Recognition of V3+/V4+/V5+ Multielectron Reactions in Na3V(PO4)2: A Potential High Energy Density Cathode for Sodium-Ion Batteries
Abstract
:1. Introduction
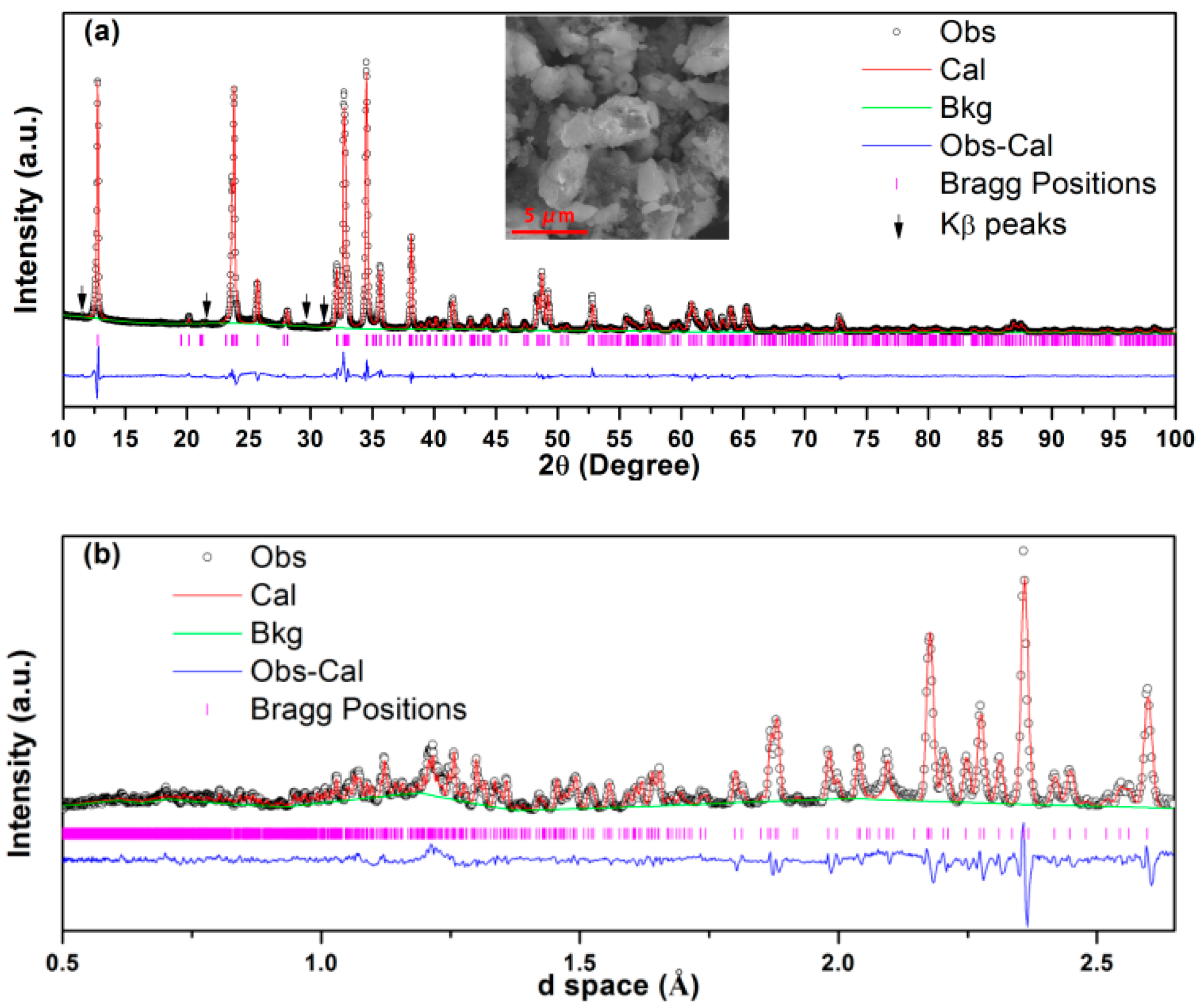
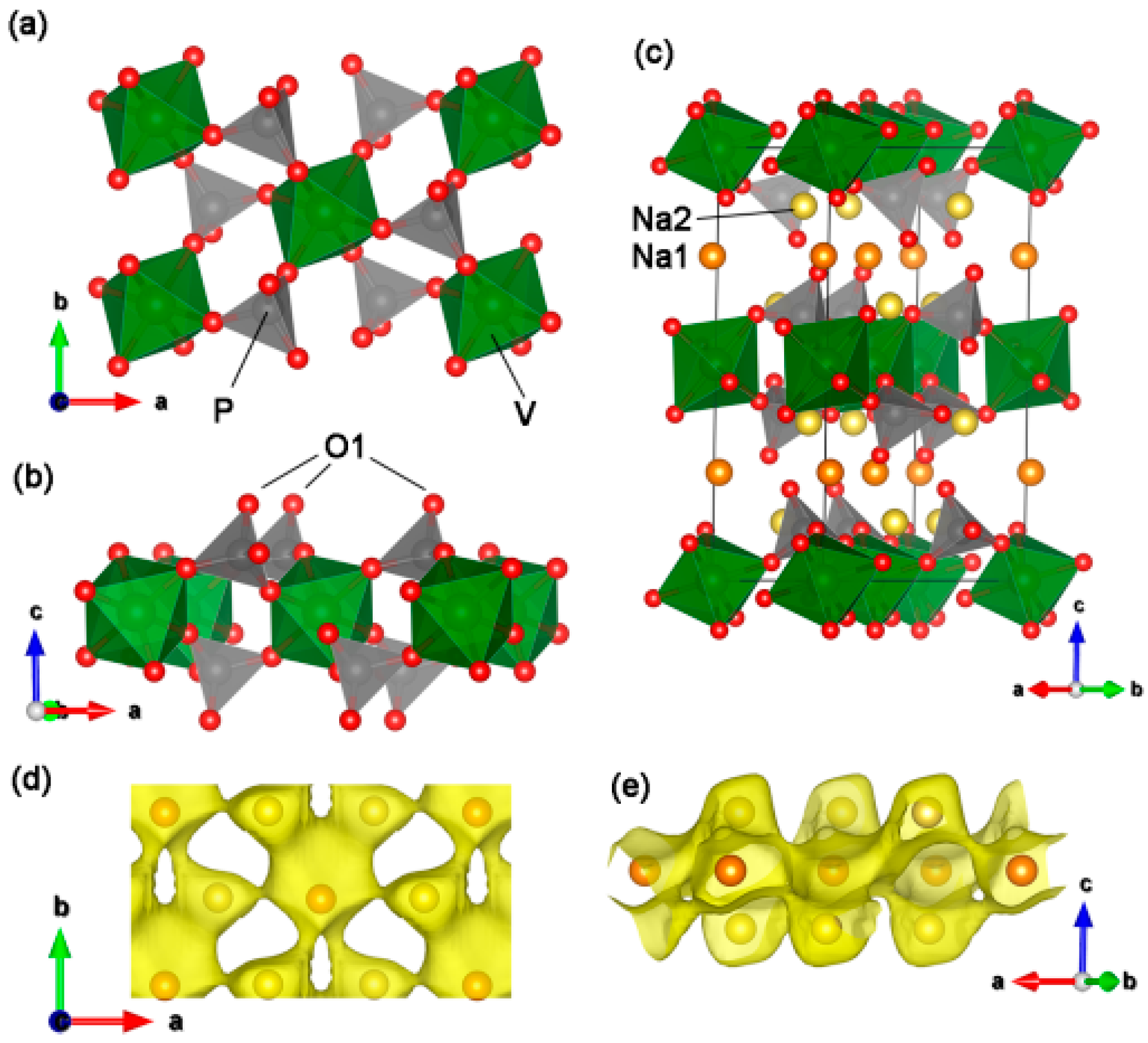
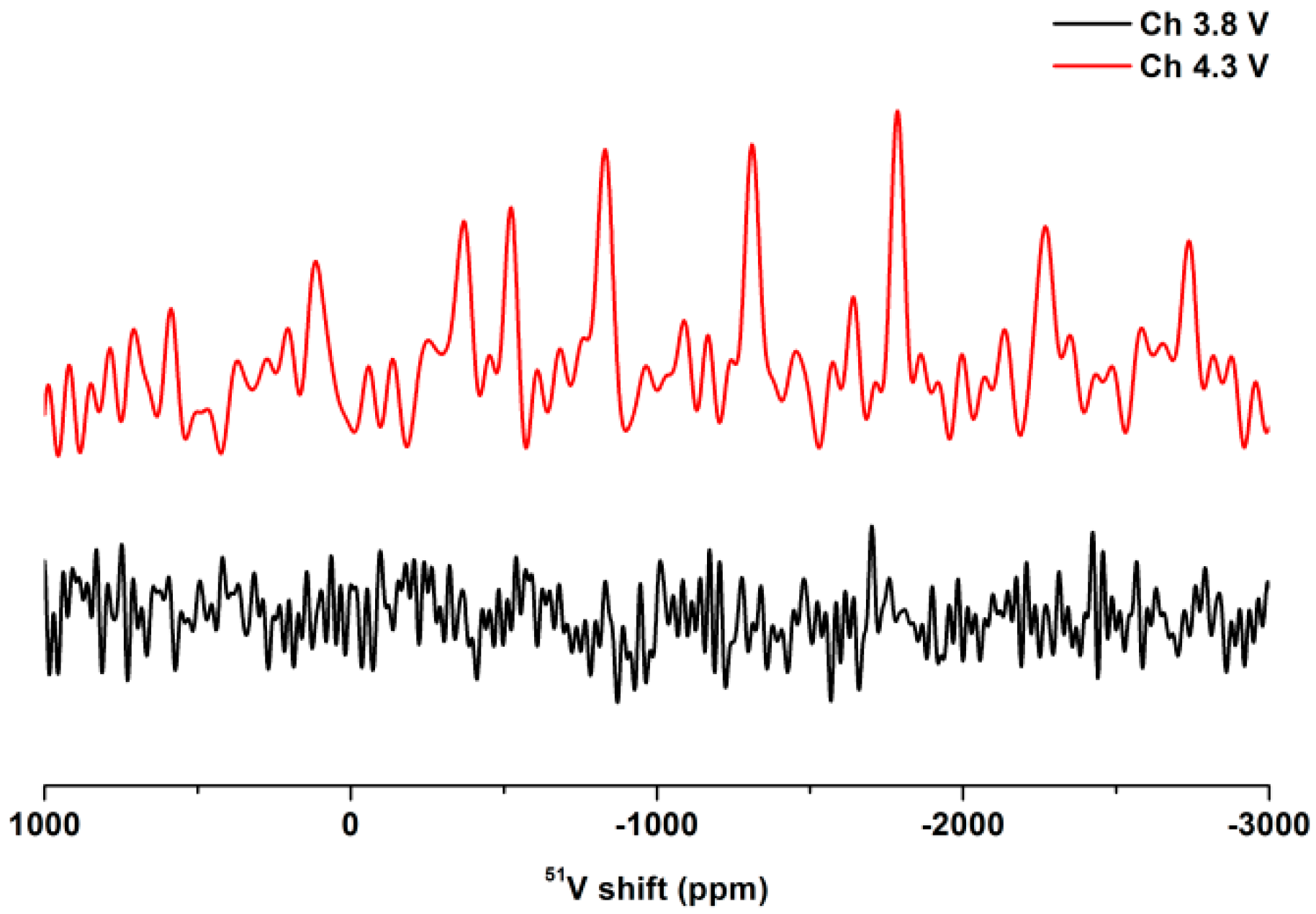
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Lu, Y.; Zhao, C.; Hu, Y.-S.; Titirici, M.-M.; Li, H.; Huang, X.; Chen, L. Recent advances of electrode materials for low-cost sodium-ion batteries towards practical application for grid energy storage. Energy Storage Mater. 2017, 7, 130–151. [Google Scholar] [CrossRef]
- You, Y.; Manthiram, A. Progress in High-Voltage Cathode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1701785. [Google Scholar] [CrossRef]
- Pu, X.; Wang, H.; Zhao, D.; Yang, H.; Ai, X.; Cao, S.; Chen, Z.; Cao, Y. Recent Progress in Rechargeable Sodium-Ion Batteries: toward High-Power Applications. Small 2019, 15, 1805427. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Zheng, Y.H.; Li, X.; Adams, F.; Luo, W.; Huang, Y.H.; Hu, L.B. Electrode Materials of Sodium-Ion Batteries toward Practical Application. ACS Energy Lett. 2018, 3, 1604–1612. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, Z.; Chen, M.; Zou, C.; Jin, H.; Wang, S.; Chou, S.-L.; Dou, S.-X. Recent Progress of Layered Transition Metal Oxide Cathodes for Sodium-Ion Batteries. Small 2019, 15, 1805381. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Y.; Zhong, G.M.; McDonald, M.J.; Gong, Z.L.; Liu, R.; Wen, W.; Yang, C.; Yang, Y. Exploring the working mechanism of Li+ in O3-type NaLi0.1Ni0.35Mn0.55O2 cathode materials for rechargeable Na-ion batteries. J. Mater. Chem. A 2016, 4, 9054–9062. [Google Scholar] [CrossRef]
- Wu, X.H.; Xu, G.L.; Zhong, G.M.; Gong, Z.L.; McDonald, M.J.; Zheng, S.Y.; Fu, R.Q.; Chen, Z.H.; Amine, K.; Yang, Y. Insights into the Effects of Zinc Doping on Structural Phase Transition of P2-Type Sodium Nickel Manganese Oxide Cathodes for High-Energy Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 22227–22237. [Google Scholar] [CrossRef]
- Masquelier, C.; Croguennec, L. Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries. Chem. Rev. 2013, 113, 6552–6591. [Google Scholar] [CrossRef]
- Ni, Q.; Bai, Y.; Wu, F.; Wu, C. Polyanion-Type Electrode Materials for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1600275. [Google Scholar] [CrossRef]
- Barpanda, P.; Lander, L.; Nishimura, S.; Yamada, A. Polyanionic Insertion Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703055. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.; Sheng, T.; Zheng, S.; Zhong, G.; Zheng, G.; Liang, Z.; Ortiz, G.F.; Zhao, W.; Mi, J.; et al. Novel 3.9 V Layered Na3V3(PO4)4 Cathode Material for Sodium Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 3603–3606. [Google Scholar] [CrossRef]
- Hurlbutt, K.; Wheeler, S.; Capone, I.; Pasta, M. Prussian Blue Analogs as Battery Materials. Joule 2018, 2, 1950–1960. [Google Scholar] [CrossRef] [Green Version]
- You, Y.; Wu, X.L.; Yin, Y.X.; Guo, Y.G. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energy Environ. Sci. 2014, 7, 1643–1647. [Google Scholar] [CrossRef]
- You, Y.; Yao, H.R.; Xin, S.; Yin, Y.X.; Zuo, T.T.; Yang, C.P.; Guo, Y.G.; Cui, Y.; Wan, L.J.; Goodenough, J.B. Subzero-Temperature Cathode for a Sodium-Ion Battery. Adv. Mater. 2016, 28, 7243. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Liu, H.D.; Xu, J.; Ma, C.Z.; Meng, Y.S. A new O3-type layered oxide cathode with high energy/power density for rechargeable Na batteries. Chem. Commun. 2015, 51, 4693–4696. [Google Scholar] [CrossRef]
- Liu, R.; Liang, Z.; Gong, Z.; Yang, Y. Research Progress in Multielectron Reactions in Polyanionic Materials for Sodium-Ion Batteries. Small Methods 2018, 3, 1800221. [Google Scholar] [CrossRef]
- de Boisse, B.M.; Ming, J.; Nishimura, S.I.; Yamada, A. Alkaline Excess Strategy to NASICON-Type Compounds towards Higher-Capacity Battery Electrodes. J. Electrochem. Soc. 2016, 163, A1469–A1473. [Google Scholar] [CrossRef]
- Zakharkin, M.V.; Drozhzhin, O.A.; Tereshchenko, I.V.; Chernyshov, D.; Abakumov, A.M.; Antipov, E.V.; Stevenson, K.J. Enhancing Na+ Extraction Limit through High Voltage Activation of the NASICON-Type Na4MnV(PO4)3 Cathode. ACS Appl. Energy Mater. 2018, 1, 5842–5846. [Google Scholar] [CrossRef]
- Chen, F.; Kovrugin, V.M.; David, R.; Mentré, O.; Fauth, F.; Chotard, J.N.; Masquelier, C. A NASICON-Type Positive Electrode for Na Batteries with High Energy Density: Na4MnV(PO4)3. Small Methods 2018, 2, 1800218. [Google Scholar] [CrossRef]
- Yan, G.C.; Mariyappan, S.; Rousse, G.; Jacquet, Q.; Deschamps, M.; David, R.; Mirvaux, B.; Freeland, J.W.; Tarascon, J.M. Higher energy and safer sodium ion batteries via an electrochemically made disordered Na3V2(PO4)2F3 material. Nat. commun. 2019, 10, 585. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.B.; Broux, T.; Camacho, P.S.; Denux, D.; Bourgeois, L.; Belin, S.; Iadecola, A.; Fauth, F.; Carlier, D.; Olchowka, J.; et al. Stability in water and electrochemical properties of the Na3V2(PO4)2F3–Na3(VO)2(PO4)2F solid solution. Energy Storage Mater. 2019, 20, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.C.; Li, Y.T.; Park, K.; Goodenough, J.B. Sodium Extraction from NASICON-Structured Na3MnTi(PO4)3 through Mn(III)/Mn(II) and Mn(IV)/Mn(III) Redox Couples. Chem. Mater. 2016, 28, 6553–6559. [Google Scholar] [CrossRef]
- Gao, H.C.; Seymour, I.D.; Xin, S.; Xue, L.G.; Henkelman, G.; Goodenough, J.B. Na3MnZr(PO4)3: A High-Voltage Cathode for Sodium Batteries. J. Am. Chem. Soc. 2018, 140, 18192–18199. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xu, G.L.; Li, Q.; Zheng, S.Y.; Zheng, G.R.; Gong, Z.L.; Li, Y.X.; Kruskop, E.; Fu, R.Q.; Chen, Z.H.; et al. Exploring Highly Reversible 1.5-Electron Reactions (V3+/V4+/V5+) in Na3VCr(PO4)3 Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 43632–43639. [Google Scholar] [CrossRef] [PubMed]
- Lalere, F.; Seznec, V.; Courty, M.; David, R.; Chotard, J.N.; Masquelier, C. Improving the energy density of Na3V2(PO4)3-based positive electrodes through V/Al substitution. J. Mater. Chem. A 2015, 3, 16198–16205. [Google Scholar] [CrossRef]
- You, Y.; Xin, S.; Asl, H.Y.; Li, W.; Wang, P.-F.; Guo, Y.-G.; Manthiram, A. Insights into the Improved High-Voltage Performance of Li-Incorporated Layered Oxide Cathodes for Sodium-Ion Batteries. Chem 2018, 4, 2124–2139. [Google Scholar] [CrossRef] [Green Version]
- Jian, Z.L.; Hu, Y.S.; Ji, X.L.; Chen, W. NASICON-Structured Materials for Energy Storage. Adv. Mater. 2017, 29, 1601925. [Google Scholar] [CrossRef]
- Chen, S.Q.; Wu, C.; Shen, L.F.; Zhu, C.B.; Huang, Y.Y.; Xi, K.; Maier, J.; Yu, Y. Challenges and Perspectives for NASICON-Type Electrode Materials for Advanced Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1700431. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kajiyama, M.; Iwatate, J.; Nishikawa, H.; Hitomi, S.; Okuyama, R.; Usui, R.; Yamada, Y.; Komaba, S. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 2012, 11, 512–517. [Google Scholar] [CrossRef]
- Aragon, M.J.; Lavela, P.; Ortiz, G.F.; Tirado, J.L. Effect of Iron Substitution in the Electrochemical Performance of Na3V2(PO4)3 as Cathode for Na-Ion Batteries. J. Electrochem. Soc. 2015, 162, A3077–A3083. [Google Scholar] [CrossRef]
- Aragon, M.J.; Lavela, P.; Alcantara, R.; Tirado, J.L. Effect of aluminum doping on carbon loaded Na3V2(PO4)3 as cathode material for sodium-ion batteries. Electrochim. Acta 2015, 180, 824–830. [Google Scholar] [CrossRef]
- Aragon, M.J.; Lavela, P.; Ortiz, G.F.; Tirado, J.L. Benefits of Chromium Substitution in Na3V2(PO4)3 as a Potential Candidate for Sodium-Ion Batteries. Chemelectrochem 2015, 2, 995–1002. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Y.-Y.; Dunstan, M.T.; Huo, H.; Hao, X.; Zou, H.; Zhong, G.; Yang, Y.; Grey, C.P. Local Structure and Dynamics in the Na Ion Battery Positive Electrode Material Na3V2(PO4)2F3. Chem. Mater. 2014, 26, 2513–2521. [Google Scholar] [CrossRef]
- Broux, T.; Bamine, T.; Simonelli, L.; Stievano, L.; Fauth, F.; Menetrier, M.; Carlier, D.; Masquelier, C.; Croguennec, L. VIV Disproportionation Upon Sodium Extraction From Na3V2(PO4)2F3 Observed by Operando X-ray Absorption Spectroscopy and Solid-State NMR. J. Phys. Chem. C 2017, 121, 4103–4111. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; David, R.; Chotard, J.-N.; Recham, N.; Masquelier, C. A High Voltage Cathode Material for Sodium Batteries: Na3V(PO4)2. Inorg. Chem. 2018, 57, 8760–8768. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, G.; Kim, H.; Park, Y.U.; Kang, K. Na3V(PO4)2: A New Layered-Type Cathode Material with High Water Stability and Power Capability for Na-Ion Batteries. Chem. Mater. 2018, 30, 3683–3689. [Google Scholar] [CrossRef]
- Dupre, N.; Gaubicher, J.; Guyomard, D.; Grey, C.P. Li-7 and V-51 MAS NMR study of the electrochemical Behavior of Li1−xV3O8. Chem. Mater. 2004, 16, 2725–2733. [Google Scholar] [CrossRef]
- Sale, M.; Avdeev, M. 3DBVSMAPPER: a program for automatically generating bond-valence sum landscapes. J. Appl. Crystallogr. 2012, 45, 1054–1056. [Google Scholar] [CrossRef]
- An, K.; Skorpenske, H.D.; Stoica, A.D.; Ma, D.; Wang, X.-L.; Cakmak, E. First In Situ Lattice Strains Measurements Under Load at VULCAN. Metall. Mater. Trans. A 2011, 42, 95–99. [Google Scholar] [CrossRef]
- An, K.; Wang, X.L.; Stoica, A.D. Vulcan Data Reduction and Interactive Visualization Software. ORNL Rep. 2012, 621, 1. [Google Scholar]
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Larson, A.C.; Dreele, R.B.V. General Structure Analysis System (GSAS); Los Alamos National Laboratory Report LAUR: Los Alamos, CA, USA, 2000; pp. 86–748.
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the compounds are available from the authors. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Liang, Z.; Xiang, Y.; Zhao, W.; Liu, H.; Chen, Y.; An, K.; Yang, Y. Recognition of V3+/V4+/V5+ Multielectron Reactions in Na3V(PO4)2: A Potential High Energy Density Cathode for Sodium-Ion Batteries. Molecules 2020, 25, 1000. https://doi.org/10.3390/molecules25041000
Liu R, Liang Z, Xiang Y, Zhao W, Liu H, Chen Y, An K, Yang Y. Recognition of V3+/V4+/V5+ Multielectron Reactions in Na3V(PO4)2: A Potential High Energy Density Cathode for Sodium-Ion Batteries. Molecules. 2020; 25(4):1000. https://doi.org/10.3390/molecules25041000
Chicago/Turabian StyleLiu, Rui, Ziteng Liang, Yuxuan Xiang, Weimin Zhao, Haodong Liu, Yan Chen, Ke An, and Yong Yang. 2020. "Recognition of V3+/V4+/V5+ Multielectron Reactions in Na3V(PO4)2: A Potential High Energy Density Cathode for Sodium-Ion Batteries" Molecules 25, no. 4: 1000. https://doi.org/10.3390/molecules25041000






