Chemical Speciation of Aluminum in Wine by LC–ICP–MS
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development for Speciation Analysis of Aluminum
2.2. Models vs. Standard Solution Analysis
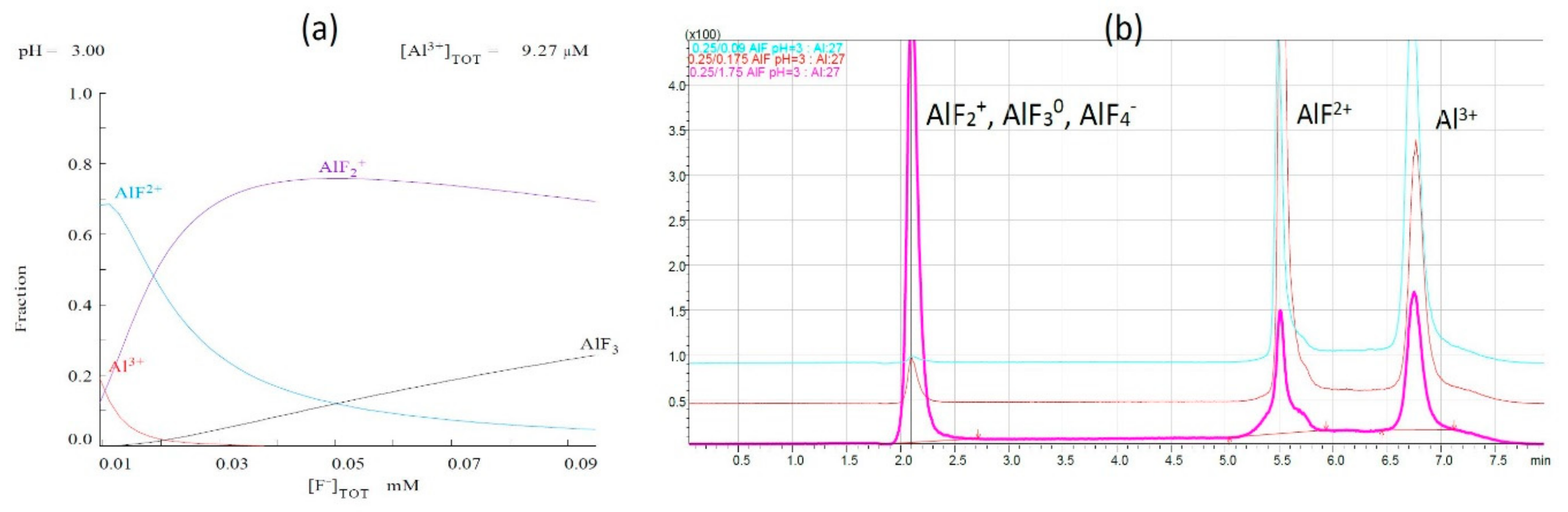
2.2.1. Aluminum Fluoride Complexes
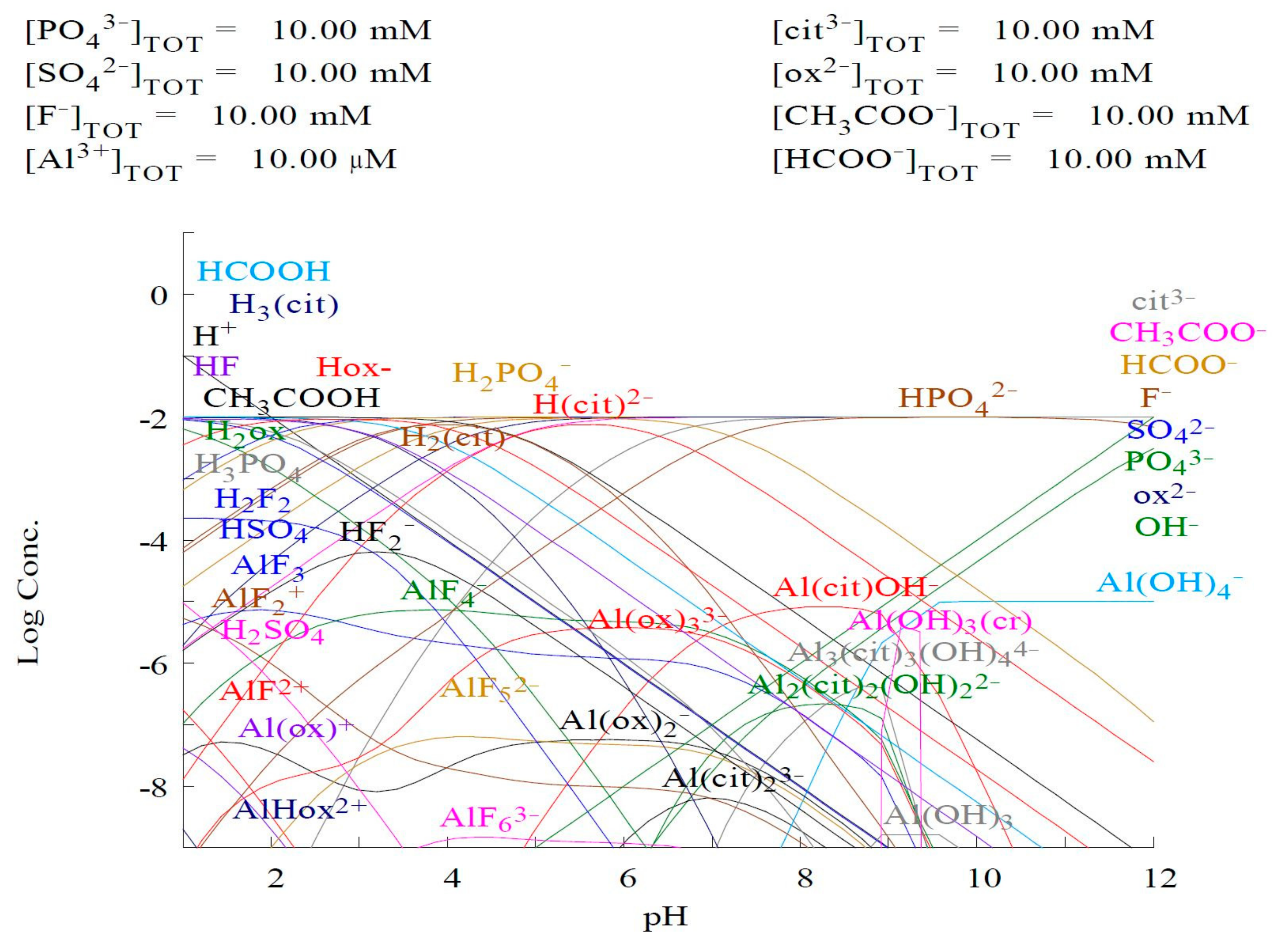
2.2.2. Aluminum Organic Complexes
2.2.3. Al/Citrate
2.2.4. Al/Oxalate
2.3. Wine Samples
2.3.1. Total Content of Aluminum, Fluorides, and Organic Ions
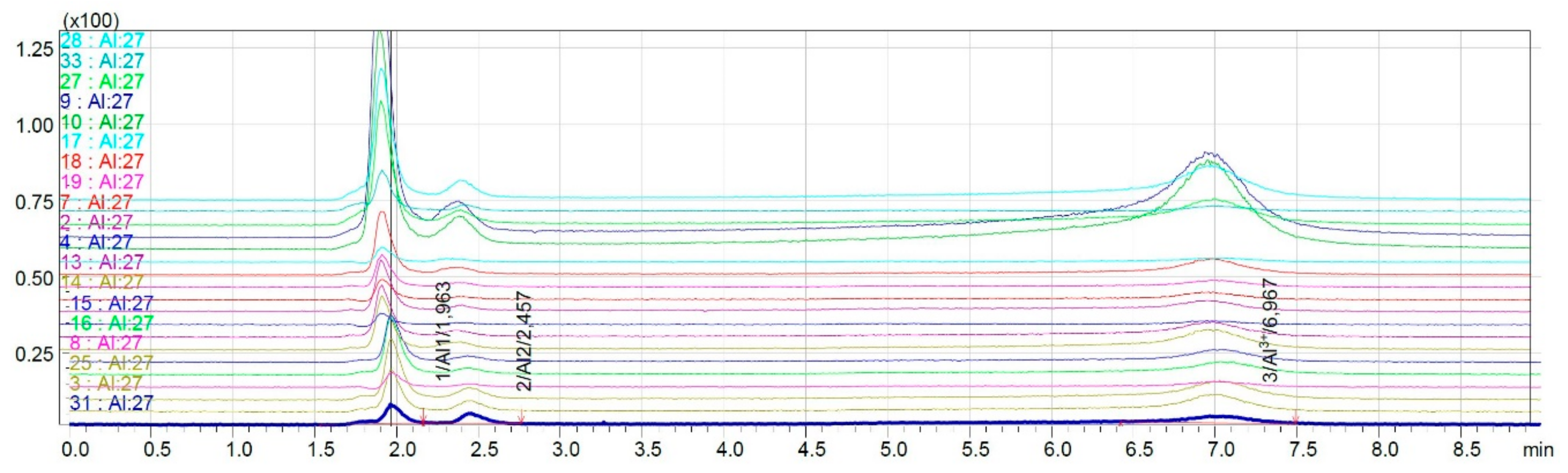
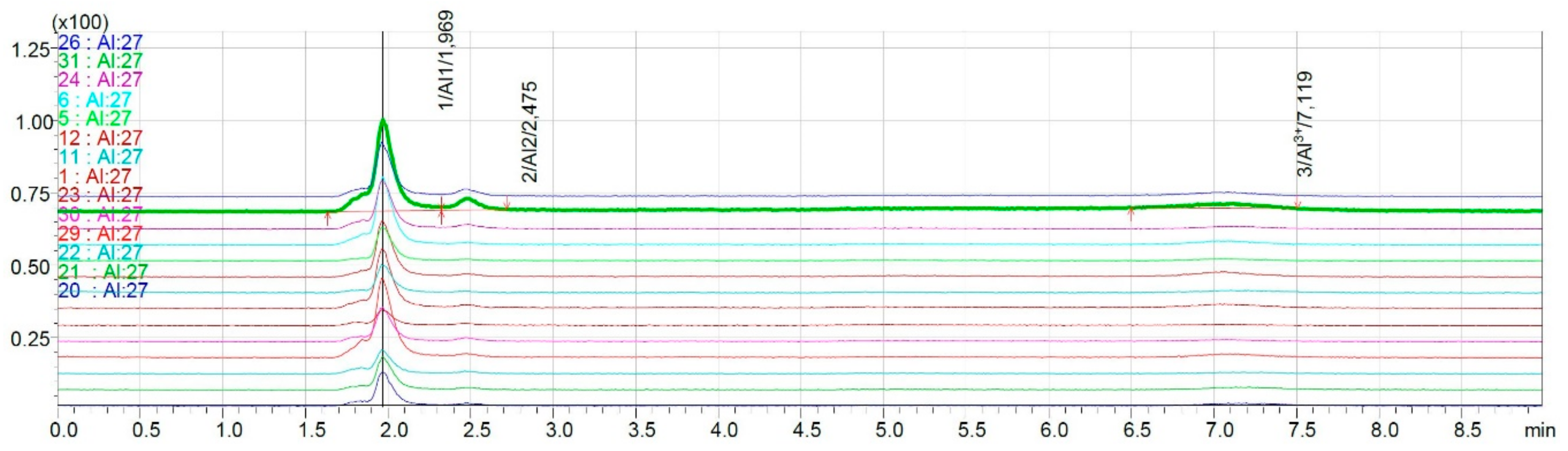
2.3.2. Speciation Analysis of Wine
3. Materials and Methods
3.1. Analytical System
3.2. Reagents and Standards
3.3. Total Content of Aluminum, Fluorides, and Organic Ions (Acetate, Citrate, Formate, and Oxalate)
3.4. Method Development for Speciation Analysis of Aluminum
3.4.1. Modeling and Model Solutions
3.4.2. pH Effect and Adjustment
3.5. Wine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frankowski, M. Aluminium and its complexes in teas and fruity brew samples, Speciation and Ions Determination by Ion Chromatography and High-Performance Liquid Chromatography-Fluorescence Analytical Methods. Food Anal. Methods 2014, 7, 1109–1117. [Google Scholar] [CrossRef]
- Magnier, A.; Fekete, V.; Van Loco, J.; Bolle, F.; Elskens, M. Speciation study of aluminium in beverages by competitive Ligand Exchange-Adsorptive Stripping Voltamperometry. Talanta 2014, 122, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.; Cabrera, C.; Lorenzo, M.; Lopez, C. Aluminium levels in wine, beer and other alcoholic beverages consumed in Spain. Sci. Total Environ. 1998, 220, 1–9. [Google Scholar] [CrossRef]
- Darbre, P.; Mannello, F.; Exley, C. Aluminium and breast cancer: Sources of exposure, tissue measurements and mechanisms of toxicological actions on breast biology. J. Inorg. Biochem. 2013, 128, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Renedo, O.; Navarro-Cuñado, A.; Ventas-Romay, E.; Asunción Alonso-Lomillo, M. Determination of aluminium using different techniques based on the Al(III)- morin complex. Talanta 2019, 196, 131–136. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathes, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.; Blaise, A. Validation and evaluation of a high performance liquid chromatographic method for the determination of aluminium in wine. J. Chromatogr. A 2006, 1134, 74–80. [Google Scholar] [CrossRef]
- Chen, B.; Zeng, Y.; Hu, B. Study on speciation of aluminum in human serum using zwitterionic bile acid derivative dynamically coated C18 column HPLC separation with UV and on-line ICP-MS detection. Talanta 2010, 81, 180–186. [Google Scholar] [CrossRef]
- Javad, M.T.; Vahidinia, A.; Samiee, F.; Elaridi, J.; Leili, M.; Faradmal, J.; Rahmani, A. Analysis of aluminum, minerals and trace elements in the milk samples from lactating mothers in Hamadan, Iran. J. Trace Elements Med. Biol. 2018, 50, 8–15. [Google Scholar] [CrossRef]
- Exley, C. What is the risk of aluminium as a neurotoxin? Expert Rev. Neurother. 2014, 14, 589–591. [Google Scholar] [CrossRef]
- Alonso-Mateos, A.; Almendral-Parra, M.J.; Curto-Serrano, Y.; Rodríguez-Martín, F.J. Online monitoring of aluminium in drinking water with Fluorimetric detection. J. Fluoresc. 2008, 18, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; Sancho, D.; Caballero, I. Aluminium content in beers and silicon sequestering effects. Food Res. Int. 2010, 43, 2432–2436. [Google Scholar] [CrossRef]
- Mold, M.; Umar, D.; King, A.; Exley, C. Aluminium in brain tissue in autism. J. Trace Elem. Med. Biol. 2018, 46, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Pugazhendhi, D.; Mannello, F. Aluminium and human breast diseases. J. Inorg. Biochem. 2011, 105, 1484–1488. [Google Scholar] [CrossRef]
- Fei-Bo, W.U.; Chen, J.-X. Influence of Aluminum and Cadmium Stresses on Mineral Nutrition and Root Exudates in Two Barley Cultivars. Pedosphere 2007, 17, 505–512. [Google Scholar]
- Zioła-Frankowska, A.; Frankowski, M. Speciation analysis of aluminium in plant parts of Betula pendula and in soil. J. Environ. Sci. 2018, 65, 153–161. [Google Scholar]
- Frankowski, M.; Zioła-Frankowska, A.; Siepak, J. From soil to leaves–aluminium fractianaton by single step extraction procedures in polluted and protected areas. J. Environ. Manag. 2013, 127, 1–9. [Google Scholar] [CrossRef]
- Zioła-Frankowska, A.; Kuta, J.; Frankowski, M. Application of a new HPLC-ICP-MS method for simultaneous determination of Al3+ and aluminium fluoride complexes. Helyion 2015, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tria, J.; Butler, E.; Haddad, P.; Bowie, A. Determination of aluminium in natural water samples. Anal. Chim. Acta 2007, 588, 153–165. [Google Scholar] [CrossRef]
- Lu, J.; Tian, J.; Guo, N.; Wang, Y.; Pan, Y. Microemulsion extraction separation and determination of aluminium apecies by spectrofluorimetry. J. Hazard. Mater. 2011, 185, 1107–1114. [Google Scholar] [CrossRef]
- Cardiano, P.; Cigala, R.; Crea, F.; Giacobello, F.; Giuffre, O.; Irto, A.; Lando, G.; Sammartano, S. Sequestration of aluminium(III) by different natural and synthetic organic and inorganic ligands in aqueous solution. Chemosphere 2017, 186, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Happel, O.; Seubert, A. Separation and characterization of aluminium malate species by ion chromatography. Anal. Bioanal. Chem. 2008, 392, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Happel, O.; Seubert, A. Characterization of stable aluminium-citrate species as reference substances for aluminium speciation by ion chromatography. J. Chromatogr. A 2006, 1108, 68–75. [Google Scholar] [CrossRef]
- Bi, S.P.; Yang, X.D.; Zhang, F.P.; Wang, X.L.; Zou, G.W. Analytical methodologies for aluminium speciation in environmental and biological samples—A review. Fresenius J. Anal. Chem. 2001, 370, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Ščančar, J.; Milačič, R. Aluminium Speciation in environmental samples-a review. Anal. Bioanal. Chem. 2006, 386, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, M.; Zioła-Frankowska, A. Speciation analysis of aluminium and aluminium fluoride complexes by HPLC-UVVIS. Talanta 2010, 82, 1763–1769. [Google Scholar] [CrossRef]
- Matúš, P.; Kubová, J. Recent developments in the determination fractionation and speciation analysis of aluminium spectrochemical analytical methods and computer modelling. J. Soil Contam. 2008, 2, 43–72. [Google Scholar]
- Goulle, J.-P.; Saussereau, E.; Mahieu, L.; Geubert, M. Current role of ICP-MS in clinical toxicology and forensic toxicology: A metallic profile. Bioanalysis 2014, 6, 2245–2259. [Google Scholar] [CrossRef]
- Popp, M.; Hann, S.; Koellensperger, G. Environmental application of elemental speciation analysis based on liquid or gas chromatography hyphenated to inductively coupled plasma mass spectrometry—A review. Anal. Chim. Acta 2010, 668, 114–129. [Google Scholar] [CrossRef]
- De Villiers, A.; Alberts, P.; Tredoux, A.; Nieuwoudt, H. Analytical techniques for wine analysis: An African perspective: A review. Anal. Chim. Acta 2012, 730, 2–23. [Google Scholar] [CrossRef]
- Pyrzynska, K. Chemical speciation and fractionation of metals in wine. Chem. Speciat. Bioavailab. 2007, 19, 1–8. [Google Scholar] [CrossRef]
- Tariba, B. Metals in wine- impact on wine quality and health outcomes. Biol. Trace Elem. Res. 2011, 144, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Grindlay, G.; Mora, J.; Gras, L.; de Loos-Vollebregt, M. Atomic spectrometry methods for wine analysis-a critical evaluation and discussion of recent applications. Anal. Chim. Acta 2011, 691, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Bao, Y.; Yu, Y.; Cui, H.; Fa, Y.; Liu, H. Simultaneous determination of organic acids, inorganic anions and alditols in wine with valve-switching ion chromatography. Chromatographia 2018, 81, 1103–1108. [Google Scholar] [CrossRef]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile composition of Merlot red wine and its contribution to the aroma: Optimization and validation of analytical method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef]
- Đurđic, S.; Pantelic, M.; Trifkovic, J.; Vukojevic, V.; Natic, M.; Tesic, Z.; Mutic, J. Elemental composition as a tool for the assessment of type, seasonable variability and graphical origin of wine and its contribution to daily elemental intake. RSC Adv. 2017, 7, 2151–2162. [Google Scholar] [CrossRef] [Green Version]
- Bantan, T.; Milaćić, R.; Pihlar, B. Quantitative determination of trace amounts of Al-citrate by anion-exchange FPLC-ETAAS. Talanta 1998, 47, 929–941. [Google Scholar] [CrossRef]
- Montes Bayón, M.; Rodríguez Garcia, A.; Ignacio Garcia, J.; Sanz-Medel, A. Indirect determination of trace amounts of fluoride in natural waters by ion chromatography: A comparison of on-line post column fluorimetry and ICP-MS detectors. Analyst 1998, 124, 27–31. [Google Scholar] [CrossRef]
- Kuta, J.; Frankowski, M. Speciation of aluminium fluoride complexes and Al3+ in environmental samples using HPLC-ICP-MS. Agil. Technol. 2013, 16, 4–6. [Google Scholar]
- Zioła-Frankowska, A.; Frankowski, M.; Siepak, J. Development of a new analytical method for online sumiltaneous qualitative determination of aluminium (free aluminium ion, aluminium-fluoride complexes) by HPLC-FAAS. Talanta 2009, 78, 623–630. [Google Scholar] [CrossRef]
- Borrmann, G.; Seubert, A. Aluminium speciation by liquid chromatography concerning hydro- and geo-chemical aspects. Anal. Chim. Acta 1996, 322, 233–239. [Google Scholar] [CrossRef]
- Cabrita, M.J.; Martins, N.; Barrulas, P.; Garcia, R.; Dias, C.; Perez-Alvarez, P.; Freitas, A.; Garde-Cerdan, T. Multi-element composition of red, white and palhete amphora wines from Alentejo by ICPMS. Food Control 2018, 92, 80–85. [Google Scholar] [CrossRef]
- Frankowski, M.; Zioła-Frankowska, A. Determination of metals and metalloids in wine using inductively coupled plasma optical emission spctrometry and mini-torch, ICP-OES. Food Anal. Methods 2017, 10, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Paz, S.; Jaudenes, J.R.; Gutièrrez, A.J.; Rubio, C.; Hardisson, A.; Revert, C. Determination of Fluoride in Organic and Non-organic Wines. Biol. Trace Elem. Res. 2017, 178, 153–159. [Google Scholar] [CrossRef]
- Gomez, M.R.; de la Torre, A.H.; Ojeda, A.B.; Marante, R.A.; Diaz-Flores, L. Fluoride levels in wines of the Canary Islands (Spain). Eur. Food Res. Technol. 2003, 216, 145–149. [Google Scholar] [CrossRef]
- Hopfer, H.; Nelson, J.; Collins, T.; Heymann, H.; Ebeler, S. The combined impact of vineyard origin and processing winery on the elemental profile of red wines. Food Chem. 2015, 172, 486–496. [Google Scholar] [CrossRef]
- Gomez, M.; Brandt, R.; Jakubowski, N.; Andersson, J. Differences in the distribution of aluminum forms may result from the variety of wine varieties and the proportion in which they have been mixed. J. Agric. Food Chem. 2004, 52, 2953–2961. [Google Scholar]
- Marisa, C.; Almeida, R.; Teresa, M.; Vasconelos, S.D. Multielemental composition of wines and their precursors including provenance soil and their potentialities as fingerprints of wine origin. J. Agric. Food Chem. 2003, 51, 4788–4798. [Google Scholar]
- Rossano, E.; Szilagyi, Z.; Malorni, A.; Pocsfalvi, G. Influence of winemaking practices on the concentration of rare earth elements in white wines studied by Inductively Coupled Plasma Mass Spectrometry. J. Agric. Food Chem. 2007, 55, 311–317. [Google Scholar] [CrossRef]
- Lopez-Lopez, J.; Albendin, G.; Arufe, M.; Manuel-Vez, M. Simplification of iron speciation in wine samples: A spectrophotometric approach. J. Agric. Food Chem. 2015, 63, 4545–4550. [Google Scholar] [CrossRef]
- Neto, J.; Porto, I.; Schneider, M.; Santos, A.; Gomes, A.; Fereira, S. Speciation analysis based on digital image colorimetry: Iron (II/III) in white wine. Talanta 2019, 194, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, C.; Nelson, J.; Ebeler, S. Current Perspective on Arsenic in wines: Analysis, speciation and changes in composition during production. J. Agric. Food Chem. 2019, 67, 4154–4159. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Programme on Chemical Safety. Environmental Health Criteria 194. 1997. Available online: www.inchem.org/documents/ehc/ehc/ehc194.htm (accessed on 3 October 2019).
- World Health Organization. Global Status Report on Alcohol and Health 2014, 2014 ed.; WHO: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/bitstream/handle/10665/112736/9789240692763_eng.pdf;jsessionid=7208B9C2A2F61CE1AC4A9823C2903A07?sequence=1 (accessed on 27 September 2019).
- GUS: Polacy Piją Coraz Więcej. Spożycie Mocnego Alkoholu w Polsce Znacząco Wzrosło. Available online: https://buisnessinsider.com.pl/wiadomosci/spozycie-alkoholu-w-polsce-dane-gus/zkndw0z (accessed on 3 October 2019).
- JAKOŚĆ GLEB. Available online: http://www.krakow.pios.gov.pl/Press/publikacje/raporty/raport16/6_gleby.pdf (accessed on 3 October 2019).
- Frankowski, M. Simultaneous determination of inorganic and organic ions in plant parts Betula pentula from two different types of ecosystems (Wielkopolski National park and Chemical Plant in Luboń, Poland). ESPR 2016, 23, 11046–11057. [Google Scholar] [PubMed] [Green Version]
- Schecher, W. Thermochemical data used in Mineql+ version 4.5. Environ. Res. Softw. 2001, 1, 1–217. [Google Scholar]
- Płotka-Wasylka, J.; Frankowski, M.; Simeonov, V.; Polkowska, Ż.; Namieśnik, J. Determination of Metals Content in Wine Samples by Inductively Coupled Plasma-Mass Spectrometry. Molecules 2018, 23, 2886. [Google Scholar] [CrossRef] [Green Version]
- Greenough, J.; Mallory-Greenough, L.; Fryer, B. Geology and Wine 9: Regional trace element fingerprinting of Canadian wines. Geosci. Can. 2005, 32, 129–137. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaś, K.; Zioła-Frankowska, A.; Frankowski, M. Chemical Speciation of Aluminum in Wine by LC–ICP–MS. Molecules 2020, 25, 1069. https://doi.org/10.3390/molecules25051069
Karaś K, Zioła-Frankowska A, Frankowski M. Chemical Speciation of Aluminum in Wine by LC–ICP–MS. Molecules. 2020; 25(5):1069. https://doi.org/10.3390/molecules25051069
Chicago/Turabian StyleKaraś, Katarzyna, Anetta Zioła-Frankowska, and Marcin Frankowski. 2020. "Chemical Speciation of Aluminum in Wine by LC–ICP–MS" Molecules 25, no. 5: 1069. https://doi.org/10.3390/molecules25051069





