Regioselective Monobromination of Phenols with KBr and ZnAl–BrO3−–Layered Double Hydroxides
Abstract
:1. Introduction
2. Results
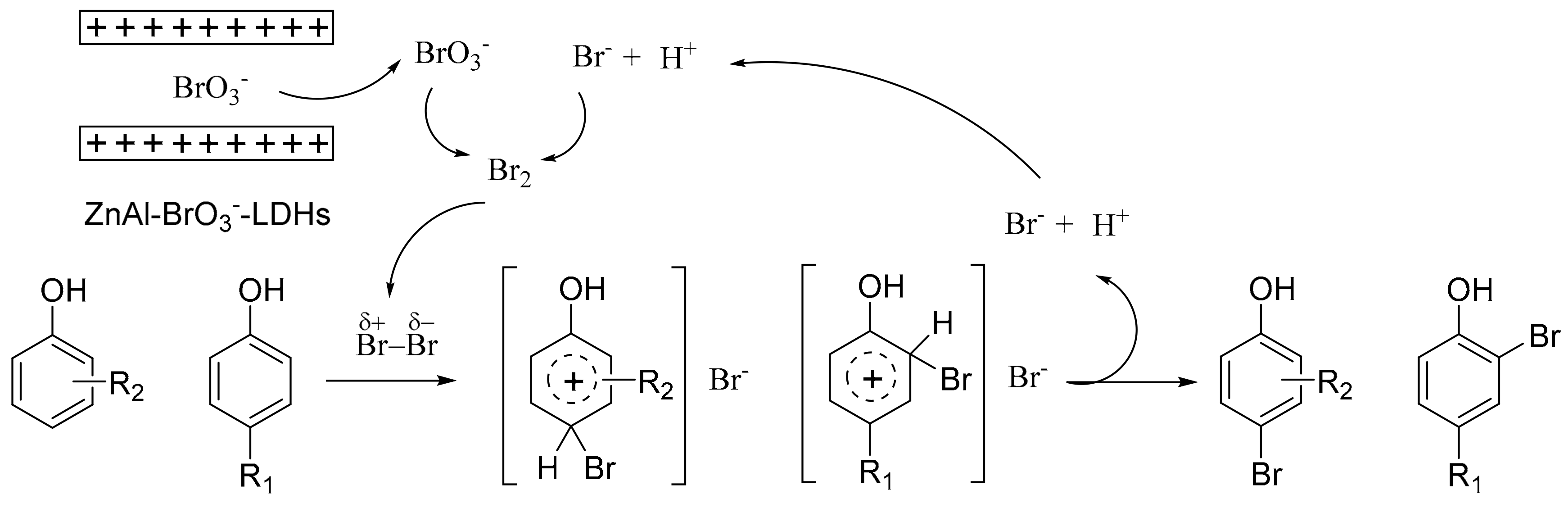
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. General Procedure for the Bromination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, F.; Zhou, Q.; Liu, D.; Fang, T.; Shi, Y.; Du, Y.; Chen, G. Dimerization of aromatic aompounds using Palladium-carbon-catalyzed Suzuki-Miyaura cross-coupling by one-pot synthesis. Synlett 2018, 29, 779–784. [Google Scholar] [CrossRef] [Green Version]
- Harada, K.; Arioka, C.; Miyakita, A.; Kubo, M.; Fukuyama, Y. Efficient synthesis of neurotrophic honokiol using Suzuki-Miyaura reactions. Tetrahedron Lett. 2014, 55, 6001–6003. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, B.; Riemer, M. Suzuki-Miyaura coupling of halophenols and phenol boronic acids: Systematic investigation of positional isomer effects and conclusions for the synthesis of phytoalexins from pyrinae. J. Org. Chem. 2014, 79, 4104–4118. [Google Scholar] [CrossRef] [PubMed]
- Schmoger, C.; Szuppa, T.; Tied, A.; Schneider, F.; Stolle, A.; Ondruschka, B. Pd on porous glass: A versatile and easily recyclable catalyst for Suzuki and Heck reactions. Chemsuschem 2008, 1, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lumb, J.-P. Phenol-directed C-H functionalization. Acs. Catal. 2019, 9, 521–555. [Google Scholar] [CrossRef]
- Jesus, A.; Correia-da-Silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and potential biological applications of haloaryl secondary metabolites from macroalgae. Mar. Drugs 2019, 17. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [Green Version]
- Oztaskin, N.; Cetinkaya, Y.; Taslimi, P.; Goksu, S.; Gulcin, I. Antioxidant and acetylcholinesterase inhibition properties of novel bromophenol derivatives. Bioorg. Chem. 2015, 60, 49–57. [Google Scholar] [CrossRef]
- Ghorpade, P.V.; Pethsangave, D.A.; Some, S.; Shankarling, G.S. Graphene oxide promoted oxidative bromination of anilines and phenols in water. J. Org. Chem. 2018, 83, 7388–7397. [Google Scholar] [CrossRef]
- Jiang, P.-P.; Yang, X.-J. A quick, mild and efficient bromination using a CFBSA/KBr system. Rsc Adv. 2016, 6, 90031–90034. [Google Scholar] [CrossRef]
- Su, P.; Fan, C.; Yu, H.; Wang, W.; Jia, X.; Rao, Q.; Fu, C.; Zhang, D.; Huang, B.; Pan, C.; et al. Synthesis of Ti-Al binary oxides and their catalytic application for C-H halogenation of phenols, aldehydes and ketones. Mol. Catal. 2019, 475. [Google Scholar] [CrossRef]
- Sim, J.; Jo, H.; Viji, M.; Choi, M.; Jung, J.-A.; Lee, H.; Jung, J.-K. Rapid, Operationally simple, and metal-free NBS mediated one-pot synthesis of 1,2-Naphthoquinone from 2-naphthol. Adv. Synth. Catal. 2018, 360, 852–858. [Google Scholar] [CrossRef]
- Sengupta, G.; Pandey, P.; De, S.; Ramapanicker, R.; Bera, J.K. A bromo-capped diruthenium(I, I) N-heterocyclic carbene compound for in situ bromine generation with NBS: catalytic olefin aziridination reactions. Dalton T. 2018, 47, 11917–11924. [Google Scholar] [CrossRef] [PubMed]
- Anjaiah, B.; Prameela, K.; Srinivas, P.; Rajanna, K.C. Synthesis, kinetics, and mechanism of bromophenols by N-bromophthalimide in aqueous acetic acid. Int. J. Chem. Kinet. 2018, 50, 804–812. [Google Scholar] [CrossRef]
- Jereb, M.; Gosak, K. Acid-promoted direct electrophilic trifluoromethylthiolation of phenols. Org. Biomol. Chem. 2015, 13, 3103–3115. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, K. Development of Highly selective organic transformation reactions using halogen lewis acids. J. Syn. Org. Chem. Jpn. 2014, 72, 137–148. [Google Scholar] [CrossRef]
- Andersh, B.; Murphy, D.L.; Olson, R.J. Hydrochloric acid catalysis of N-bromosuccinimide (NBS) mediated nuclear aromatic brominations in acetone. Synth. Commun. 2000, 30, 2091–2098. [Google Scholar] [CrossRef]
- Oberhauser, T. A new bromination method for phenols and anisoles: NBS/HBF4 center dot Et2O in CH3CN. J. Org. Chem. 1997, 62, 4504–4506. [Google Scholar] [CrossRef]
- Zysman-Colman, E.; Arias, K.; Siegel, J.S. Synthesis of arylbromides from arenes and N-bromosuccinimide (NBS) in acetonitrile - A convenient method for aromatic bromination. Can. J. Chem. 2009, 87, 440–447. [Google Scholar] [CrossRef]
- Khazaei, A.; Rostami, A.; Raiatzadeh, A. N-bromosuccinimide (NBS): a mild and efficient catalyst for tetrahydropyranylation of alcohols and Phenols under solvent-free conditions. J. Chin. Chem. Soc. 2007, 54, 1029–1032. [Google Scholar] [CrossRef]
- Semwal, R.; Ravi, C.; Kumar, R.; Meena, R.; Adimurthy, S. Sodium salts (NaI/NaBr/NaCl) for the halogenation of imidazo-fused heterocycles. J. Org. Chem. 2019, 84, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Sun, X.; Li, X.; Yuan, Y.; Jiao, N. Efficient and practical oxidative bromination and iodination of arenes and heteroarenes with DMSO and hydrogen halide: a mild protocol for late-Stage functionalization. Org. Lett. 2015, 17, 2886–2889. [Google Scholar] [CrossRef] [PubMed]
- Karki, M.; Magolan, J. Bromination of olefins with HBr and DMSO. J. Org. Chem. 2015, 80, 3701–3707. [Google Scholar] [CrossRef] [PubMed]
- Barhate, N.B.; Gajare, A.S.; Wakharkar, R.D.; Bedekar, A.V. Simple and practical halogenation of arenes, alkenes and alkynes with hydrohalic acid/H2O2 (or TBHP). Tetrahedron 1999, 55, 11127–11142. [Google Scholar] [CrossRef]
- Bora, U.; Bose, G.; Chaudhuri, M.K.; Dhar, S.S.; Gopinath, R.; Khan, A.T.; Patel, B.K. Regioselective bromination of organic substrates by tetrabutylammonium bromide promoted by V2O5-H2O2: An environmentally favorable synthetic protocol. Org. Lett. 2000, 2, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.; Gayen, K.S.; Khamarui, S.; Chatterjee, N.; Maiti, D.K. Addition of halide to π-bond directly from aqueous NaX solution: A general strategy for installation of two different functional groups. Chem. Commun. 2011, 47, 6933–6935. [Google Scholar] [CrossRef]
- Truchan, N.; Jandl, C.; Pothig, A.; Breitenlechner, S.; Bach, T. Access to biphenyls by Palladium-catalyzed oxidative coupling of phenyl carbamates and phenols. Synthesis 2019, 51, 3060–3076. [Google Scholar] [CrossRef]
- Khatun, R.; Biswas, S.; Ghosh, S.; Islam, S.M. Polymer-anchored Fe(III)Azo complex: An efficient reusable catalyst for oxidative bromination and multi-components reaction for the synthesis of spiropiperidine derivatives. J. Organomet. Chem. 2018, 858, 37–46. [Google Scholar] [CrossRef]
- Song, S.; Huang, X.; Liang, Y.-F.; Tang, C.; Li, X.; Jiao, N. From simple organobromides or olefins to highly value-added bromohydrins: a versatile performance of dimethyl sulfoxide. Green Chem. 2015, 17, 2727–2731. [Google Scholar] [CrossRef]
- Huang, Z.; Li, F.; Chen, B.; Lu, T.; Yuan, Y.; Yuan, G. A sustainable process for catalytic oxidative bromination with molecular oxygen. Chemsuschem 2013, 6, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; Rohitha, C.N.; Kulkarni, S.J.; Narender, N. Bromination of aromatic aompounds using ammonium bromide and oxone (R). Synthesis 2010, 1629–1632. [Google Scholar] [CrossRef]
- Adimurthy, S.; Ramachandraiah, G.; Bedekar, A.V.; Ghosh, S.; Ranu, B.C.; Ghosh, P.K. Eco-friendly and versatile brominating reagent prepared from a liquid bromine precursor. Green Chem. 2006, 8, 916–922. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Khodykin, S.V.; Krylov, I.B.; Ogibin, Y.N.; Nikishin, G.I. A convenient synthesis of 2,2-dibromo-1-arylethanones by bromination of 1-arylethanones with the H2O2-HBr system. Synthesis 2006, 1087–1092. [Google Scholar] [CrossRef]
- Hemati, R.; Shahvelayati, A.S.; Yadollahzadeh, K. o-Xylylene Bis(Triethyl Ammonium Tribromide) as a mild and ecyclable reagent for rapid and regioselective bromination of anilines and phenols. Lett. Org. Chem. 2018, 15, 682–687. [Google Scholar] [CrossRef]
- Bovonsombat, P.; Teecomegaet, P.; Kulvaranon, P.; Pandey, A.; Chobtumskul, K.; Tungsirisurp, S.; Sophanpanichkul, P.; Losuwanakul, S.; Soimaneewan, D.; Kanjanwongpaisan, P.; et al. Regioselective monobromination of aromatics via a halogen bond acceptor-donor interaction of catalytic thioamide and N -bromosuccinimide. Tetrahedron 2017, 73, 6564–6572. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Jiang, M.; Wang, M.; Tang, L.; Wei, M.; Zhou, Q. Mild and regioselective bromination of phenols with TMSBr. Eur. J. Org. Chem. 2019, 2019, 4593–4596. [Google Scholar] [CrossRef]
- Satkar, Y.; Ramadoss, V.; Nahide, P.D.; García-Medina, E.; Juárez-Ornelas, K.A.; Alonso-Castro, A.J.; Chávez-Rivera, R.; Jiménez-Halla, J.O.C.; Solorio-Alvarado, C.R. Practical, mild and efficient electrophilic bromination of phenols by a new I(iii)-based reagent: the PIDA–AlBr3 system. RSC Adv. 2018, 8, 17806–17812. [Google Scholar] [CrossRef] [Green Version]
- Georgiev, D.; Saes, B.W.H.; Johnston, H.J.; Boys, S.K.; Healy, A.; Hulme, A.N. Selective and efficient generation of ortho-brominated para-substituted phenols in ACS-grade methanol. Molecules 2016, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Bovonsombat, P.; Ali, R.; Khan, C.; Leykajarakul, J.; Pla-on, K.; Aphimanchindakul, S.; Pungcharoenpong, N.; Timsuea, N.; Arunrat, A.; Punpongjareorn, N. Facile p-toluenesulfonic acid-promoted para-selective monobromination and chlorination of phenol and analogues. Tetrahedron 2010, 66, 6928–6935. [Google Scholar] [CrossRef]
- Alinezhad, H.; Tavakkoli, S.M.; Salehian, F. Efficient, rapid, and regioselective bromination of phenols and anilines with N-bromosaccharin using tungstophosphoric acid as a heterogeneous recyclable catalyst. Synth. Commun. 2010, 40, 3226–3232. [Google Scholar] [CrossRef]
- Narender, N.; Naresh, M.; Arun Kumar, M.; Mahender Reddy, M.; Swamy, P.; Nanubolu, J. Fast and efficient bromination of aromatic compounds with ammonium bromide and oxone. Synthesis 2013, 45, 1497–1504. [Google Scholar] [CrossRef] [Green Version]
- Mokhtary, M.; Lakouraj, M.M. Polyvinylpolypyrrolidone–bromine complex: Mild and efficient polymeric reagent for bromination of activated aromatic compounds. Chinese Chem. Lett. 2011, 22, 13–17. [Google Scholar] [CrossRef]
- Borikar, S.P.; Daniel, T.; Paul, V. Mild, Efficient, and regioselective monobromination of arylamines and phenols using [BBIm]Br3 as a new reagent. Synth. Commun. 2010, 40, 647–653. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Tajbakhsh, M.; Mohadjerani, M.; Lasemi, Z. Efficient and regioselective Bromination of aromatic compounds with ethylenebis(N-methylimidazolium) ditribromide (EBMIDTB). Synth. Commun. 2010, 40, 868–876. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Zargarani, D. Regioselective bromination of aromatic amines and phenols using N-benzyl-DABCO tribromide. Synth. Commun. 2009, 39, 4212–4220. [Google Scholar] [CrossRef]
- Borikar, S.P.; Daniel, T.; Paul, V. An efficient, rapid, and regioselective bromination of anilines and phenols with 1-butyl-3-methylpyridinium tribromide as a new reagent/solvent under mild conditions. Tetrahedron Lett. 2009, 50, 1007–1009. [Google Scholar] [CrossRef]
- Stropnik, T.; Bombek, S.; Kočevar, M.; Polanc, S. Regioselective bromination of activated aromatic substrates with a ZrBr4/diazene mixture. Tetrahedron Lett. 2008, 49, 1729–1733. [Google Scholar] [CrossRef]
- Suresh, P.; Annalakshmi, S.; Pitchumani, K. Regioselective monobromination of substituted phenols in the presence of β-cyclodextrin. Tetrahedron 2007, 63, 4959–4967. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Jalili, H. Mild and regioselective bromination of aromatic compounds with N,N,N′,N′-tetrabromobenzene-1,3-disulfonylamide and poly(N-bromobenzene-1,3-disulfonylamide). Synthesis 2005, 1099–1102. [Google Scholar] [CrossRef]
- Singh, P.P.; Thatikonda, T.; Kumar, K.A.A.; Sawant, S.D.; Singh, B.; Sharma, A.K.; Sharma, P.R.; Singh, D.; Vishwakarma, R.A. Cu-Mn Spinel oxide catalyzed regioselective halogenation of phenols and N-heteroarenes. J. Org. Chem. 2012, 77, 5823–5828. [Google Scholar] [CrossRef] [PubMed]
- Wischang, D.; Hartung, J. Bromination of phenols in bromoperoxidase-catalyzed oxidations. Tetrahedron 2012, 68, 9456–9463. [Google Scholar] [CrossRef]
- Chen, A.-J.; Wong, S.-T.; Hwang, C.-C.; Mou, C.-Y. Highly efficient and regioselective halogenation over well dispersed Rhenium-promoted mesoporous zirconia. ACS Catal. 2011, 1, 786–793. [Google Scholar] [CrossRef]
- Baharfar, R.; Alinezhad, H.; Azimi, S.; Salehian, F. Regioselective and high-yielding bromination of phenols and anilins using N-bromosaccharin and amberlyst-15. J. Chil. Chem. Soc. 2011, 56, 863–865. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.-J.; Chen, X.-R.; Mou, C.-Y. Highly Regioselective oxybromination in an aqueous system using SBA-15 supported sulfated zirconia catalyst. J. Chin. Chem. Soc. 2010, 57, 820–828. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Li, J.-H. An efficient copper-catalysed aerobic oxybromination of arenes in water. Green Chem. 2010, 12, 2124–2126. [Google Scholar] [CrossRef]
- Bhatt, S.; Nayak, S.K. Copper(II) Bromide: A simple and selective monobromination reagent for electron-rich aromatic compounds. Synth. Commun. 2007, 37, 1381–1388. [Google Scholar] [CrossRef]
- Chhattise, P.K.; Ramaswamy, A.V.; Waghmode, S.B. Regioselective, photochemical bromination of aromatic compounds using N-bromosuccinimide. Tetrahedron Lett. 2008, 49, 189–194. [Google Scholar] [CrossRef]
- Das, B.; Venkateswarlu, K.; Majhi, A.; Siddaiah, V.; Reddy, K.R. A facile nuclear bromination of phenols and anilines using NBS in the presence of ammonium acetate as a catalyst. J. Mol. Catal. A- Chem. 2007, 267, 30–33. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Bromide-assisted oxidation of substituted phenols with hydrogen peroxide to the corresponding p-quinol and p-quinol ethers over WO42--exchanged layered double hydroxides. Angew. Chem. Int. Edit. 2005, 44, 310–313. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, Z.; Jiang, B.; Sun, Y.; Chen, Z.; Gao, X.; Yang, N. Ni-Al layered double hydroxides (LDHs) coated superhydrophobic mesh with flower-like hierarchical structure for oil/water separation. Appl. Surf. Sci. 2019, 490, 145–156. [Google Scholar] [CrossRef]
- Wang, B.; Shang, J.; Guo, C.; Zhang, J.; Zhu, F.; Han, A.; Liu, J. A general method to ultrathin bimetal-MOF nanosheets arrays via in situ transformation of layered double hydroxides arrays. Small 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Na, W.; Kim, J.; Lee, S.; Jang, J. Improved electrochemical performances of MOF-derived Ni-Co layered double hydroxide complexes using distinctive hollow-in-hollow structures. J. Mater. Chem. A 2019, 7, 17637–17647. [Google Scholar] [CrossRef]
- Guo, D.; Song, X.; Tan, L.; Ma, H.; Sun, W.; Pang, H.; Zhang, L.; Wang, X. A facile dissolved and reassembled strategy towards sandwich-like rGO@NiCoAl-LDHs with excellent supercapacitor performance. Chem. Eng. J. 2019, 356, 955–963. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Huang, R.; Zhou, Y.; Wu, Y.; Hu, Y.; Ostrikov, K. Ni-Co hydroxide nanosheets on plasma-reduced Co-based metal-organic nanocages for electrocatalytic water oxidation. J. Mater. Chem. A 2019, 7, 4950–4959. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, F.; Jiang, C.; Ni, Z. New reagent system for bromination built with Zn/Al-BrO3-- -LDHs as carrier. J. Chin. Cera. Soc. 2015, 43, 672–677. [Google Scholar]
- Wang, L.; Yu, Q.; Feng, C.; Zhang, Y.; Hu, J. Efficient synthesis of dibromoalkanes and iodoacetates from olefins using ZnAl-XO3(-)-LDHs/LiX (X= Br, I) as halogen sources. Chin. J. Org. Chem. 2019, 39, 1787–1793. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Yu, Q.; Feng, C.; Hu, J. Green synthesis of haloformates from olefins using formic acid as reactant, protonic acid, and solvent. Synlett 2018, 29, 1611–1616. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chen, L.; Zhang, H.; Yu, Q. Selective oxidative bromination of anilines using potassium bromide and ZnAl-BrO3--LDHs. Chin. J. Org. Chem. 2017, 37, 3186–3190. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


 | |||||
|---|---|---|---|---|---|
| Entry | Oxidant (equiv.) | Bromide (equiv.) | Solvent 2 | Temperature | Yield 3 |
| 1 | ZnAl-BrO3--LDHs (0.2) | LiBr (1.4) | AcOH | 25 °C | 71 |
| 2 | ZnAl-BrO3--LDHs (0.2) | LiBr (1.2) | AcOH | 25 °C | 73 |
| 3 | ZnAl-BrO3--LDHs (0.2) | LiBr (1.0) | AcOH | 25 °C | 76 |
| 4 | ZnAl-BrO3--LDHs (0.2) | LiBr (0.8) | AcOH | 25 °C | 68 |
| 5 | ZnAl-BrO3--LDHs (0.2) | NaBr (1.0) | AcOH | 25 °C | 80 |
| 6 | ZnAl-BrO3--LDHs (0.2) | KBr (1.0) | AcOH | 25 °C | 83 |
| 7 | ZnAl-BrO3--LDHs (0.2) | ZnBr2 (0.5) | AcOH | 25 °C | 73 |
| 8 | ZnAl-BrO3--LDHs (0.2) | KBr (1.0) | MeOH | 25 °C | - |
| 9 | ZnAl-BrO3--LDHs (0.2) | KBr (1.0) | EtOH | 25 °C | - |
| 10 | ZnAl-BrO3--LDHs (0.2) | KBr (1.0) | DCM | 25 °C | - |
| 11 | ZnAl-BrO3--LDHs (0.2) | KBr (1.0) | EA | 25 °C | - |
| 12 | ZnAl-BrO3--LDHs (0.2) | KBr (1.0) | AcOH/H2O | 25 °C | 86 |
| 13 | ZnAl-BrO3--LDHs (0.2) | KBr (1.0) | AcOH/H2O | 30 °C | 85 |
| 14 | ZnAl-BrO3--LDHs (0.2) | KBr (1.0) | AcOH/H2O | 35 °C | 91 |
| 15 | ZnAl-BrO3--LDHs (0.2) | KBr (1.0) | AcOH/H2O | 40 °C | 81 |
| 16 | ZnAl-BrO3--LDHs (0.2) | KBr (1.0) | AcOH/H2O | 45 °C | 77 |
| 17 | ZnAl-BrO3--LDHs (0.4) | KBr (2.0) | AcOH/H2O | 35°C | 76 (17) |
| 18 | KBrO3 (0.4) | KBr (2.0) | AcOH/H2O | 35 °C | 22 (61) |
| 19 | KBrO3 (0.2) | KBr (1.0) | AcOH/H2O | 35 °C | 35 (28) |
| 20 | - | KBr (1.0) | AcOH/H2O | 35 °C | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Feng, C.; Zhang, Y.; Hu, J. Regioselective Monobromination of Phenols with KBr and ZnAl–BrO3−–Layered Double Hydroxides. Molecules 2020, 25, 914. https://doi.org/10.3390/molecules25040914
Wang L, Feng C, Zhang Y, Hu J. Regioselective Monobromination of Phenols with KBr and ZnAl–BrO3−–Layered Double Hydroxides. Molecules. 2020; 25(4):914. https://doi.org/10.3390/molecules25040914
Chicago/Turabian StyleWang, Ligeng, Chun Feng, Yan Zhang, and Jun Hu. 2020. "Regioselective Monobromination of Phenols with KBr and ZnAl–BrO3−–Layered Double Hydroxides" Molecules 25, no. 4: 914. https://doi.org/10.3390/molecules25040914






