Characterization of the Volatiles and Quality of Hybrid Grouper and Their Relationship to Changes of Microbial Community During Storage at 4 °C
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Analysis
2.2. Free Amino Acid Analysis
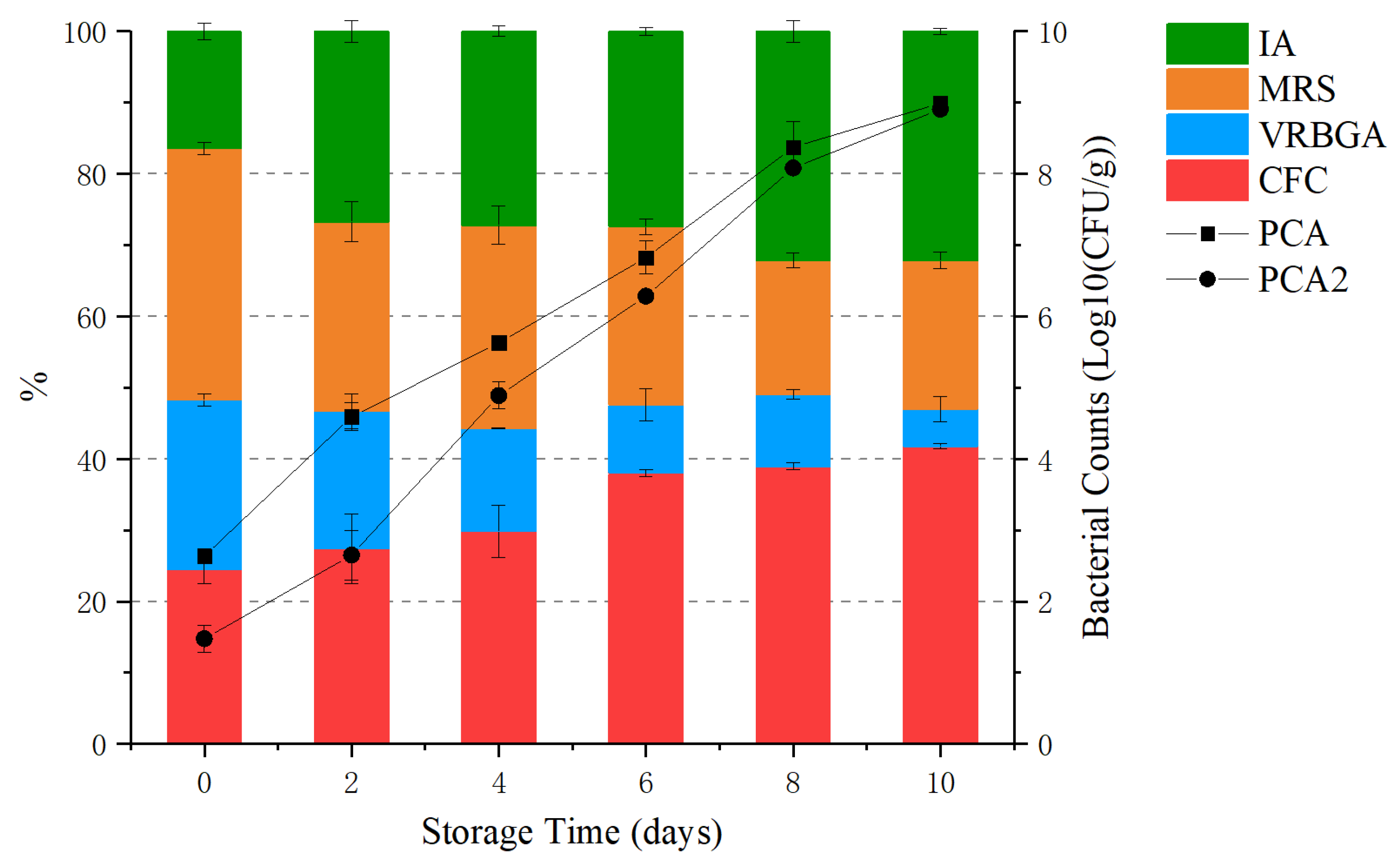
2.3. Total Viable Count and Bacterial Distribution
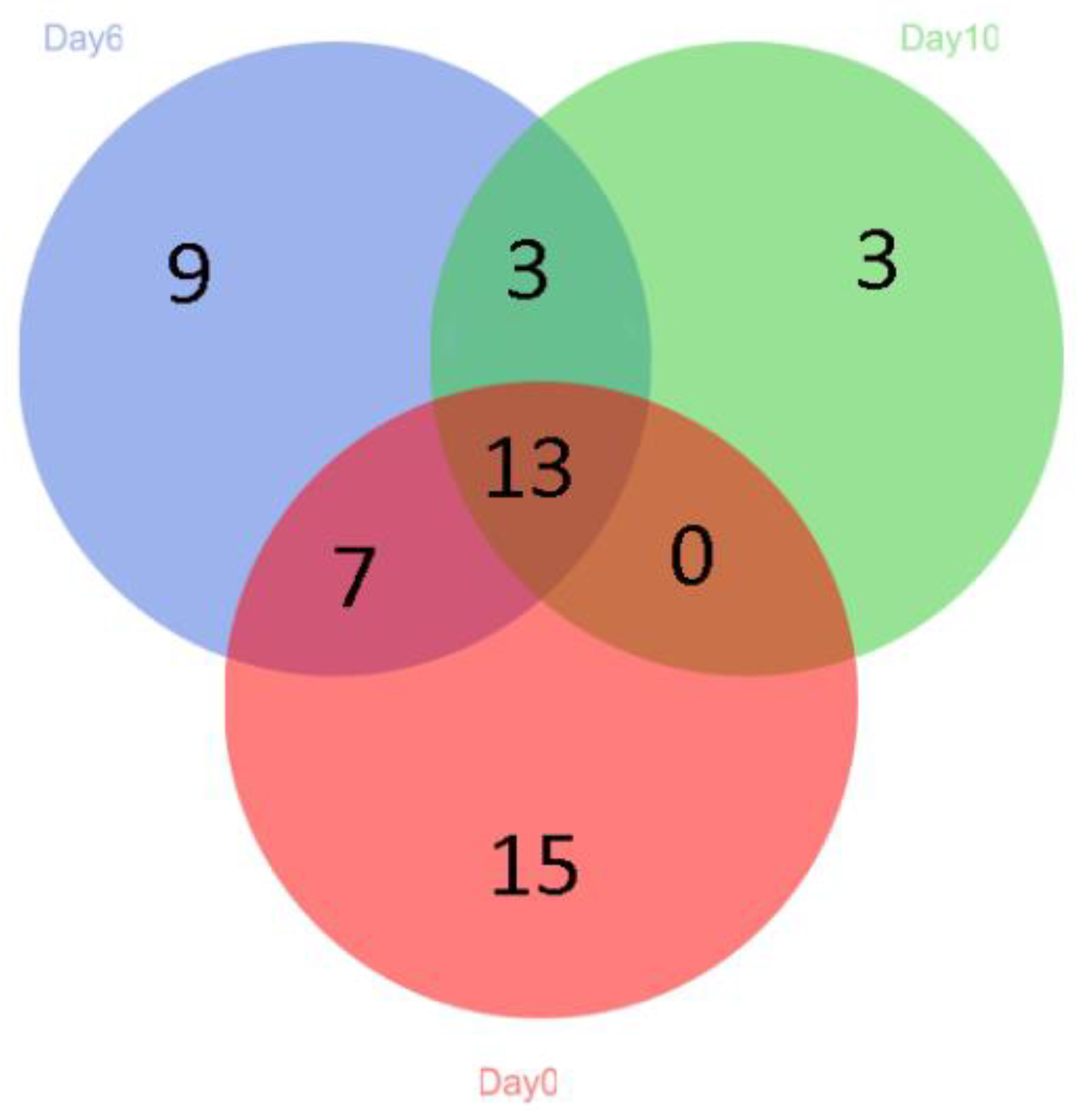
2.4. Diversity of Microbial Community
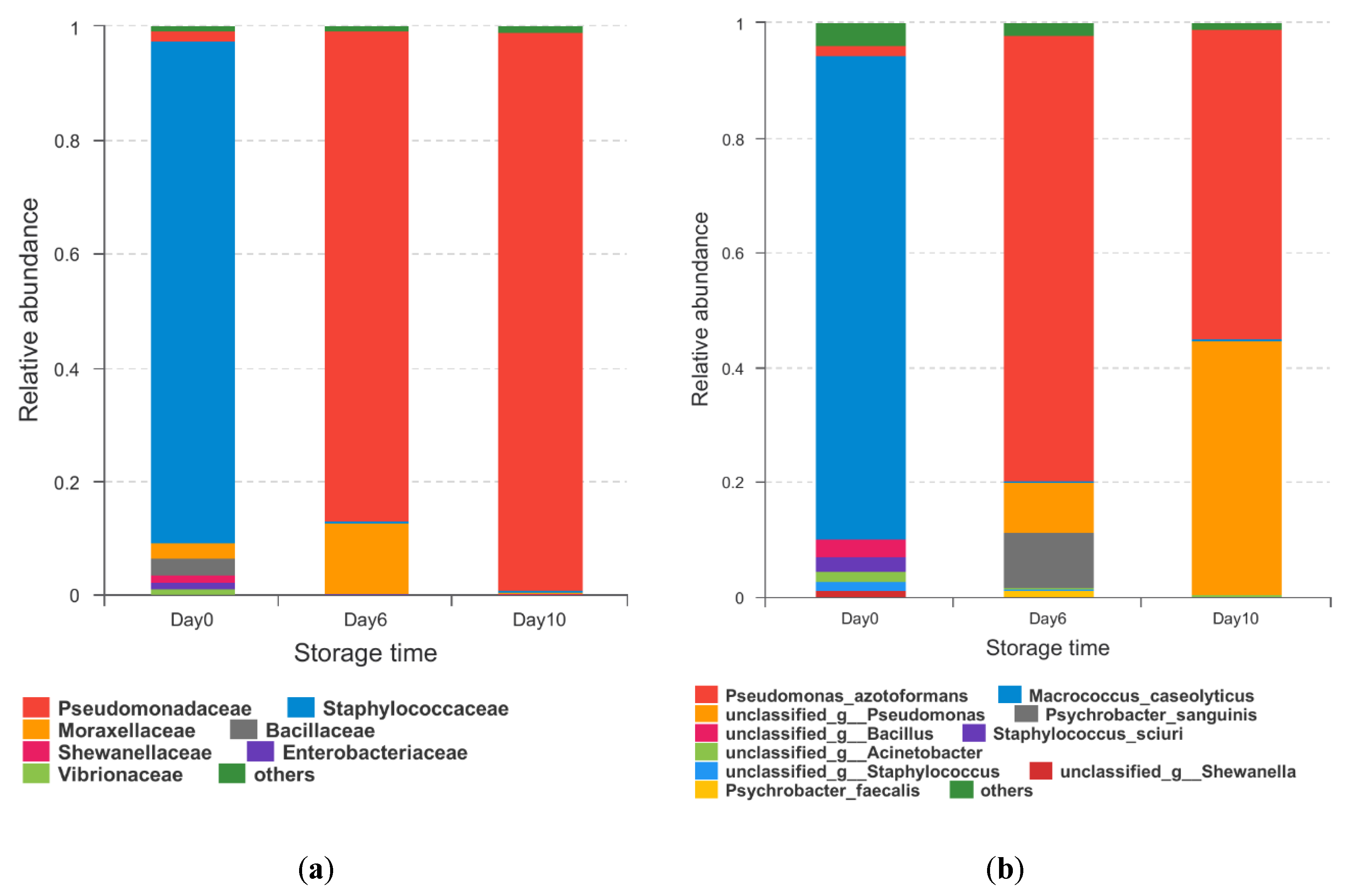
2.5. Bacterial Community Diversity and Succession in Grouper Fillets at Family and Genus Levels
2.6. Microbiological Analysis and Amplicon Sequencing
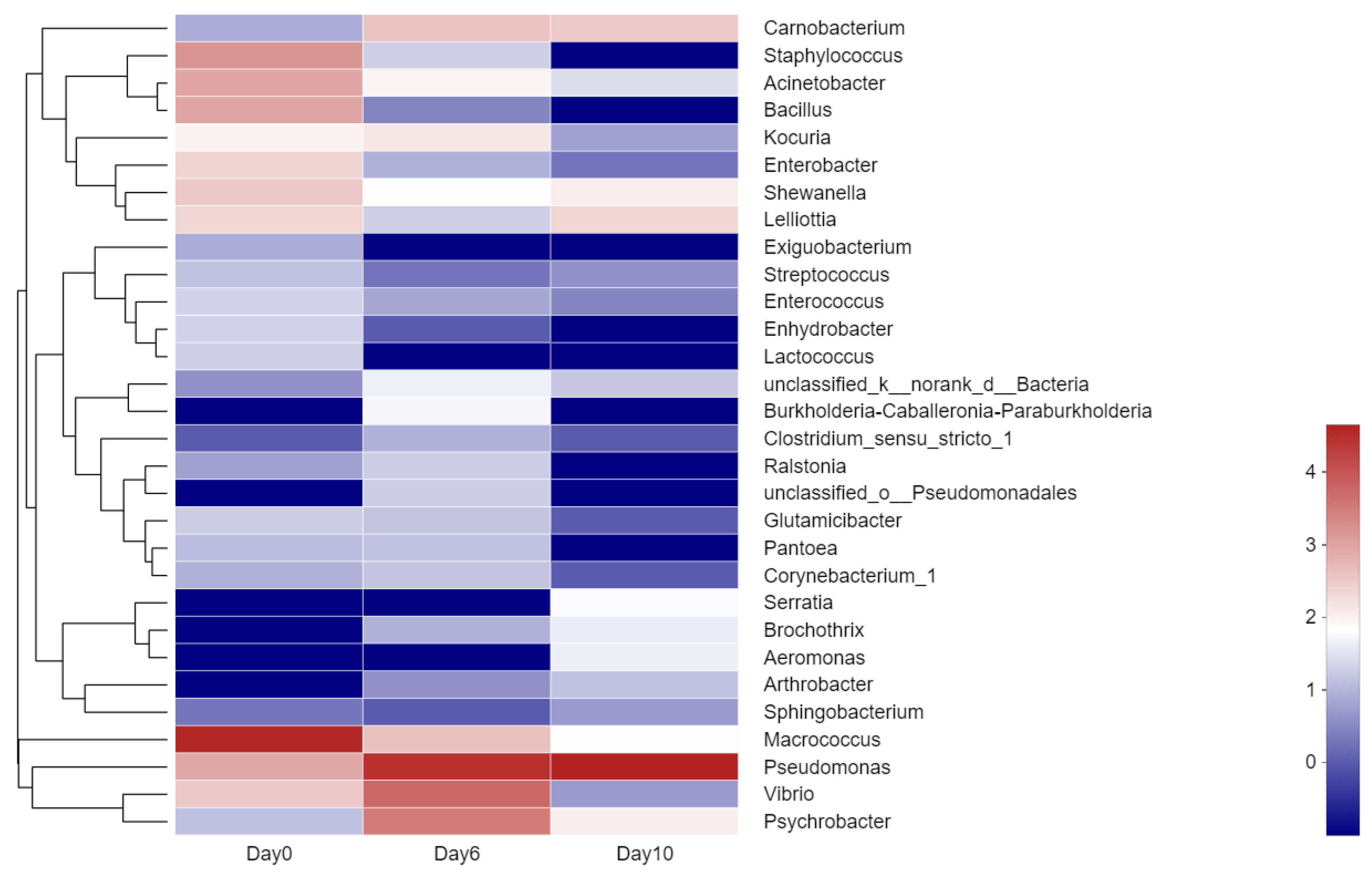
2.7. Heatmap Analysis
2.8. VOCs Analysis on Grouper Fillets
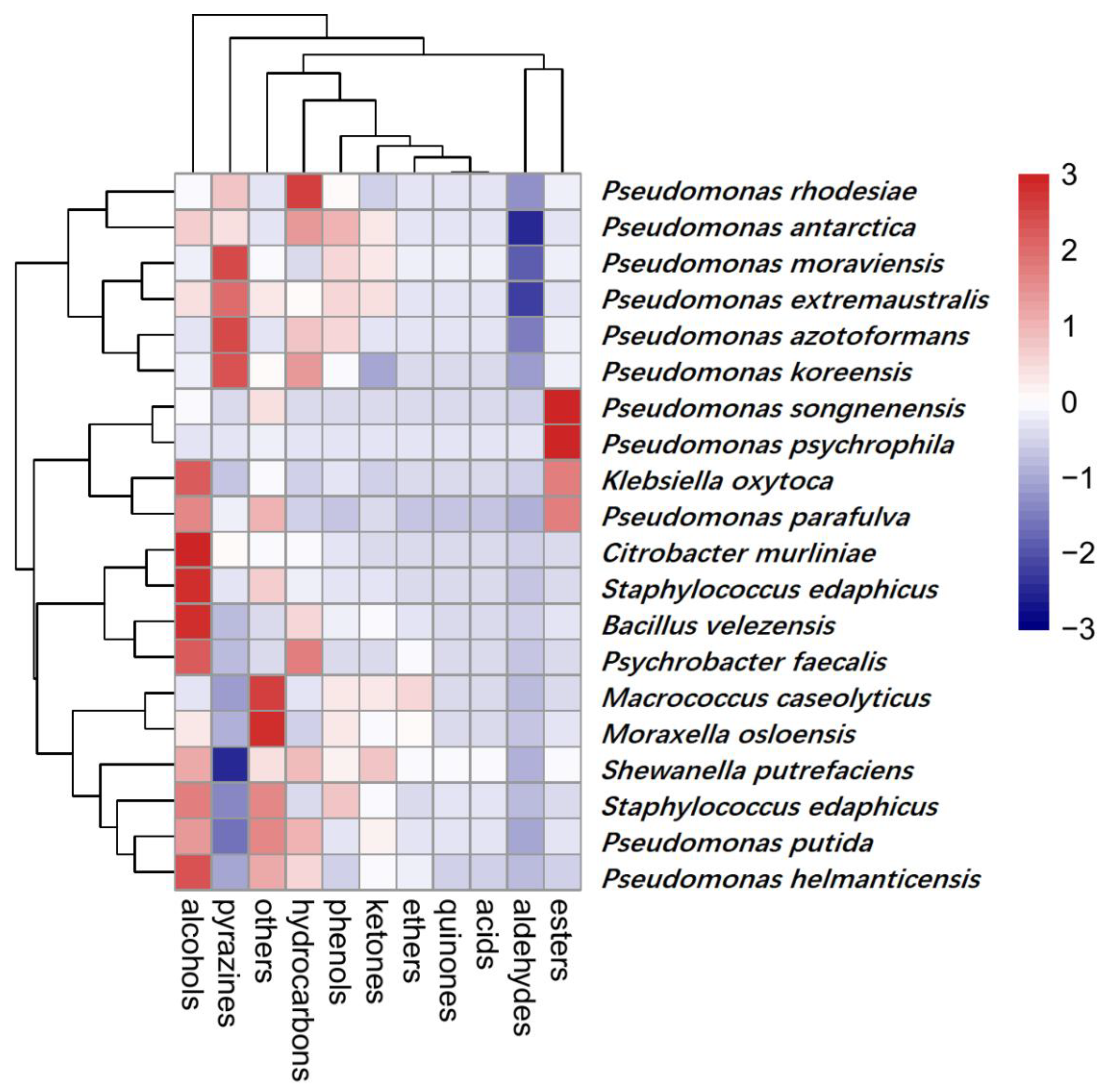
2.9. VOCs Analysis of Bacteria
3. Materials and Methods
3.1. Grouper Fillet Provision and Storage
3.2. Physico-Chemical Analysis
3.3. FAAs Analysis
3.4. Determination of VOCs in Grouper Fillets
3.5. Microbial Diversity Analysis
3.6. Microbiological Analysis
3.6.1. Enumeration and Purification of Bacteria
3.6.2. Isolation and Identification of Spoilage Bacteria
3.6.3. HS-SPME-GC-MS analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oehlenschläger, J. Seafood quality assessment. Seaf. Process. Technol. Qual. Saf. 2014, 359–386. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Microbiological spoilage and investigation of volatile profile during storage of sea bream fillets under various conditions. Int. J. Food Microbiol. 2014, 189, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Olafsdóttir, G.; Martinsdóttir, E.; Oehlenschläger, J.; Dalgaard, P.; Jensen, B.; Undeland, I.; Mackie, I.M.; Henehan, G.; Nielsen, J.; Nilsen, H. Methods to evaluate fish freshness in research and industry. Trends Food Sci. Technol. 1997, 8, 258–265. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Volatile organic compounds of microbial and non-microbial origin produced on model fish substrate un-inoculated and inoculated with gilt-head sea bream spoilage bacteria. Lwt - Food Sci. Technol. 2017, 78, 54–62. [Google Scholar] [CrossRef]
- Edirisinghe, R.K.B.; Graffham, A.J.; Taylor, S.J. Characterisation of the volatiles of yellowfin tuna (Thunnus albacares) during storage by solid phase microextraction and GC–MS and their relationship to fish quality parameters. Int. J. Food Sci. Technol. 2007, 42, 1139–1147. [Google Scholar] [CrossRef]
- Marta, M.-K.; Yoon, Y.-J.; Ukuku, D.O.; Yuk, H.-G. Volatile chemical spoilage indexes of raw Atlantic salmon (Salmo salar) stored under aerobic condition in relation to microbiological and sensory shelf lives. Food Microbiol. 2016, 53, 182–191. [Google Scholar] [CrossRef]
- Duflos, G.; Coin, V.M.; Cornu, M.; Antinelli, J.F.; Malle, P. Determination of volatile compounds to characterize fish spoilage using headspace/mass spectrometry and solid-phase microextraction/gas chromatography/mass spectrometry. J. Sci. Food Agric. 2010, 86, 600–611. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef]
- Iglesias, J.; Medina, I.; Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Study of the volatile compounds useful for the characterisation of fresh and frozen-thawed cultured gilthead sea bream fish by solid-phase microextraction gas chromatography–mass spectrometry. Food Chem. 2009, 115, 1473–1478. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Seafood spoilage microbiota and associated volatile organic compounds at different storage temperatures and packaging conditions. Int. J. Food Microbiol. 2018, 280, S0168160517305603. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liang, L.; Xia, W.; Regenstein, J.M.; Zhou, P. Biochemical and physical changes of grass carp ( Ctenopharyngodon idella ) fillets stored at −3 and 0 °C. Food Chem. 2013, 140, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, Q.; Ma, T.; Bao, K.; Yu, X.; Duan, Z.; Shen, X.; Li, C. Effect of EGCG-gelatin biofilm on the quality and microbial composition of tilapia fillets during chilled storage. Food Chem. 2020, 305, 125454. [Google Scholar] [CrossRef] [PubMed]
- Chéret, R.; Delbarre-Ladrat, C.; Lamballerie-Anton, M.D.; Verrez-Bagnis, V. Calpain and cathepsin activities in post mortem fish and meat muscles. Food Chem. 2007, 101, 1474–1479. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Ng, V.K.; Mikš-Krajnik, M.; Yang, H. Effects of Fish Gelatin and Tea Polyphenol Coating on the Spoilage and Degradation of Myofibril in Fish Fillet During Cold Storage. Food Bioprocess. Technol. 2017, 10, 89–102. [Google Scholar] [CrossRef]
- Yilin, L.; Shaoyang, L.; David, C.; Shengshun, C.; Yifen, W.; Bell, L.N. Chemical treatments for reducing the yellow discoloration of channel catfish (Ictalurus punctatus) fillets. J. Food Sci. 2013, 78, S1609–S1613. [Google Scholar] [CrossRef]
- Jun-Hu, C.; Da-Wen, S.; Zhong, H.; Xin-An, Z. Texture and Structure Measurements and Analyses for Evaluation of Fish and Fillet Freshness Quality: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 52–61. [Google Scholar] [CrossRef]
- Haaland, H.; Njaa, L.R. Total volatile nitrogen — A quality criterion for fish silage? Aquaculture 1989, 79, 311–316. [Google Scholar] [CrossRef]
- Fan, W.; Chi, Y.; Zhang, S. The use of tea polyphenol dip to extend the shelf life of silver carp (Hypophthalmicthys molitrix) during storage in ice. Food Chem. 2008, 108, 148–153. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Liu, D.; Fu, M.; Xi, Y.; Guo, Y. The preservative effects of chitosan film incorporated with thinned young apple polyphenols on the quality of grass carp ( Ctenopharyngodon idellus ) fillets during cold storage: Correlation between the preservative effects and the active properties of the film. Food Packag. Shelf Life 2018, 17, 1–10. [Google Scholar] [CrossRef]
- Zeng, X.; Xia, W.; Fang, Y.; Jiang, Q. Changes of biogenic amines in Chinese low-salt fermented fish pieces (Suan yu) inoculated with mixed starter cultures. Int. J. Food Sci. Technol. 2013, 48, 685–692. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Murata, M.; Kawai, A. Changes in free amino acids and creatine contents in yellowtail (Seriola quinqueradiata) muscle during ice storage. J. Food Sci. 2010, 47, 1662–1666. [Google Scholar] [CrossRef]
- Ababouch, L.; Afilal, M.E.; Benabdeljelil, H.; Busta, F.F. Quantitative changes in bacteria, amino acids and biogenic amines in sardine (Sardina pilchardus) stored at ambient temperature (25–28 °C) and in ice. Int. J. Food Sci. Technol. 2010, 26, 297–306. [Google Scholar] [CrossRef]
- Shi, C.; Cui, J.; Na, Q.; Luo, Y.; Han, L.; Hang, W. Effect of ginger extract and vinegar on ATP metabolites, IMP-related enzyme activity, reducing sugars and phosphorylated sugars in silver carp during postslaughter storage. Int. J. Food Sci. Technol. 2017, 52, 413–423. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Changes in free amino acids during chilled storage of hake (Merluccius merluccius L.) in controlled atmospheres and their use as a quality control index. Eur. Food Res. Technol. 2001, 212, 302–307. [Google Scholar] [CrossRef]
- Castro-Giráldez, M.; Toldrá, F.; Fito, P. Low frequency dielectric measurements to assess post-mortem ageing of pork meat. Lwt-Food Sci. Technol. 2011, 44, 1465–1472. [Google Scholar] [CrossRef]
- Özden, Ö. Changes in amino acid and fatty acid composition during shelf-life of marinated fish. J. Sci. Food Agric. 2010, 85, 2015–2020. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Kormas, K.A.; Boziaris, I.S. Microbiological changes, shelf life and identification of initial and spoilage microbiota of sea bream fillets stored under various conditions using 16S rRNA gene analysis. Food Agric. 2015, 95, 2386–2394. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, X.; Jia, S.; Luo, Y. The effect of essential oils on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets during chilled storage. Food Control. 2017, 82, 316–324. [Google Scholar] [CrossRef]
- Yang, S.P.; Xie, J.; Cheng, Y.; Zhang, Z.; Zhao, Y.; Qian, Y.F. Response of Shewanella putrefaciens to low temperature regulated by membrane fluidity and fatty acid metabolism. Lwt-Food Sci Technol 2020, 117, 108638. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Tao, X.Y.; Zhang, H.; Rao, S.Q.; Gao, L.; Pan, Z.M.; Jiao, X.A. Isolation and characterization of virulent phages infecting Shewanella baltica and Shewanella putrefaciens, and their application for biopreservation of chilled channel catfish (Ictalurus punctatus). Int. J. Food Microbiol. 2019, 292, 107–117. [Google Scholar] [CrossRef]
- El-Barbary, I.M. First recording of Shewanella putrefaciens in cultured Oreochromis niloticus and its identification by 16Sr RNA in Egypt. Egypt. J. Aquat. Res. 2017, 43, 101–107. [Google Scholar] [CrossRef]
- Jia, S.; Huang, Z.; Lei, Y.; Zhang, L.; Li, Y.; Luo, Y. Application of Illumina-MiSeq high throughput sequencing and culture-dependent techniques for the identification of microbiota of silver carp ( Hypophthalmichthys molitrix ) treated by tea polyphenols. Food Microbiol. 2018, 76, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Spoilage microbial community profiling by 16S rRNA amplicon sequencing of modified atmosphere packaged live mussels at cold storage temperature. Food Res. Int. 2018, 121, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, E.; Jakobsen, L.M.A.; Hultman, J.; Eggers, N.; Bertram, H.C.; Björkroth, J. Metabolomics and bacterial diversity of packaged yellowfin tuna (Thunnus albacares) and salmon (Salmo salar) show fish species-specific spoilage development during chilled storage. Int. J. Food Microbiol. 2019, 293, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Emborg, J.; Laursen, B.G.; Rathjen, T.; Dalgaard, P. Microbial spoilage and formation of biogenic amines in fresh and thawed modified atmosphere-packed salmon (Salmo salar) at 2 °C. J. Appl. Microbiol. 2002, 92, 790–799. [Google Scholar] [CrossRef]
- Danilo, E.; Ilario, F.; Antonella, N.; Maurice, N.; Pamela, V.; Antonietta, L.S.; Luca, L.; Gianluigi, M.; M Elisabetta, G.; Francesco, V. Monitoring of microbial metabolites and bacterial diversity in beef stored under different packaging conditions. Appl. Environ. Microbiol. 2011, 77, 7372–7381. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Hong, H.; Jia, S.; Liu, Y.; Luo, Y. Effects of phytic acid and lysozyme on microbial composition and quality of grass carp (Ctenopharyngodon idellus) fillets stored at 4 °C. Food Microbiol. 2020, 86, 103313. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Verdos, G.I.; Haroutounian, S.A.; Boziaris, I.S. The dynamics of Pseudomonas and volatilome during the spoilage of gutted sea bream stored at 2 °C. Food Control. 2015, 55, 257–265. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, L.; Luo, Y. Changes in microbial communities and quality attributes of white muscle and dark muscle from common carp ( Cyprinus carpio ) during chilled and freeze-chilled storage. Food Microbiol. 2018, 73, 237–244. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Y.; Huang, H.; Xu, Q. Microbial succession of grass carp (Ctenopharyngodon idellus) filets during storage at 4 °C and its contribution to biogenic amines’ formation. Int. J. Food Microbiol. 2014, 190, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Taylor, K.D.A.; Shahidi, F. Comparison of volatiles of cultured and wild sea bream (Sparus aurata) during storage in ice by dynamic headspace analysis/gas chromatography–mass spectrometry. J. Agric. Food Chem. 2005, 53, 2616–2622. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Scanlan, R.A.; Lee, J.S.; Libbey, L.M. Volatile Compounds Produced in Ground Muscle Tissue of Canary Rockfish. J. Fish. Res. Board Can. 1973, 29, 1125–1129. [Google Scholar] [CrossRef]
- Lerke, P.A.; Huck, R.W. Objective determination of canned tuna quality: Identification of ethanol as a potentially useful index. J. Food Sci. 1977, 42, 755–758. [Google Scholar] [CrossRef]
- Josephson, D.B.; Lindsay, R.C. Enzymic Generation of Volatile Aroma Compounds from Fresh Fish. In Proceedings of the Acs Symposium Series-american Chemical Society, Washington, DC, USA, 25 August 1986; pp. 201–219. [Google Scholar]
- Curioni, P.M.G.; Bosset, J.O. Key odorants in various cheese types as determined by gas chromatography-olfactometry. Int. Dairy J. 2002, 12, 0–984. [Google Scholar] [CrossRef]
- Insausti, K.; Beriain, M.J.; Gorraiz, C.; Purroy, A. Volatile Compounds of Raw Beef from 5 Local Spanish Cattle Breeds Stored Under Modified Atmosphere. J. Food Sci. 2006, 67, 1580–1589. [Google Scholar] [CrossRef]
- Calkins, C.R.; Hodgen, J.M. A fresh look at meat flavor. Meat Sci. 2007, 77, 63–8053. [Google Scholar] [CrossRef]
- Marsili, R.T. Comparison of Solid-Phase Microextraction and Dynamic Headspace Methods for the Gas Chromatographic--Mass Spectrometric Analysis of Light-Induced Lipid Oxidation Products in Milk. J. Chromatogr. Sci. 1999, 37, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Marsili, R.T. SPME-MS-MVA as an electronic nose for the study of off-flavors in milk. J. Agric. Food Chem. 1999, 47, 648–654. [Google Scholar] [CrossRef]
- Kawai, T.; Sakaguchi, M. Fish flavor. C R C Crit. Rev. Food Technol. 1996, 36, 257–298. [Google Scholar] [CrossRef]
- Wang, G.Y.; Wang, H.H.; Han, Y.W.; Xing, T.; Ye, K.P.; Xu, X.L.; Zhou, G.H. Evaluation of the spoilage potential of bacteria isolated from chilled chicken invitro and in situ. Food Microbiol. 2017, 63, 139. [Google Scholar] [CrossRef] [PubMed]
- Yuxiang, Z.; Jianping, W.; Yahong, Y.; Tianli, Y. Diversity and characterization of spoilage-associated psychrotrophs in food in cold chain. Int. J. Food Microbiol. 2019, 290, 86–95. [Google Scholar] [CrossRef]
- Ercolini, D.; Casaburi, A.; Nasi, A.; Ferrocino, I.; Monaco, R.D.; Ferranti, P.; Mauriello, G.; Villani, F. Different molecular types of Pseudomonas fragi have the same overall behaviour as meat spoilers. Int. J. Food Microbiol. 2010, 142, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Gudrun, O.; Rosa, J.; Lauzon, H.L.; Joop, L.; Kristberg, K. Characterization of volatile compounds in chilled cod (Gadus morhua) fillets by gas chromatography and detection of quality indicators by an electronic nose. J. Agric. Food Chem. 2005, 53, 10140–10147. [Google Scholar] [CrossRef]
- Ferrocino, I.; La, S.A.; Torrieri, E.; Musso, S.S.; Mauriello, G.; Villani, F.; Ercolini, D. Antimicrobial packaging to retard the growth of spoilage bacteria and to reduce the release of volatile metabolites in meat stored under vacuum at 1 °C. J. Food Prot. 2013, 76, 52–58. [Google Scholar] [CrossRef]
- Argyri, A.A.; Mallouchos, A.; Panagou, E.Z.; Nychas, G.J.E. The dynamics of the HS/SPME–GC/MS as a tool to assess the spoilage of minced beef stored under different packaging and temperature conditions. Int. J. Food Microbiol. 2015, 193, 51–58. [Google Scholar] [CrossRef]
- Benjakul, S.; Visessanguan, W.; Tueksuban, J. Changes in physico-chemical properties and gel-forming ability of lizardfish (Saurida tumbil) during post-mortem storage in ice. Food Chem. 2003, 80, 535–544. [Google Scholar] [CrossRef]
- Fan, H.; Luo, Y.; Yin, X.; Bao, Y.; Feng, L. Biogenic amine and quality changes in lightly salt- and sugar-salted black carp ( Mylopharyngodon piceus ) fillets stored at 4 °C. Food Chem. 2014, 159, 20–28. [Google Scholar] [CrossRef]
- Chaillou, S.; Chaulottalmon, A.; Caekebeke, H.; Cardinal, M.; Christieans, S.; Denis, C.; Desmonts, M.H.; Dousset, X.; Feurer, C.; Hamon, E. Origin and ecological selection of core and food-specific bacterial communities associated with meat and seafood spoilage. Isme J. 2015, 9, 1105–1118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yue, T.; Yuan, Y. Alicyclobacillus contamination in the production line of kiwi products in China. PLoS ONE 2013, 8, e67704. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |
| Time (days) | L * | a * | b * | W * | TVB-N (mg/100 g) |
|---|---|---|---|---|---|
| 0 | 41.55 ± 1.39 c | −0.46 ± 0.14bc | −2.78 ± 1.50 bc | 41.46 ± 1.37 c | 9.60 ± 0.41 e |
| 2 | 43.52 ± 0.34 bc | 0.53 ± 0.09a | −3.63 ± 0.26 c | 43.40 ± 0.33 bc | 10.27 ± 0.27 e |
| 4 | 44.21 ± 0.91 b | −0.28 ± 0.37bc | −4.01 ± 0.18 c | 44.06 ± 0.91 b | 12.53 ± 0.86 d |
| 6 | 43.36 ± 1.20 bc | −0.86 ± 0.21c | −3.30 ± 1.09 bc | 43.25 ± 1.26 bc | 14.26 ± 1.08 c |
| 8 | 46.79 ± 1.90 a | 0.89 ± 0.86a | 3.86 ± 0.55 a | 46.63 ± 1.94 a | 20.21 ± 0.99 b |
| 10 | 47.49 ± 0.76 a | 0.16 ± 0.36ab | −1.87 ± 0.60 b | 47.45 ± 0.77 a | 21.36 ± 2.07 a |
| Amino Acids | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 |
|---|---|---|---|---|---|---|
| Aspartic acid | 1.50 ± 0.04 d | 1.94 ± 0.08 c | 1.05 ± 0.09 e | 3.04 ± 0.02 b | 1.66 ± 0.00 d | 3.48 ± 0.02 a |
| Threonine | 13.31 ± 1.42 d | 21.89 ± 0.15 a | 19.95 ± 0.96 ab | 16.99 ± 1.32 bc | 17.37 ± 0.72 bc | 14.39 ± 0.04 cd |
| Serine | 6.20 ± 0.64 b | 16.02 ± 0.03 a | 16.60 ± 0.87 a | 4.73 ± 3.68 b | 8.39 ± 0.36 b | 6.33 ± 0.01 b |
| Glutamic acid | 17.95 ± 1.76a bc | 5.53 ± 0.03 d | 20.30 ± 0.60 ab | 17.61 ± 1.80 bc | 21.63 ± 0.63 a | 16.04 ± 0.39 c |
| Glycine | 69.69 ± 7.22 cd | 129.94 ± 1.19 a | 95.6 ± 2.75 b | 86.88 ± 6.35 b | 85.25 ± 4.36 bc | 58.37 ± 1.01 d |
| Alanine | 30.14 ± 3.46 c | 55.06 ± 0.09 a | 42.06 ± 1.93 b | 45.19 ± 3.56 b | 33.73 ± 1.70 c | 33.07 ± 0.48 c |
| Valine | 4.30 ± 0.40 b | 2.32 ± 0.02 c | 3.97 ± 0.20 b | 4.36 ± 0.28 b | 4.72 ± 0.25 ab | 5.43 ± 0.09 a |
| Methionine | 2.37 ± 0.20 ab | 0.74 ± 0.01 d | 1.66 ± 0.09 c | 2.05 ± 0.13 bc | 2.15 ± 0.15 b | 2.61 ± 0.03 a |
| Isoleucine | 2.90 ± 0.20 b | 1.56 ± 0.01 d | 2.08 ± 0.09 c | 2.99 ± 0.17 b | 3.07 ± 0.18 b | 3.66 ± 0.05 a |
| Leucine | 4.44 ± 0.33 b | 2.56 ± 0.01 d | 3.49 ± 0.13 c | 4.83 ± 0.25 b | 5.09 ± 0.28 b | 6.06 ± 0.11 a |
| Tyrosine | 1.86 ± 0.09 b | 1.03 ± 0.01 d | 1.50 ± 0.07 c | 1.88 ± 0.08 b | 1.98 ± 0.16 b | 2.37 ± 0.06 a |
| Phenylalanine | 2.07 ± 0.12 c | 0.96 ± 0.07 d | 1.34 ± 0.08 c | 1.73 ± 0.09 b | 1.71 ± 0.05 b | 1.88 ± 0.01 bc |
| Lysine | 30.74 ± 2.84 b | 21.06 ± 0.00 c | 20.57 ± 0.93 c | 27.46 ± 1.52 b | 31.02 ± 1.15 b | 36.44 ± 0.16 a |
| Histidine | 2.86 ± 0.3 b | 2.65 ± 0.06 b | 3.88 ± 0.26 a | 3.88 ± 0.31 a | 3.95 ± 0.12 a | 3.19 ± 0.05 ab |
| Arginine | 5.91 ± 0.53 bc | 3.68 ± 0.00 e | 5.45 ± 0.09 cd | 6.77 ± 0.40 b | 4.48 ± 0.20 de | 8.24 ± 0.02 a |
| Total | 196.24 ± 27.66 c | 266.93 ± 2.11 a | 239.48 ± 12.88 ab | 230.4 ± 17.75 bc | 226.21 ± 14.56 bc | 201.56 ± 2.72 c |
| Sample | OTUs | Shannon | Simpson | ACE | Chao | Coverage |
|---|---|---|---|---|---|---|
| Day 0 | 53 | 1.359056 | 0.388594 | 105.7616 | 138.25 | 0.9997 |
| Day 6 | 51 | 0.929429 | 0.614348 | 141.148483 | 146 | 0.9996 |
| Day 10 | 39 | 0.845352 | 0.621279 | 50.48075 | 46.58333 | 0.9999 |
| VOCs | Day 0 | Day 2 | Day 4 | Day 6 | Day 8 | Day 10 |
|---|---|---|---|---|---|---|
| Alcohols | ||||||
| 1-Penten-3-ol | 2.43 ± 0.01 c | 2.40 ± 0.05 c | 3.91 ± 0.16 d | ND | 3.12 ± 0.1 b | 3.84 ± 0.83 a |
| 1-Heptanol | ND | ND | 13.11 ± 2.67 a | 8.71 ± 4.33 b | ND | ND |
| 1-Hexanol | ND | ND | ND | ND | 7.71 ± 4.24 a | ND |
| 1-Octen-3-ol | 18.00 ± 2.83 c | 21.92 ± 14.21 bc | 33.47 ± 9.47 ab | 39.19 ± 4.00 a | 16.59 ± 4.64 c | 40.85 ± 3.61 a |
| 2-Ethyl-1-hexanol | 207.39 ± 17.32 c | 127.28 ± 2.00 d | 137.07 ± 8.22 d | 568 ± 0.01 a | 227.46 ± 8.87 c | 421.15 ± 19.95 b |
| 1-Pentanol | ND | ND | ND | 5.72 ± 5.00 a | ND | ND |
| Aldehydes | ||||||
| Hexanal | 51.97 ± 7.07 d | 84.76 ± 11.82 bc | 111.25 ± 7.91 a | 64.91 ± 4.7 c | 34.91 ± 3.27 e | ND |
| Heptanal | 15.03 ± 3.99 bc | 16.43 ± 2.00 bc | 21.58 ± 2.59 a | 16.64 ± 2.15 b | 10.70 ± 3.61 c | 3.78 ± 1.04 d |
| Benzaldehyde | 16.39 ± 2.6 ab | 13.53 ± 0.95 b | 21.97 ± 7.10 a | 13.31 ± 4.03 b | ND | ND |
| (E)-2-Octenal | ND | ND | 6.78 ± 0.00 a | ND | ND | ND |
| Nonanal | 152.70 ± 11.36 a | 31.20 ± 8.51 cd | 55.33 ± 1.05 b | 41.89 ± 5.99 c | 18.32 ± 3.95 e | 20.76 ± 1.07 de |
| Decanal | 4.60 ± 1.41 a | ND | 3.80 ± 0.94 a | ND | ND | 3.27 ± 0.33 a |
| Ketones | ||||||
| Acetylacetone | ND | ND | ND | ND | ND | 1.11 ± 0.99 a |
| 2-Nonanone | ND | ND | 3.63 ± 0.58 b | 2.79 ± 0.10 c | ND | 6.15 ± 0.99 a |
| Others | ||||||
| Trimethylamine | ND | ND | ND | ND | 0.40 ± 0.52a | ND |
| Hydrocarbon | ||||||
| Nonane | 6.34 ± 0.98 bc | ND | ND | ND | ND | 8.43 ± 0.95 a |
| Decane | ND | ND | ND | ND | ND | 19.26 ± 1.34 a |
| Undecane | 9.11 ± 1.78 a | ND | 7.61 ± 0.01 b | ND | 6.33 ± 0.80 b | ND |
| Naphthalene | 4.90 ± 0.1 a | ND | 6.59 ± 0.92 a | ND | 4.60 ± 3.61 a | 5.50 ± 2.52 a |
| Dodecane | 28.07 ± 6.04 a | 8.46 ± 2.18 c | 17.09 ± 2.65 b | 6.51 ± 0.87 c | 7.96 ± 1.00 c | 4.23 ±0.10 c |
| Tridecane | 53.45 ± 0.31 a | 11.94 ± 0.83 c | 30.09 ± 7.51 b | 9.19 ±1.00 c | 13.08 ± 1.89 c | 8.61 ± 0.52 c |
| Tetradecane | 15.24 ± 5.29 ab | ND | 11.63 ± 0.00 bc | 15.27 ± 1.99 ab | 10.47 ± 1.00 c | 18.63 ± 1.42 a |
| Pentadecane | 328.65 ± 24.97 a | 37.98 ± 2.04 b | 312.99 ± 60.00 a | 338.00 ± 18.22 a | 103.08 ± 7.92 b | 365.61 ± 60.00 a |
| Hexadecane | 7.95 ± 0.00 a | ND | 6.23 ± 0.00 a | ND | ND | 9.09 ± 0.00 a |
| Heptadecane | 54.73 ± 4.58 a | 36.32 ± 3.82 c | 45.26 ± 4.30 b | 42.83 ± 6.99 bc | 6.61 ± 1.92 d | 54.25 ± 0.00 a |
| Pentadecane | 111.4 ± 2.56 a | 46.73 ± 1.92 d | 84.22 ± 0.21 c | 99.72 ± 7.01 b | 23.66 ± 0.34 e | ND |
| Styrene | 5.14 ± 0.86 bc | ND | ND | 3.9 ± 1.20 c | 5.91 ± 0.57 b | 11.17 ± 2.02 a |
| Caryophyllene | 18.86 ± 0.01 a | ND | 13.69 ± 0.24 b | 17.49 ± 1.06 ab | ND | 21.97 ± 5.94 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Xie, J. Characterization of the Volatiles and Quality of Hybrid Grouper and Their Relationship to Changes of Microbial Community During Storage at 4 °C. Molecules 2020, 25, 818. https://doi.org/10.3390/molecules25040818
Huang W, Xie J. Characterization of the Volatiles and Quality of Hybrid Grouper and Their Relationship to Changes of Microbial Community During Storage at 4 °C. Molecules. 2020; 25(4):818. https://doi.org/10.3390/molecules25040818
Chicago/Turabian StyleHuang, Wenbo, and Jing Xie. 2020. "Characterization of the Volatiles and Quality of Hybrid Grouper and Their Relationship to Changes of Microbial Community During Storage at 4 °C" Molecules 25, no. 4: 818. https://doi.org/10.3390/molecules25040818






