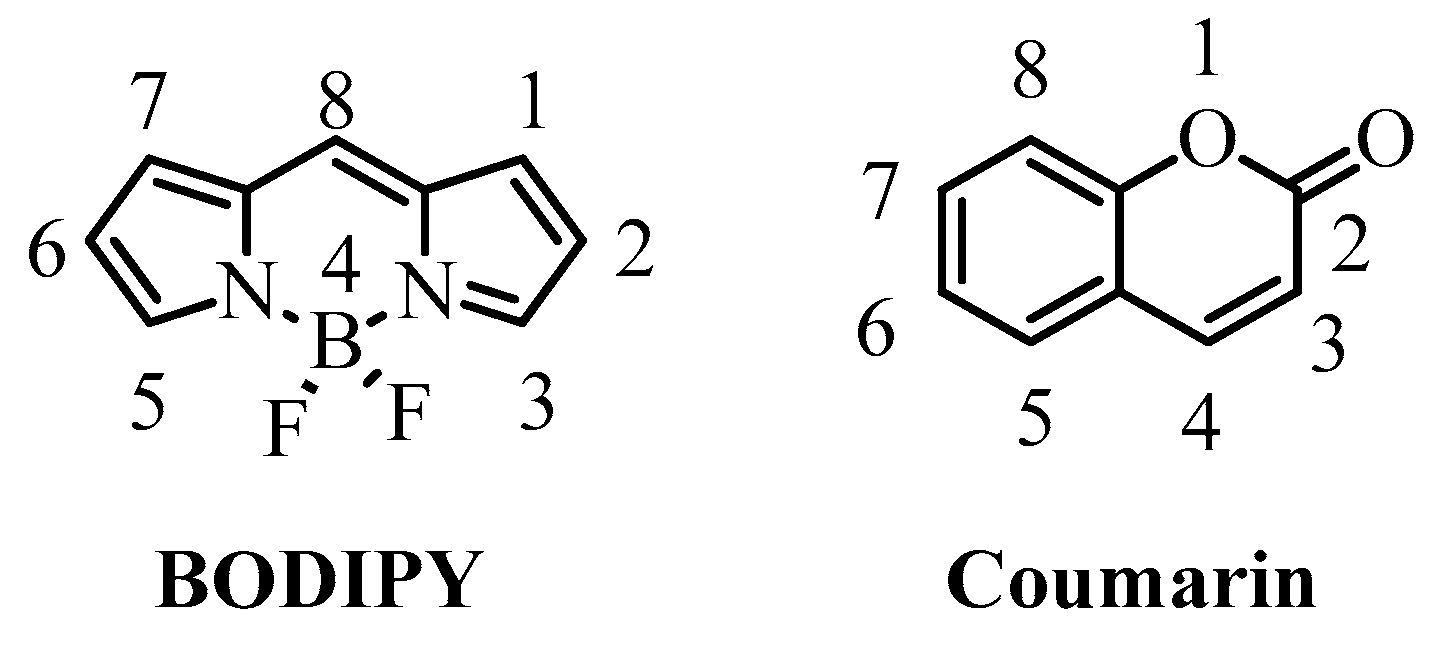
Ready Access to Molecular Rotors Based on Boron Dipyrromethene Dyes-Coumarin Dyads Featuring Broadband Absorption
Abstract
:1. Introduction
2. Results and Discussion
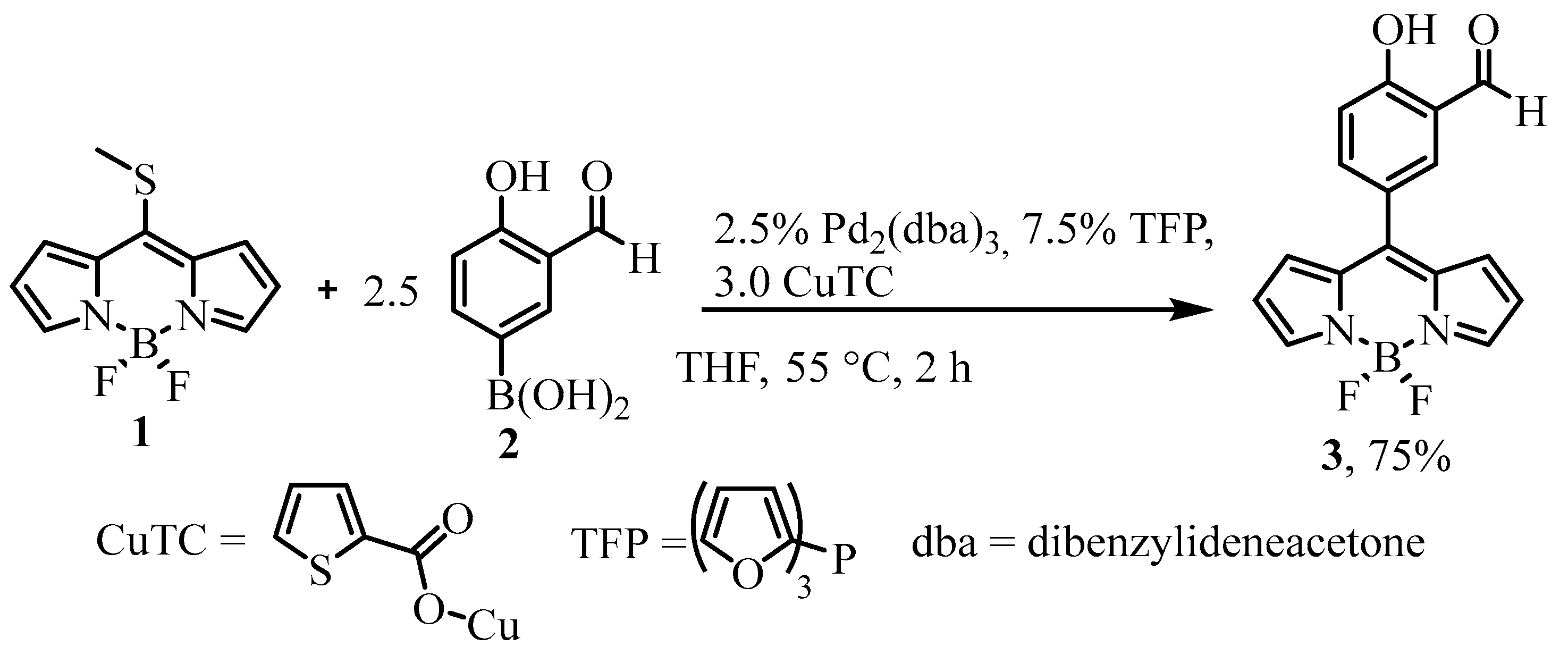
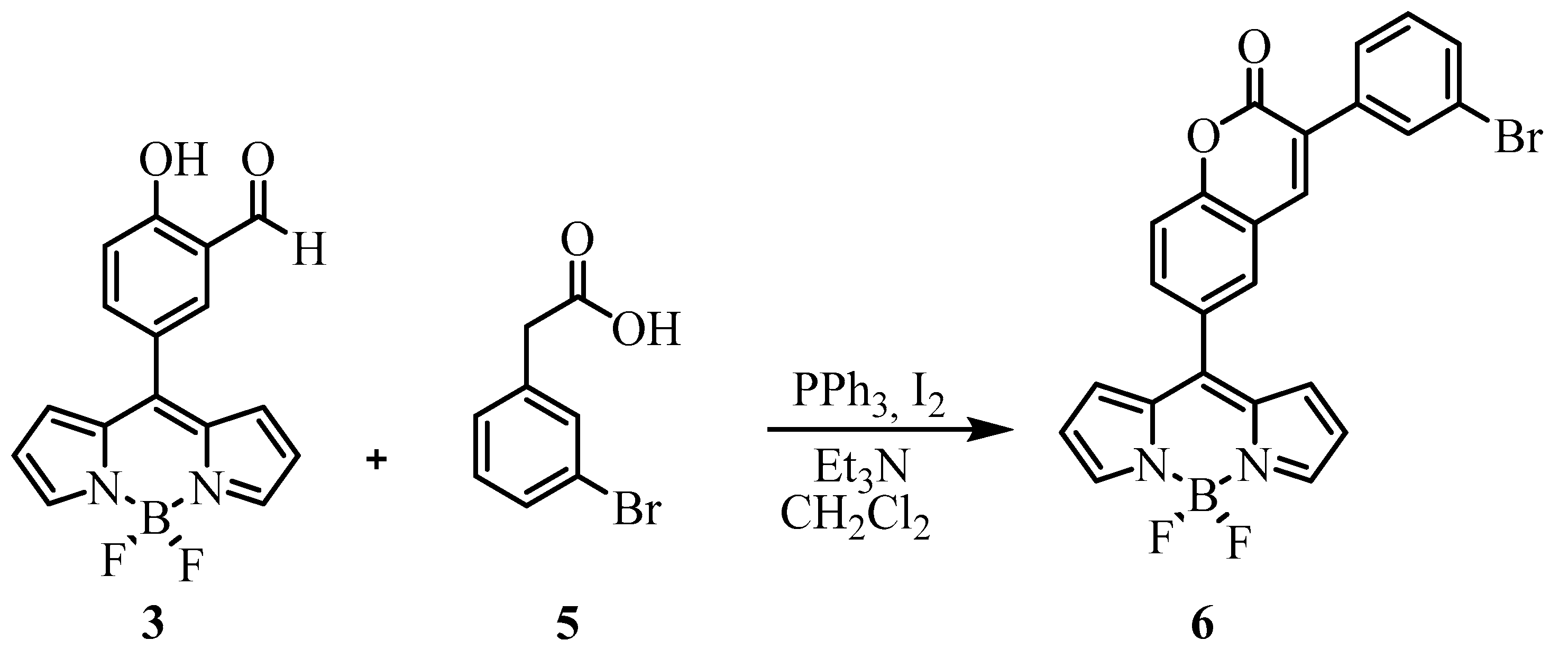
2.1. Synthesis
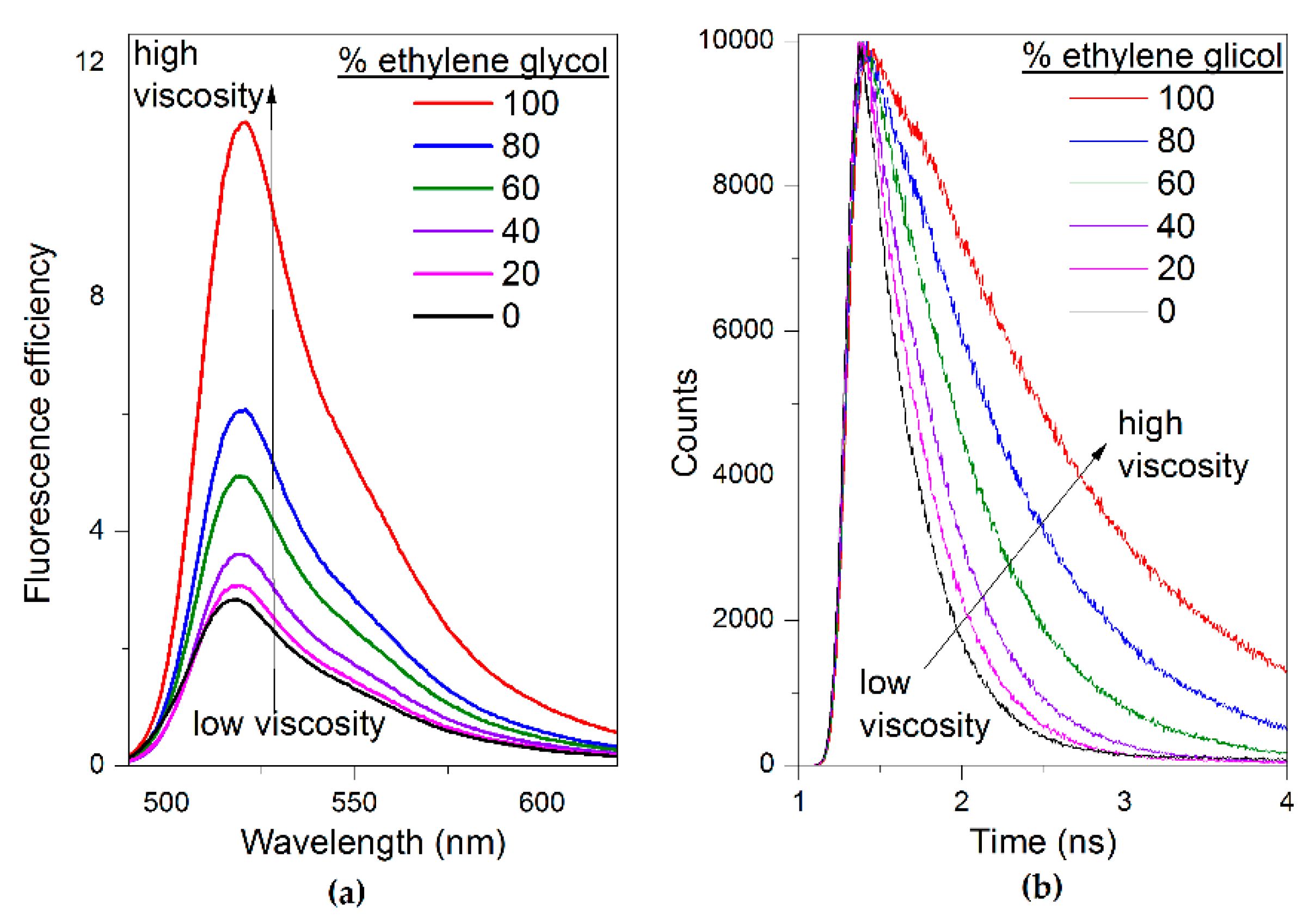
2.2. Photophysical Properties
3. Materials and Methods
3.1. Materials
3.2. General Procedure for the Suzuki Reaction
3.3. Synthesis and Characterization
3.4. Spectroscopic Measurements
3.5. Computational Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Terenziani, F.; Parthasarathy, V.; Pla-Quintana, A.; Maishal, T. Cooperative Two-Photon Absorption Enhancement by Through-Space Interactions in Multichromophoric Compounds. Angew. Chem. Int. Ed. 2009, 48, 8691–8694. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.; Le Gac, S.; Cornelissen, J.J.L.M.; Nolte, R.J.M.; Alan, E.; Rowan, A.E. Macromolecular Multi-chromophoric scaffolding. Chem. Soc. Rev. 2010, 39, 1576–1599. [Google Scholar] [CrossRef] [PubMed]
- Teo, Y.N.; Kool, E.T. DNA-Multichromophore Systems. Chem. Rev. 2012, 112, 4221–4245. [Google Scholar] [CrossRef] [Green Version]
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; Van Grondelle, R.; Govindjee; Scholes, G.D. Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem. Rev. 2017, 117, 249–293. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hu, M.; Zhang, P.; Peng, X. Energy transfer cassettes based on organic fluorophores: Construction and applications in ratiometric sensing. Chem. Soc. Rev. 2013, 42, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Sen, E.; Meral, K.; Atılgan, S. From Dark to Light to Fluorescence Resonance Energy Transfer (FRET): Polarity-Sensitive Aggregation-Induced Emission (AIE)-Active Tetraphenylethene-Fused BODIPY Dyes with a Very Large Pseudo-Stokes Shift. Chem. Eur. J. 2016, 22, 736–745. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, F.; Smith, P.M.; Zandler, M.E.; McCarty, A.L.; Itou, M.; Araki, Y.; Ito, O. Energy transfer followed by electron transfer in a supramolecular triad composed of boron dipyrrin, zinc porphyrin, and fullerene: A model for the photosynthetic antenna-reaction center complex. J. Am. Chem. Soc. 2004, 126, 7898–7907. [Google Scholar] [CrossRef] [PubMed]
- Iehl, J.; Nierengarten, J.-F.; Harriman, A.; Bura, T.; Ziessel, R. Artificial light-harvesting arrays: Electronic energy migration and trapping on a sphere and between spheres. J. Am. Chem. Soc. 2012, 134, 988–998. [Google Scholar] [CrossRef]
- Qu, X.; Liu, Q.; Ji, X.; Chen, H.; Zhou, Z.; Shen, Z. Enhancing the Stokes’ shift of BODIPY dyes via through-bond energy transfer and its application for Fe3+-detection in live cell imaging. Chem. Commun. 2012, 48, 4600–4602. [Google Scholar] [CrossRef]
- Han, J.; Engler, A.; Qi, J.; Tung, C.-H. Ultra pseudo-Stokes shift near infrared dyes based on energy transfer. Tetrahedron Lett. 2013, 54, 502–505. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Zhu, S.; Liu, Y.; Xie, Y.; Xu, Q.; Wei, H.; Lin, W. Broadband light-harvesting molecular triads with high FRET efficiency on the coumarin-rhodamine-BODIPY platform. Chem. Eur. J. 2015, 21, 12181–12187. [Google Scholar] [CrossRef] [PubMed]
- Bessette, A.; Hanan, G.S. Design, synthesis and photophysical studies of dipyrromethene-based materials: Insights into their applicattions in organic photovoltaic devices. Chem. Soc. Rev. 2014, 43, 3342–3405. [Google Scholar] [CrossRef] [PubMed]
- El-Khouly, M.E.; El-Mohsnawy, E.; Fukuzumi, S. Solar energy conversion: From natural to artificial photosynthesis. J. Photochem. Photobiol. C 2017, 31, 36–83. [Google Scholar] [CrossRef]
- Alstrum-Acevedo, J.H.; Brennaman, M.K.; Meyer, T.J. Chemical approaches to artificial photosynthesis. 2. Inorg. Chem. 2005, 44, 6802–6827. [Google Scholar] [CrossRef] [Green Version]
- Benniston, A.C.; Harriman, A. Artificial photosynthesis. Mater. Today 2008, 11, 26–34. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY Dye, the Most Versatile Fluorophore Ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef]
- Patil, P.O.; Bari, S.B.; Firke, S.D.; Deshmukh, P.K.; Donda, S.T.; Patil, D.A. A comprehensive review on synthesis and designing aspects of coumarin derivatives as monoamine oxidase inhibitors for depression and Alzheimer’s disease. Bioorg. Med. Chem. 2013, 21, 2434–2450. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Cao, X.; Lin, W.; Yu, Q.; Wang, J. Ratiometric Sensing of Fluoride Anions Based on a BODIPY-Coumarin Platform. Org. Lett. 2011, 13, 6098–6101. [Google Scholar] [CrossRef]
- Bai, Y.; Shi, X.; Chen, Y.; Zhu, C.; Jiao, Y.; Han, Z.; He, W.; Guo, Z. Coumarin/BODIPY hybridization for ratiometric sensing of intracellular polarity oscillation. Chem. Eur. J. 2018, 24, 7513–7524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Lv, X.; Liu, Y.; Chen, M.; Wang, P.; Liu, J.; Guo, W. Through-bond energy transfer cassettes based on coumarin–Bodipy/distyryl Bodipy dyads with efficient energy efficiences and large pseudo-Stokes’ shifts. J. Mater. Chem. 2011, 21, 13168–13171. [Google Scholar] [CrossRef]
- Esnal, I.; Duran-Sampedro, G.; Agarrabeitia, A.R.; Bañuelos, J.; García-Moreno, I.; Macias, M.A.; Peña-Cabrera, E.; López-Arbeloa, I.; De la Moya, S.; Ortiz, M.J. Coumarin-BODIPY hybrids by heteroatom linkage: Versatile, tunable and photostable dye lasers for UV irradiation. Phys. Chem. Chem. Phys. 2015, 17, 8239–8247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Yang, Z.; Wi, Y.; Kim, T.W.; Verwilst, P.; Lee, Y.H.; Han, G.-i.; Kang, C.; Kim, J.S. BODIPY-coumarin conjugate as an endoplamic reticulum membrane fluidity sensor and its application to ER stress models. Bionconjug. Chem. 2015, 26, 2474–2480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, N.; Li, Y.; Yang, Z.; Chen, L.; Sun, T.; Xie, Z. Comparative study of two near-infrared coumarin-BODIPY dyes for bioimaging and phototermal therapy of cancer. J. Mater. Chem. C 2019, 7, 4717–4724. [Google Scholar]
- Wang, P.; Guo, S.; Wang, H.-J.; Chen, K.-K.; Zhang, N.; Zhang, Z.-M.; Lu, T.-B. A broadband and strong visible-light-absorbing photosensitizer boosts hydrogen evolution. Nat. Comm. 2019, 10, 3155. [Google Scholar] [CrossRef] [Green Version]
- Momahed Heravi, M.; Zadsirjan, V.; Mollaiye, M.; Heydari, M.; Taheri Kal Koshvandi, A. Salicylaldehydes as privileged synthons in multicomponent reactions. Russ. Chem. Rev. 2018, 87, 553–585. [Google Scholar] [CrossRef]
- Alamiry, M.A.H.; Benniston, A.C.; Copley, G.; Elliott, K.J.; Harriman, A.; Stewart, B.; Zhi, Y.-Z. A molecular rotor based on an unhindered boron dipyrromethene (Bodipy) dye. Chem. Mater. 2008, 20, 4024–4032. [Google Scholar] [CrossRef]
- Aswathy, P.R.; Sharma, S.; Tripathi, N.P.; Sengupta, S. Regioisomeric BODIPY benzodithiophene dyads and triads with tunable red emission as ratiometric temperature and viscosity sensor. Chem. Eur. J. 2019, 25, 14870–14880. [Google Scholar] [CrossRef]
- Miao, W.; Yu, C.; Hao, E.; Jiao, L. Functionalized BODIPYs as fluorescent molecular rotors for viscosity detection. Front. Chem. 2019, 26, 825. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.-G.; Chen, H.; Liu, Y.; Zhou, Q. The Liebeskind-Srogl Cross-Coupling Reaction and its Synthetic Applications. Asian J. Org. Chem. 2018, 7, 490–508. [Google Scholar] [CrossRef] [Green Version]
- Gary, A.; Molander, G.A.; Trice, S.L.J.; Kennedy, S.M. Scope of the Two-Step, One-Pot Palladium-Catalyzed Borylation/Suzuki Cross-Coupling Reaction Utilizing Bis-Boronic Acid. J. Org. Chem. 2012, 77, 8678–8688. [Google Scholar]
- Phakhodee, W.; Duangkamol, C.; Yamano, D.; Pattarawarapan, M. Ph3P/I2-Mediated Synthesis of 3-Aryl-Substituted and 3,4-Disubstituted Coumarins. Synlett 2017, 28, 825–830. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 4332–4353. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar. Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Jiang, Z.; Yan, M.; Wang, X. Recent progress of BODIPY dyes with aggregation-induced emission. Front. Chem. 2019, 7, 712. [Google Scholar] [CrossRef]
- Kee, H.L.; Kirmaier, C.; Yu, L.; Thamyongkit, P.; Youngblood, W.J.; Calder, M.E.; Ramos, L.; Noll, B.C.; Bocian, D.F.; Scheidt, W.R.; et al. Structural control of the photodynamics of boron-dipyrrin complexes. J. Phys. Chem. B 2005, 109, 20433–20443. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Xu, G.; Prasad, P.N. Conformationally restricted dipyrromethene boron difluoride (BODIPY) dyes: Highly fluorescent, multicolored probes for cellular imaging. Chem. Eur. J. 2008, 14, 5812–5819. [Google Scholar] [CrossRef]
- Reddy, G.; Duvva, N.; Seetharaman, S.; D’Souza, F.; Giribabu, L. Photoinduced energy transfer in carbazole-BODIPY based dyads. Phys. Chem. Chem. Phys. 2018, 20, 27418–27428. [Google Scholar] [CrossRef]
- Li, F.; Yang, S.I.; Ciringh, Y.; Seth, J.; Martin III, C.H.; Singh, D.L.; Kim, D.; Birge, R.R.; Bocian, D.F.; Holten, D.; et al. Design, synthesis, and photodynamics of light-harvesting arrays comprised of a porphyrin and one, two or eight boron-dipyrrin accessory pigments. J. Am. Chem. Soc. 1998, 120, 10001–10017. [Google Scholar] [CrossRef]
- Ramírez-Ornelas, D.E.; Alvarado-Martínez, E.; Bañuelos, J.; López-Arbeloa, I.; Arbeloa, T.; Mora-Montes, H.M.; Pérez-García, L.A.; Peña-Cabrera, E. FormylBODIPYs: Privileged building blocks for multicomponent reactions. The case of Passerini reaction. J. Org. Chem. 2016, 81, 2888–2898. [Google Scholar] [CrossRef] [PubMed]
- Prlj, A.; Vannay, L.; Corminboeuf, C. Fluorescence quenching in BODIPY dyes: The role of intramolecular interactions and charge transfer. Helv. Chim. Acta 2017, 100, e1700093. [Google Scholar] [CrossRef]
- Ramírez-Ornelas, D.E.; Sola-Llano, R.; Bañuelos, J.; López Arbeloa, I.; Martínez-Álvarez, J.A.; Mora-Montes, H.M.; Franco, B.; Peña-Cabrera, E. Synthesis, photophysical study, and biological application analysis of complex borondipyrromethene dyes. ACS Omega 2018, 3, 7783–7797. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.D.; Adamo, C.; Jacquemin, D. Dye chemistry with time-dependent density functional theory. Phys. Chem. Chem. Phys. 2014, 16, 14334–14356. [Google Scholar] [CrossRef]
- Momeni, M.R.; Brown, A. Why do TD-DFT excitation energies of BODIPY/aza-BODIPY families largely deviate from experiment? Answers from electron correlated and multireference methods. J. Chem. Theory Comput. 2015, 11, 2619–2632. [Google Scholar] [CrossRef]
- Mao, M.; Song, Q.-H. The structure-property relationships of D-π-A dyes for dye-sensitized solar cells. Chem. Rec. 2016, 16, 719–733. [Google Scholar] [CrossRef]
- Greene, L.E.; Lincoln, R.; Cosa, G. Tuning photoinduced electron transfer efficiency of fluorogenic BODIPY-α-tocopherol analogues. Photochem. Photobiol. 2019, 95, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Scholz, N.; Jadhav, A.; Screykar, M.; Behnke, T.; Nirmalananthan, N.; Resch-Genger, U.; Sekar, N. Coumarin-rhodamine hybrids–Novel probes for the optical measurement of viscosity and polarity. J. Fluoresc. 2017, 7, 1949–1956. [Google Scholar] [CrossRef]
- Dwivedi, B.K.; Singh, V.D.; Kumar, Y.; Pandey, D.S. Photophysical properties of some novel tetraphenylimidazole derived BODIPY based fluorescent molecular rotors. Dalton Trans. 2020, 49, 438–452. [Google Scholar] [CrossRef]
- Kuimova, M.K.; Yahioglu, G.; Levitt, J.A.; Suhling, K. Molecular rotor measures viscosity of live cells via fluorescence lifetime imaging. J. Am. Chem. Soc. 2008, 130, 6672–6673. [Google Scholar] [CrossRef] [Green Version]
- Kuimova, M.K.; Botchway, S.W.; Parker, A.W.; Balaz, M.; Collins, H.A.; Anderson, H.L.; Suhling, K.; Ogilby, P.R. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 2009, 1, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |

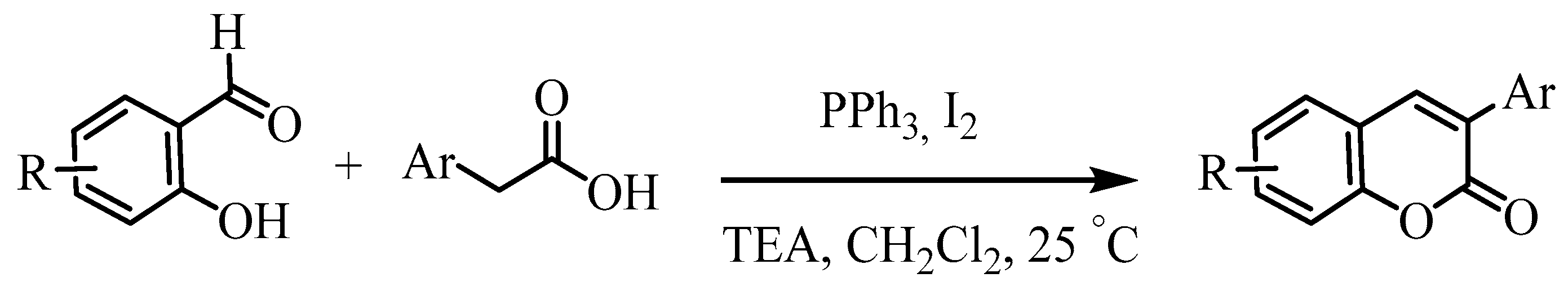




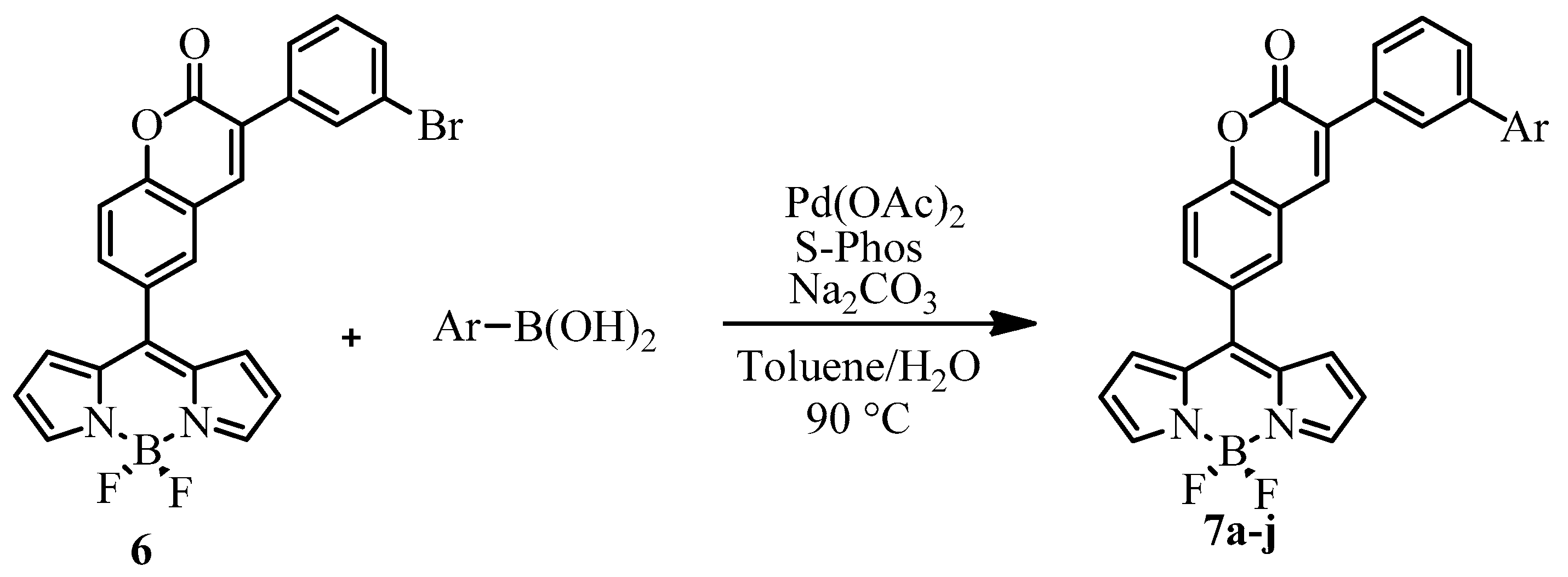
| Entry | Ar- | Reaction time (h) | % yield b | cpd |
|---|---|---|---|---|
| 1 | 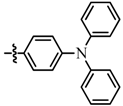 | 21 | 75 | 7a |
| 2 |  | 16 | 85 | 7b |
| 3 | 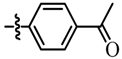 | 19 | 85 | 7c |
| 4 | 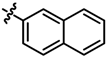 | 20 | 68 | 7d |
| 5 |  | 20 | 76 | 7e |
| 6 |  | 17 | 62 | 7f |
| 7 |  | 18 | 68 | 7g |
| 8 | 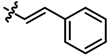 | 16 | 93 | 7h |
| 9 | 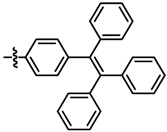 | 6.5 | 82 | 7i |
| 10 | 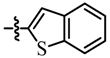 | 7 | 77 | 7j |
| 11 | 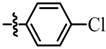 | - | - c | 7k |
| 12 | 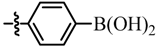 | - | - d | 7l |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enríquez-Palacios, E.; Arbeloa, T.; Bañuelos, J.; Bautista-Hernández, C.I.; Becerra-González, J.G.; López-Arbeloa, I.; Peña-Cabrera, E. Ready Access to Molecular Rotors Based on Boron Dipyrromethene Dyes-Coumarin Dyads Featuring Broadband Absorption. Molecules 2020, 25, 781. https://doi.org/10.3390/molecules25040781
Enríquez-Palacios E, Arbeloa T, Bañuelos J, Bautista-Hernández CI, Becerra-González JG, López-Arbeloa I, Peña-Cabrera E. Ready Access to Molecular Rotors Based on Boron Dipyrromethene Dyes-Coumarin Dyads Featuring Broadband Absorption. Molecules. 2020; 25(4):781. https://doi.org/10.3390/molecules25040781
Chicago/Turabian StyleEnríquez-Palacios, Ernesto, Teresa Arbeloa, Jorge Bañuelos, Claudia I. Bautista-Hernández, José G. Becerra-González, Iñigo López-Arbeloa, and Eduardo Peña-Cabrera. 2020. "Ready Access to Molecular Rotors Based on Boron Dipyrromethene Dyes-Coumarin Dyads Featuring Broadband Absorption" Molecules 25, no. 4: 781. https://doi.org/10.3390/molecules25040781








