PEM Fuel Cell Catalyst Layers
Abstract
:1. Introduction
2. Experimental
2.1. Production of Catalyst Coated Membranes
2.2. In situ Analysis
- Polarization curves—the polarization curves were recorded at 80 °C cell temperature with fully humidified gases on the anode and cathode (i.e., 80 °C dew point) and an operating pressure of 2.0 bara. A constant flow rate was used with 2.0 nL/min H2 (norm liter per minute) on the anode and 5.0 nL/min air on the cathode. The polarization curves were recorded in potentiostatic mode from 0.2 V to OCV with 0.05 V increments from 0.20–0.75 V and 0.02 V increments from 0.78 - OCV. The holding times at each potential were potential dependent (U > 0.90 V: 30 s, 0.70 V < U ≤ 0.90 V: 60 s, and U ≤ 0.70 V: 5 min). A recovery procedure was also followed, where PtO was reduced at a voltage of 0.40 V for 5 s for load points above 0.75.
- Cyclic voltammetry—the cyclic voltammetry was run between 0.05 and 0.60 V at 100 mV/s with a total of 5 cycles. For the ECSA calculation, an average of the last three cycles is taken. The operating temperature was 80 °C with fully humidified gases and at 1.0 bara.
- Humidity Sweeps—the humidity sweeps were measured at 80 °C cell temperature and 1.5 bara pressure. The cell was operated at a constant load of 1 A/cm2 with fully humidified gases on the anode while the relative gas humidity on the cathode was varied between 20 % and 120 %. The voltage change was measured after each humidity step was conditioned for 7 min.
3. Results and Discussion
3.1. Ex Situ Analysis
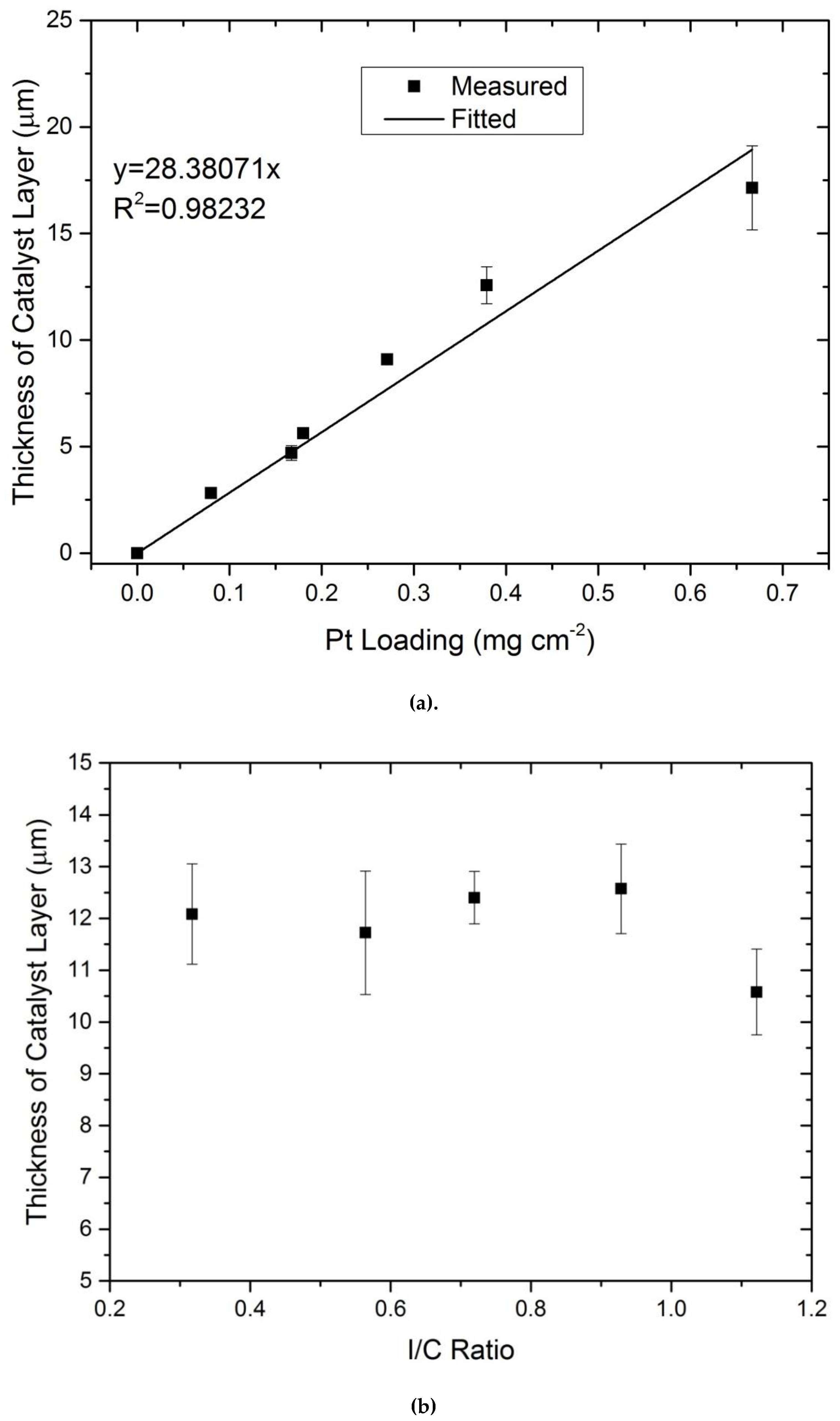
3.2. Reproducibility of Produced CCMs
3.3. In situ Analysis
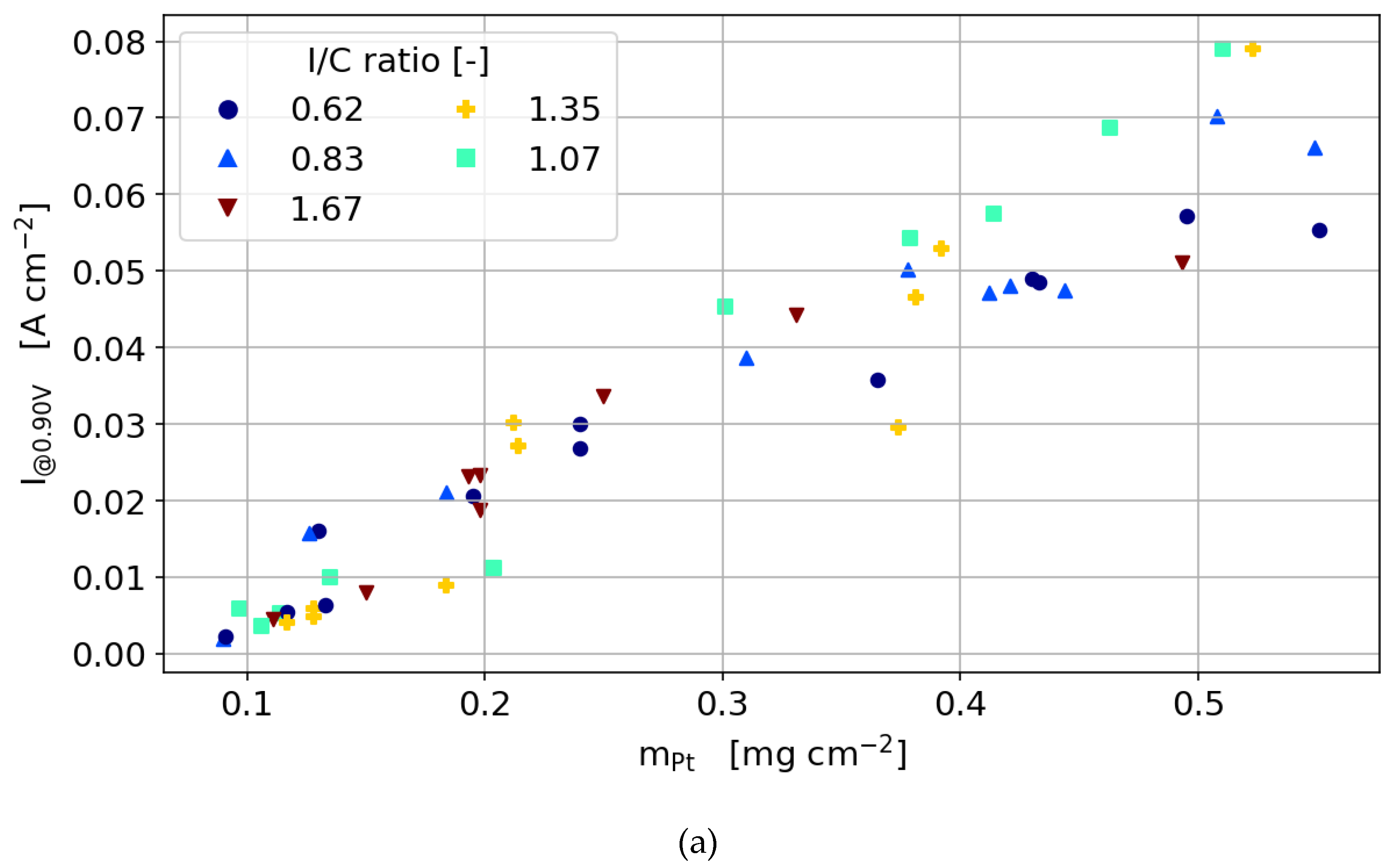
3.3.1. Cyclic Voltammetry
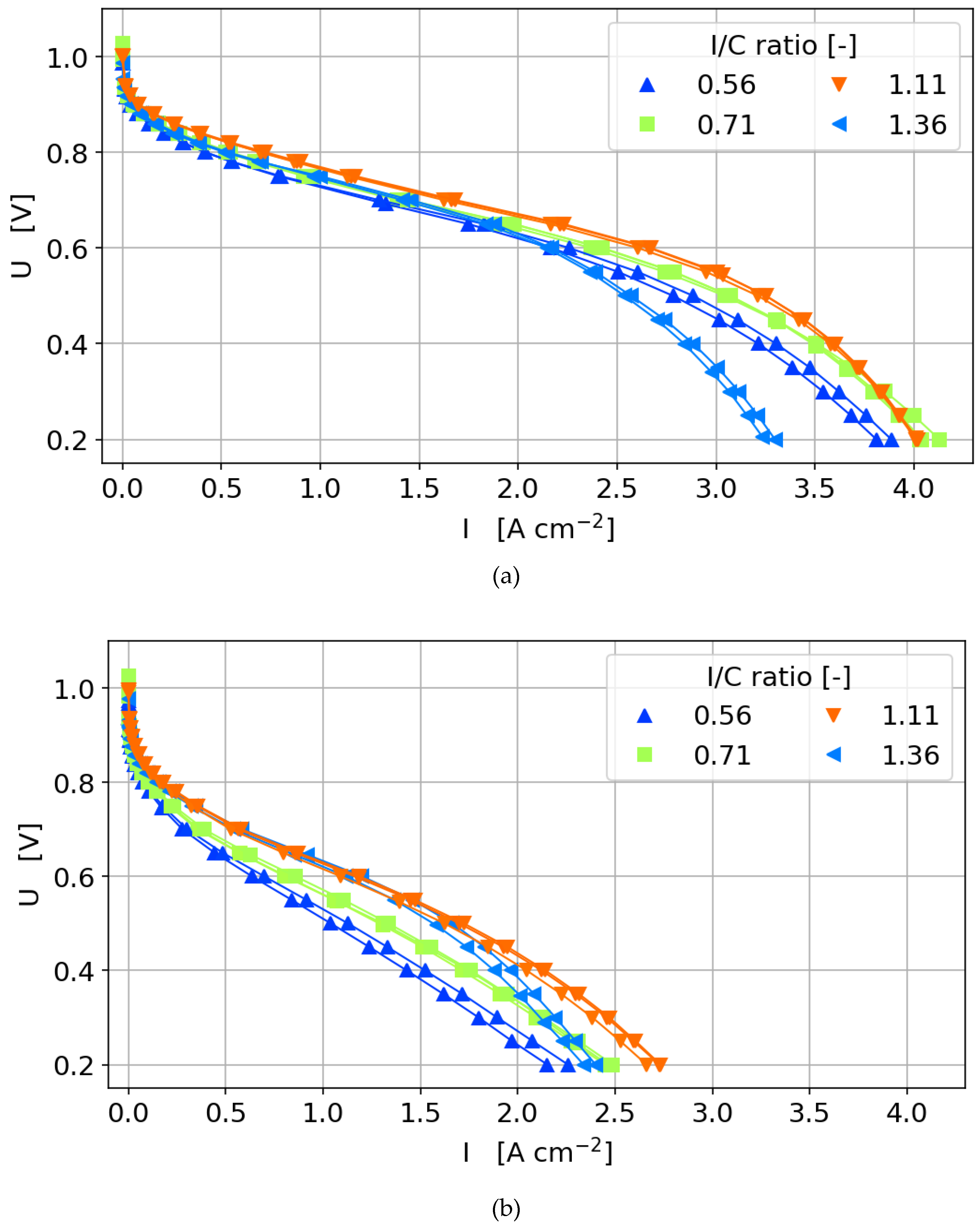
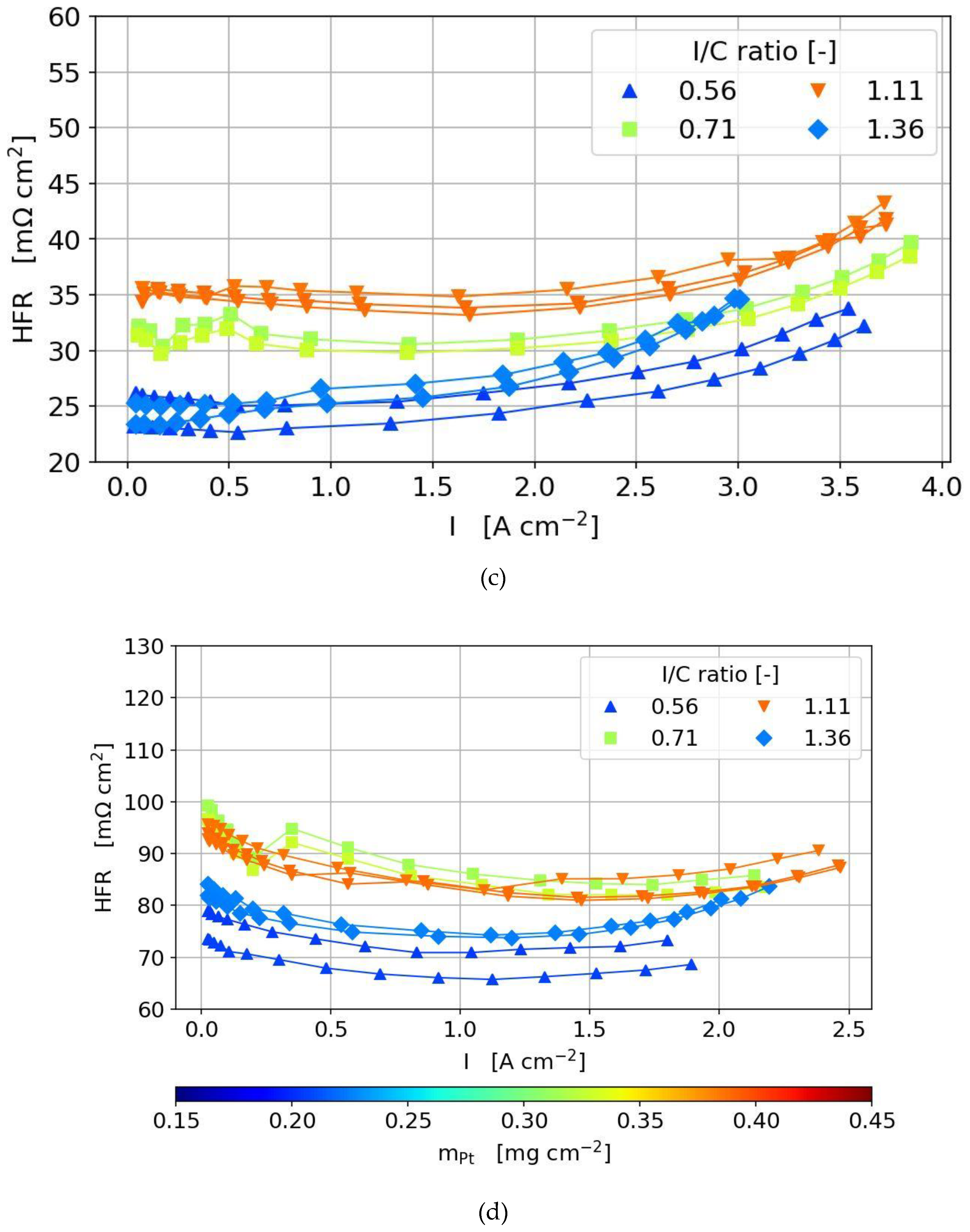
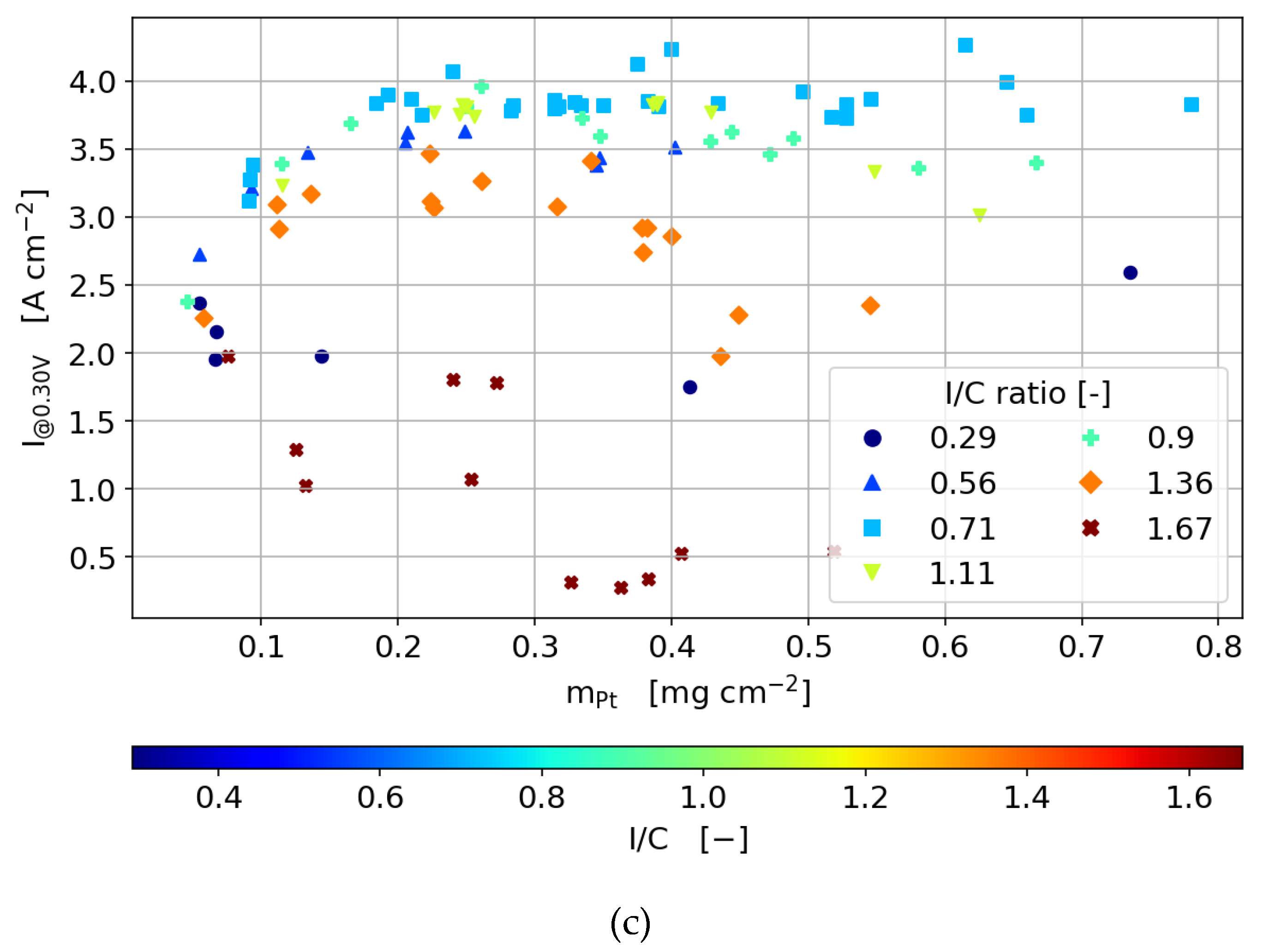
3.3.2. Polarization Curves
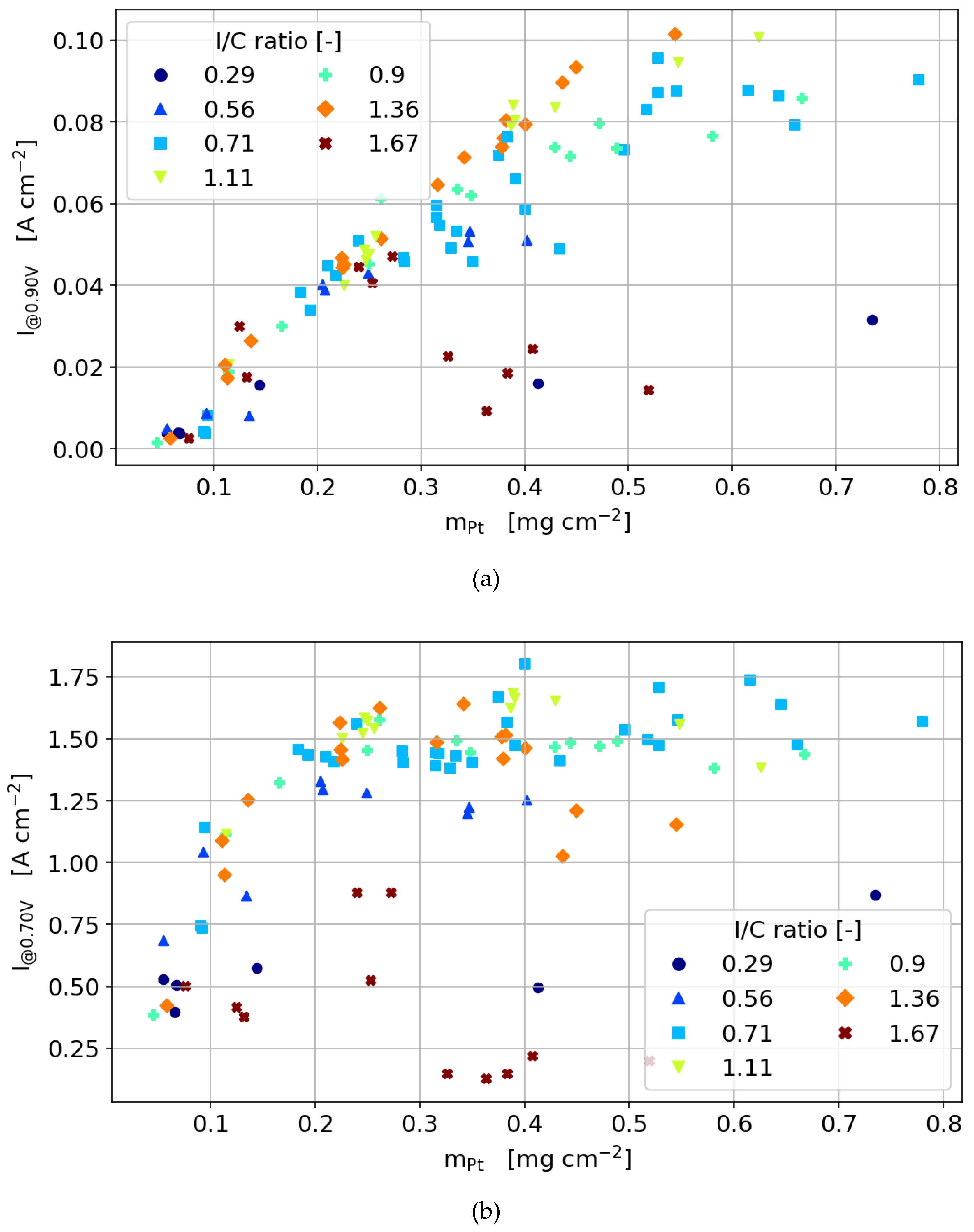
3.3.3. 40 wt% Catalyst
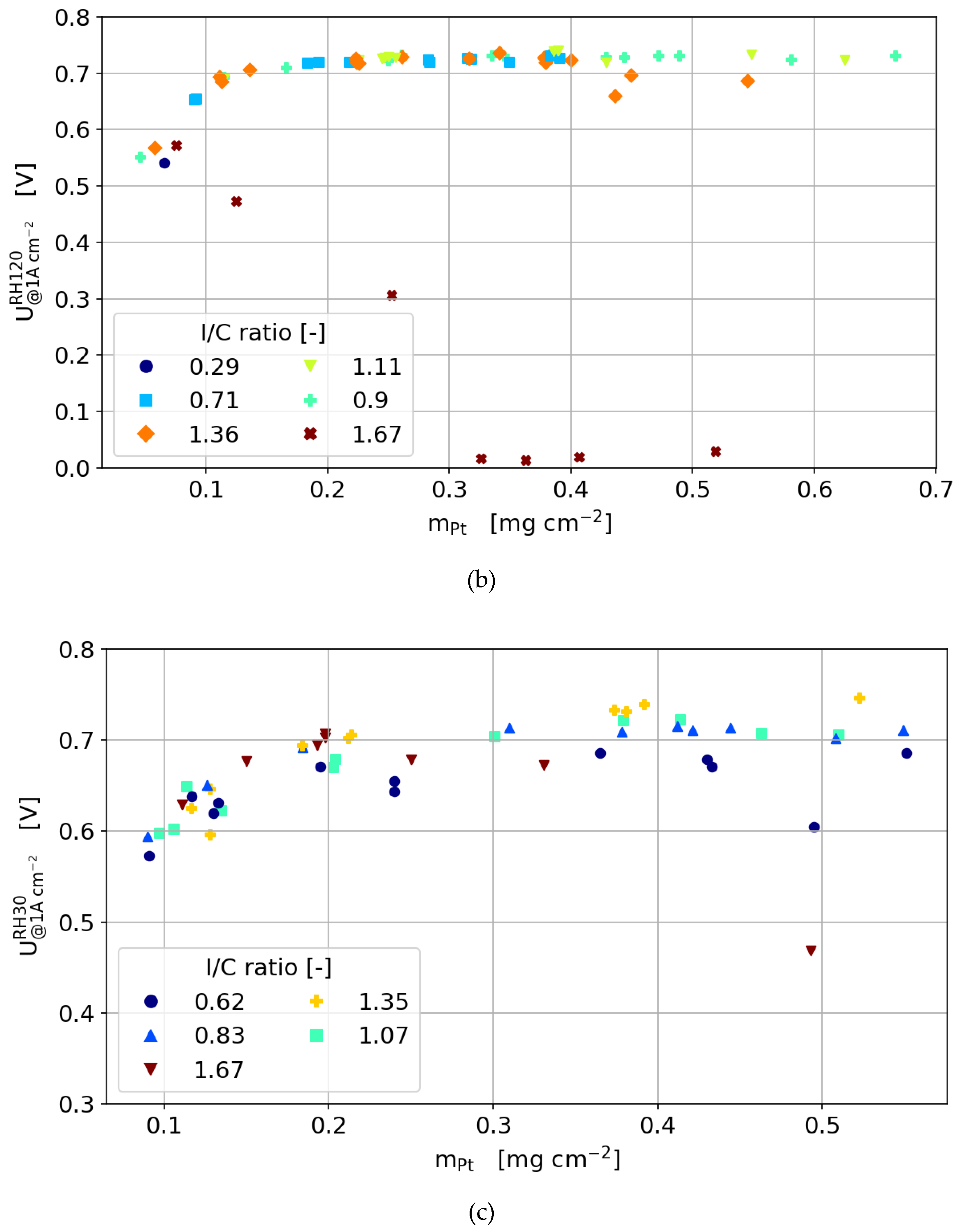
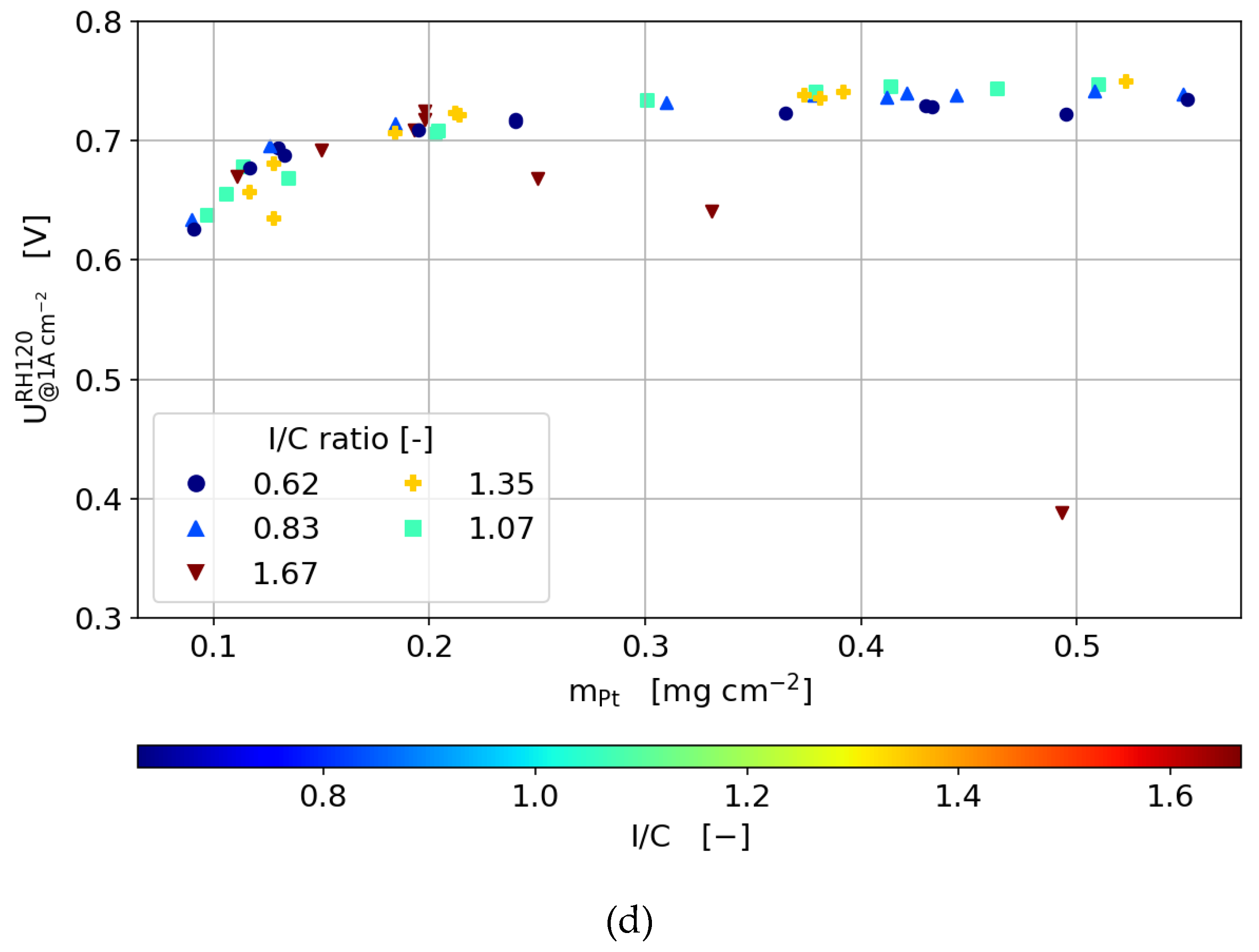
3.3.4. Humidity Sweeps
3.3.5. 60 wt % Catalyst
4. Conclusions
- Electrical and protonic contact is established regardless of the printing of layers on topic of each other. This conclusion was established based on SEM images that illustrated a homogeneous distribution of the Pt, C and F. The linear dependency of the ECSA on Pt loading for various I/C ratios without any discontinuity in the slope once again proves the establishment of the electrical and protonic contact between all layers.
- The optimum ionomer content is not only dependent on the catalyst layer thickness, but also on the load and gas humidity.
- In the activation region, regardless of which type of catalyst is used, the dependency of the current density on Pt loading is linear as long as the ionomer content is not too low to hinder proton conductivity and is not too high to hinder oxygen diffusion significantly.
- In the ohmic and mass transport region of the polarization curve, increasing the ionomer content to a value higher than the optimum results in a decrease in current density with higher Pt loading. The diffusion losses outweigh the improvement by the increasing catalytically active area.
- At high Pt loading and ionomer content, through-plane diffusion losses become more limiting than the proton conductivity.
- General trends are the same for 40 and 60 wt.% Pt/C catalysts, but the dependency on ionomer content is lower due to reduced catalyst layer thickness.
Author Contributions
Funding
Conflicts of Interest
References
- Zamel, N. The catalyst layer and its dimensionality—A look into its ingredients and how to characterize their effects. J. Power Sources 2016, 309, 141–159. [Google Scholar] [CrossRef]
- Alvar, E.N.; Zhou, B.; Eichhorn, S.H. Composite-supported Pt catalyst and electrosprayed cathode catalyst layer for polymer electrolyte membrane fuel cell. Int. J. Energy Res. 2017, 41, 1626–1641. [Google Scholar] [CrossRef]
- Chan, S.; Jankovic, J.; Susac, D.; Saha, M.S.; Tam, M.; Yang, H. Electrospun carbon nanofiber catalyst layers for polymer electrolyte membrane fuel cells: Structure and performance. J. Power Sources 2018, 392, 239–250. [Google Scholar] [CrossRef]
- Handbook of Fuel Cells—Fundamentals, Technology and Applications. Novel Catalysts, Catalysts Support and Catalysts Coated Membrane Methods; Vielstich, W.; Lamm, A.; Gasteiger, H. (Eds.) John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 978-0-471-49926-8. [Google Scholar]
- O’Hayre, R.; Lee, S.-J.; Cha, S.-W.; Prinz, F. A sharp peak in the performance of sputtered platinum fuel cells at ultra-low platinum loading. J. Power Sources 2002, 109, 483–493. [Google Scholar] [CrossRef]
- Saha, M.S.; Gullá, A.F.; Allen, R.J.; Mukerjee, S. High performance polymer electrolyte fuel cells with ultra-low Pt loading electrodes prepared by dual ion-beam assisted deposition. Electrochim. Acta 2006, 51, 4680–4692. [Google Scholar] [CrossRef]
- Hatzell, K.B.; Dixit, M.B.; Berlinger, S.A.; Weber, A.Z. Understanding inks for porous-electrode formation. J. Mater. Chem. A 2017, 5, 20527–20533. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.-Y.; Wang, C.; Lei, Y.-J.; Zhang, J. Gradient design of Pt/C ratio and Nafion content in cathode catalyst layer of PEMFCs. Int. J. Hydrog. Energy 2017, 42, 29960–29965. [Google Scholar] [CrossRef]
- Yoon, Y.-G.; Yang, T.-H.; Park, G.-G.; Lee, W.-Y.; Kim, C.-S. A multi-layer structured cathode for the PEMFC. J. Power Sources 2003, 118, 189–192. [Google Scholar] [CrossRef]
- Xing, L.; Shi, W.; Das, P.K.; Scott, K. Inhomogeneous Distribution of Platinum and Ionomer in the Porous Cathode to Maximize the Performance of a PEM Fuel Cell. AIChE J. 2017, 63, 4895–4910. [Google Scholar] [CrossRef]
- Herden, S.; Hirschfeld, J.A.; Lohri, C.; Perchthaler, M.; Haase, S. Ionomer equivalent weight structuring in the cathode catalyst layer of automotive fuel cells: Effect on performance, current density distribution and electrochemical impedance spectra. J. Power Sources 2017, 364, 449–457. [Google Scholar] [CrossRef]
- Herden, S.; Riewald, F.; Hirschfeld, J.A.; Perchthaler, M. In-plane structuring of proton exchange membrane fuel cell cathodes: Effect of ionomer equivalent weight structuring on performance and current density distribution. J. Power Sources 2017, 355, 36–43. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Li, X. The impact of short side chain ionomer on polymer electrolyte membrane fuel cell performance and durability. Appl. Energy 2018, 217, 295–302. [Google Scholar] [CrossRef]
- Shahgaldia, S.; Ozdena, A.; Li, X.; Hamdullahpur, F. Cathode catalyst layer design with gradients of ionomer distribution for proton exchange membrane fuel cells. Energy Convers. Manag. 2018, 171, 1476–1486. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Alaefour, I.; Li, X.L. Impact of manufacturing processes on proton exchange membrane fuel cell performance. Appl. Energy 2018, 225, 1022–1032. [Google Scholar] [CrossRef]
- Sassin, M.B.; Garsany, Y.; Gould, B.; Swider-Lyons, K.E. Fabrication Method for Laboratory-Scale High-Performance Membrane Electrode Assemblies for Fuel Cells. Anal. Chem. 2017, 89, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Roller, J.M.; Mustain, W.E.; Maric, R. Influence of the ionomer/carbon ratio for low-Pt loading catalyst layer prepared by reactive spray deposition technology. J. Power Sources 2015, 283, 84–94. [Google Scholar] [CrossRef]
- Garsany, Y.; Atkinson, R.W., III; Sassin, M.B.; Hjelm, R.M.E. Improving PEMFC Performance Using Short-Side-Chain Low-Equivalent-Weight PFSA Ionomer in the Cathode Catalyst Layer. J. Electrochem. Soc. 2018, 165, F381–F391. [Google Scholar] [CrossRef]
- Ozdena, A.; Shahgaldi, S.; Li, X.; Hamdullahpur, F. The impact of ionomer type on the morphological and microstructural degradations of proton exchange membrane fuel cell electrodes under freeze-thaw cycles. Appl. Energy 2019, 238, 1048–1059. [Google Scholar] [CrossRef]
- Shukla, S.; Stanier, D.; Saha, M.S.; Stumper, J.; Secanell, M. Analysis of Inkjet Printed PEFC Electrodes with Varying Platinum Loading. J. Electrochem. Soc. 2016, 163, F677–F687. [Google Scholar] [CrossRef]
- Salari, S.; Tam, M.; McCague, C.; Stumper, J.; Bahrami, M. The ex-situ and in-situ gas diffusivities of polymer electrolyte membrane fuel cell catalyst layer and contribution of primary pores, secondary pores, ionomer and water to the total oxygen diffusion resistance. J. Power Sources 2019, 449, 227479. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alink, R.; Singh, R.; Schneider, P.; Christmann, K.; Schall, J.; Keding, R.; Zamel, N. Full Parametric Study of the Influence of Ionomer Content, Catalyst Loading and Catalyst Type on Oxygen and Ion Transport in PEM Fuel Cell Catalyst Layers. Molecules 2020, 25, 1523. https://doi.org/10.3390/molecules25071523
Alink R, Singh R, Schneider P, Christmann K, Schall J, Keding R, Zamel N. Full Parametric Study of the Influence of Ionomer Content, Catalyst Loading and Catalyst Type on Oxygen and Ion Transport in PEM Fuel Cell Catalyst Layers. Molecules. 2020; 25(7):1523. https://doi.org/10.3390/molecules25071523
Chicago/Turabian StyleAlink, Robert, Rajveer Singh, Patrick Schneider, Kläre Christmann, Johannes Schall, Roman Keding, and Nada Zamel. 2020. "Full Parametric Study of the Influence of Ionomer Content, Catalyst Loading and Catalyst Type on Oxygen and Ion Transport in PEM Fuel Cell Catalyst Layers" Molecules 25, no. 7: 1523. https://doi.org/10.3390/molecules25071523





