Redox-Active Monolayers Self-Assembled on Gold Electrodes—Effect of Their Structures on Electrochemical Parameters and DNA Sensing Ability
Abstract
:1. Introduction
2. Results
2.1. Electrochemical Characterization of Gold Electrodes Modified with Symmetric TPY/M(II)/TPY Complexes
2.2. Electrochemical Characterization of Gold Electrodes Modified with Asymmetric DPM/M(II)/TPY Complexes
2.3. Comparison of Electrochemical Parameters of Symmetric TPY/M(II)/TPY and Asymmetric DPM/M(II)/TPY Sensing Platforms Functionalized with Ethanolamine and ssDNA
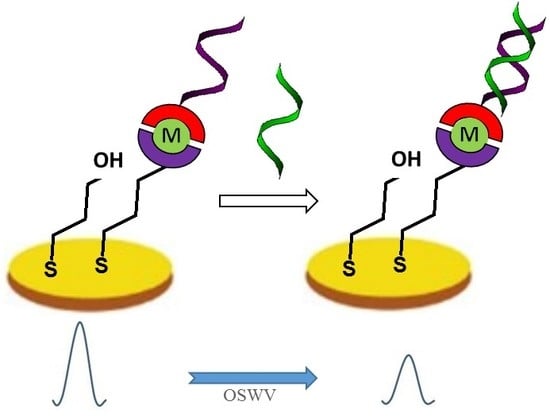
2.4. Electrochemical Determination of Target ssDNA Using Gold Electrodes Modified with Symmetric TPY/M(II)/TPY-ssDNA and Asymmetric DPM/M(II)/TPY-ssDNA Complexes
3. Discussion
4. Materials and Methods
- The spontaneous self-assembly of 0.01 mM AHT and 1 mM MBL layer formation—3 h, room temperature (RT), DCM: MeOH (1:1, v/v); the electrodes were immersed in tubes containing 180 µL of modifying solution; after modification electrodes were carefully rinsed with a mixture of DCM: MeOH
- Reaction between the amine groups of AHT and NHS of 0.1 mM TPY-NHS—1h, RT, DCM: MeOH (1:1); the electrodes were immersed in tubes containing 180 µL of modifying solution; after modification electrodes were carefully rinsed with a mixture of DCM: MeOH
- Complexation of 1mM Me (II) (Co (II) or Cu (II)) metal ions by 0.1 mM TPY-NHS—1h, RT, DCM: MeOH (1:1); the electrodes were immersed in tubes containing 180 µL of modifying solution; after modification electrodes were carefully rinsed with a mixture of DCM: MeOH
- The closure of the coordination sphere of the Me (II) metal ions by 0.1 mM TPY-NHS—1h, RT, DCM: MeOH (1:1 volume ratio); the electrodes were immersed in tubes containing 180 µL of modifying solution; after modification electrodes were carefully rinsed with: a mixture of DCM: MeOH, MeOH, sterilized Milli-Q water and MES buffer pH 7.0
- Reaction between amine groups of 10 μM NH2-NC3 probe and NHS of 0.1 mM TPY-NHS—1h, RT, MES pH 7.0, the electrodes were fixed upside down, 5 µL droplets of solution were spotted on each surface and the electrodes were covered with tubes; after modification electrodes were carefully rinsed with MES pH 7.0 and PBS pH 7.4
- Deactivation of unbound NHS groups with 1 M solution of EA, 10 min PBS pH 7.4, the electrodes were fixed upside down, 5 µL droplets of solution were spotted on each surface and electrodes were covered with tubes.
- The spontaneous self-assembly of 0.01 mM DPM-SH and 1 mM MBL layer formation—3 h, RT; the electrodes were immersed in tubes containing 180 µL of modifying solution; after modification electrodes were carefully rinsed with a mixture of DCM: MeOH
- Complexation of 1 mM M (II) (Co (II) or Cu (II)) metal ions by 0.1 mM TPY-NHS—1 h, RT; the electrodes were immersed in tubes containing 180 µL of modifying solution; after modification electrodes were carefully rinsed with a mixture of DCM: MeOH
- The closure of the coordination sphere of the M(II) metal ions by 0.1 mM TPY-NHS—1 h, RT; DCM: MeOH (1:1) the electrodes were immersed in tubes containing 180 µL of modifying solution; after modification electrodes were carefully rinsed with: a mixture of DCM: MeOH; MeOH, sterilized Milli-Q water and MES buffer pH 7.0
- Reaction between amine groups of 10 μM NH2-NC3 probe and NHS of 0.1 mM TPY-NHS—1 h, RT; MES pH 7.0; the electrodes were fixed upside down, 5 µL droplets of solution were spotted on each surface and the electrodes were covered with tubes; after modification electrodes were carefully rinsed with MES pH 7.0 and PBS pH 7.4
- Deactivation of unbound NHS groups with 1 M solution of EA, 10 min, PBS pH 7.4, RT; the electrodes were fixed upside down, 5 µL droplets of solution were spotted on each surface and the electrodes were covered with tubes.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lehn, J.M. Supramolecular Chemistry: Concepts and Perspectives; Wiley–VCH: Weinheim, Germany, 1995. [Google Scholar]
- Nuzzo, R.G.; Allara, D.L. Adsorption of bifunctional organic disulfides on gold surfaces. J. Am. Chem. Soc. 1983, 105, 4481–4483. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Radecka, H.; Radecki, J. Development of electrochemical sensors for DNA analysis. In DNA in Supramolecular Chemistry and Nanotechnology; Stulz, E., Clever, G.H., Eds.; Wiley: New York, NY, USA, 2015; Chapter 3.1; pp. 140–157. [Google Scholar] [CrossRef]
- Radecka, H.; Radecki, J. Electrochemical sensors for detections of influenza viruses: Fundamentals and applications. In Steps Forwards in Diagnosing and Controlling Influenza; Baddour, M.M., Ed.; IntechOpen: London, UK, 2016; Chapter 3; pp. 47–59. [Google Scholar] [CrossRef] [Green Version]
- Kurzątkowska, K.; Sirko, A.; Zagoórski-Ostoja, W.; Dehaen, W.; Radecka, H.; Radecki, J. Electrochemical label-free and reagentless genosensor based on an ion barrier switch-off system for DNA sequence-specific detection of the Avian Influenza Virus. Anal. Chem. 2015, 87, 9702–9709. [Google Scholar] [CrossRef] [PubMed]
- Malecka, K.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Dehaen, W.; Radecka, H.; Radecki, J. New redox-active layer create via epoxy–amine reaction—The base of genosensor for the detection of specific DNA and RNA sequences of avian influenza virus H5N1. Biosens. Bioelectron. 2015, 65, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malecka, K.; Świętoń, E.; Verwilst, P.; Stachyra, A.; Sirko, A.; Dehaen, W.; Radecki, J.; Radecka, H. Ultrasensitive electrochemical genosensor for direct detection of specific RNA sequences derived from avian influenza viruses present in biological samples. Acta Biochim. Pol. 2019, 66, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Laviron, E.J. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Oliveira-Brett, A.M. Electrochemistry Principles, Methods, and Applications; Oxford University Press: Oxford, UK, 1993; p. 112. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, 2nd ed.; Wiley: New York, NY, USA, 1980; p. 591. [Google Scholar]
- Maccá, C.; Wang, J. Experimental procedures for the determination of amperometric selectivity coefficients. Anal. Chim. Acta 1995, 303, 265–274. [Google Scholar] [CrossRef]
- Umezawa, Y.; Umezawa, K.; Sato, H. Selectivity coefficients for ion-selective electrodes: Recommended methods for reporting KA,B pot values. Pure Appl. Chem. 1995, 67, 507–518. [Google Scholar] [CrossRef]
- Swartz, M.E.; Krull, S. Handbook of Analytical Validation, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Boca Raton, FL, USA, 2012; pp. 70–71. [Google Scholar]
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 2019, 385, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Nishimori, Y.; Kanaizuka, K.; Kurita, T.; Nagatsu, T.; Segawa, Y.; Toshimitsu, F.; Muratsugu, S.; Utsuno, M.; Kume, S.; Murata, M.; et al. Superior electron-transport ability of pi-conjugated redox molecular wires prepared by the stepwise coordination method on a surface. Chem. Asian J. 2009, 4, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, H. Coordination programming: A new concept for the creation of multifunctional molecular systems. Chem. Lett. 2014, 43, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Maskus, M.; Abruña, H.D. Synthesis and characterization of redox-active metal complexes sequentially self-assembled onto gold electrodes via a new thiol-terpyridine ligand. Langmuir 1996, 12, 4455–4462. [Google Scholar] [CrossRef]
- Campagnoli, E.; Hjelm, J.; Milios, C.J.; Sjodin, M.; Pikramenou, Z.; Forster, R.J. Adsorption dynamics and interfacial properties of thiol-based cobalt terpyridine monolayers. Electrochim. Acta 2007, 52, 6692–6699. [Google Scholar] [CrossRef]
- Kaur, B.; Malecka, K.; Cristaldi, D.A.; Chay, C.S.; Mames, I.; Radecka, H.; Radecki, J.; Stulz, E. Approaching single DNA molecule detection with an ultrasensitive electrochemical genosensor based on gold nanoparticles and Cobalt-porphyrin DNA conjugates. Chem. Commun. 2018, 54, 11108–11111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the Terpy NHS ester are available from the authors. |






| TPY | TPY/Co(II) | TPY/Co(II)/TPY/EA | TPY/Co(II)/TPY/ssDNA | ||||
|---|---|---|---|---|---|---|---|
| CV Reduction | |||||||
| ERED, mV | IRED, µA | ERED, mV | IRED, µA | ERED, mV | IRED, µA | ERED, mV | IRED, µA |
| 271 ± 9 | 0.08 ± 0.01 | 263 ± 3 | 0.23 ± 0.006 | 266 ± 278 | 0.14 ± 0.05 | 294 ± 14 | 0.21 ± 0.03 |
| CV Oxidation | |||||||
| EOX, mV | IOX, µA | EOX, mV | IOX, µA | EOX, mV | IOX, µA | EOX, mV | IOX, µA |
| 317 ± 7 | 0.007 ± 0.0005 | 309 ± 2 | 0.09 ± 0.002 | 346 ± 9 | 0.09 ± 0.006 | 320 ± 8 | 0.11 ± 0.005 |
| OSWV | |||||||
| E, mV | I, µA | E, mV | I, µA | E, mV | I, µA | E, mV | I, µA |
| 326 ± 5 | 1.43 ± 0.07 | 273 ± 4 | 1.94 ± 0.07 | 329 ± 24 | 1.90 ± 0.27 | 288 ± 16 | 1.20 ± 0.23 |
| TPY | TPY/Cu(II) | TPY/Cu(II)/TPY/EA | TPY/Cu(II)/TPY/ssDNA | ||||
| CV Reduction | |||||||
| ERED, mV | IRED, µA | ERED, mV | IRED, µA | ERED, mV | IRED, µA | ERED, mV | IRED, µA |
| 271 ± 5 | 0.08 ± 0.02 | 181 ± 21 | 0.11 ± 0.03 | 216 ± 34 | 0.09 ± 0.03 | 196 ± 10 | 0.09 ± 0.02 |
| CV Oxidation | |||||||
| EOX, mV | IOX, µA | EOX, mV | IOX, µA | EOX, mV | IOX, µA | EOX, mV | IOX, µA |
| 317 ± 7 | 0.007 ± 0.0005 | 242 ± 10 | 0.25 ± 0.06 | 250 ± 17 | 0.12 ± 0.02 | 250 ± 7 | 0.12 ± 0.01 |
| OSWV | |||||||
| E, mV | I, µA | E, mV | I, µA | E, mV | I, µA | E, mV | I, µA |
| 326 ± 5 | 1.43 ± 0.07 | 292 ± 47 | 2.80 ± 0.92 | 304 ± 24 | 1.91 ± 0.60 | 330 ± 36 | 1.51 ± 0.09 |
| DPM | DPM/Co(II) | DPM/Co(II)/TPY/EA | DPM/Co(II)/TPY/ssDNA | ||||
|---|---|---|---|---|---|---|---|
| CV Reduction | |||||||
| ERED, mV | IRED, µA | ERED, mV | IRED, µA | ERED, mV | IRED, µA | ERED, mV | IRED, µA |
| 252 ± 7 | 0.26 ± 0.05 | 247 ± 7 | 0.24 ± 0.02 | 267 ± 15 | 0.15 ± 0.02 | 273 ± 17 | 0.14 ± 0.02 |
| CV Oxidation | |||||||
| EOX, mV | IOX, µA | EOX, mV | IOX, µA | EOX, mV | IOX, µA | EOX, mV | IOX, µA |
| 316 ± 5 | 0.12 ± 0.01 | 341 ± 5 | 0.09 ± 0.002 | 304 ± 18 | 0.08 ± 0.02 | 314 ± 13 | 0.12 ± 0.002 |
| OSWV | |||||||
| E, mV | I, µA | E, mV | I, µA | E, mV | I, µA | E, mV | I, µA |
| 335 ± 2 | 1.64 ± 0.27 | 267 ± 3 | 1.70 ± 0.70 | 304 ± 13 | 1.79 ± 0.20 | 321 ± 23 | 1.34 ± 0.24 |
| DPM | DPM/Cu(II) | DPM/Cu(II)/TPY/EA | DPM/Cu(II)/TPY/ssDNA | ||||
| CV Reduction | |||||||
| ERED, mV | IRED, µA | ERED, mV | IRED, µA | ERED, mV | IRED, µA | ERED, mV | IRED, µA |
| 252 ± 7 | 0.13 ± 0.05 | 230 ± 4 | 0.17 ± 0.02 | 212 ± 25 | 0.07 ± 0.02 | 201 ± 8 | 0.07 ± 0.03 |
| CV Oxidation | |||||||
| EOX, mV | IOX, µA | EOX, mV | IOX, µA | EOX, mV | IOX, µA | EOX, mV | IOX, µA |
| 275 ± 5 | 0.05 ± 0.01 | 359 ± 3 | 0.29 ± 0.02 | 241 ± 13 | 0.11 ± 0.01 | 265 ± 8 | 0.14 ± 0.05 |
| OSWV | |||||||
| E, mV | I, µA | E, mV | I, µA | E, mV | I, µA | E, mV | I, µA |
| 335 ± 2 | 1.64 ± 0.27 | 331 ± 14 | 3.57 ± 1.41 | 295 ± 12 | 1.85 ± 0.11 | 275 ± 6 | 1.45 ± 0.25 |
| TPY-Co(II)-TPY | TPY-Cu(II)-TPY | DPM-Co(II)-TPY | DPM-Cu(II)-TPY | |||||
|---|---|---|---|---|---|---|---|---|
| EA | ssDNA | EA | ssDNA | EA | ssDNA | EA | ssDNA | |
| α | 0.52 ± 0.006 | 0.63 ± 0.02 | 0.24 ± 0.05 | 0.27 ± 0.006 | 0.60 ± 0.01 | 0.77 ± 0.003 | 0.23 ± 0.02 | 0.38 ± 0.03 |
| k [s−1] | 1.06 ± 0.01 | 1.71 ± 0.20 | 0.84 ± 0.01 | 1.11 ± 0.16 | 1.08 ± 0.12 | 1.41 ± 0.09 | 1.22 ± 0.13 | 1.25 ± 0.07 |
| Surface Coverage (Γ) [×10−11 mol/cm2] | ||||||||
|---|---|---|---|---|---|---|---|---|
| TPY-M(II)-TPY | DPM-M(II)-TPY | |||||||
| EA | ssDNA | EA | ssDNA | |||||
| ox | red | ox | red | ox | red | ox | red | |
| Co(II) | 4.29 | 4.61 | 4.81 | 6.54 | 2.28 | 4.37 | 3.44 | 5.17 |
| Cu(II) | 3.53 | 2.35 | 3.69 | 2.77 | 2.88 | 1.76 | 3.57 | 2.45 |
| Type of SAM | LOD, fM | Rij = Sj/Si | The Tested Linear Concentration Range, fM |
|---|---|---|---|
| TPY-Co(II)-TPY-ssDNA | 2.13 | 0.22 | From 1 to 20 |
| TPY-Cu(II)-TPY-ssDNA | 1.58 | 0.13 | From 1 to 20 |
| DPM-Co(II)-TPY-ssDNA | 5.43 | 0.23 | From 1 to 20 |
| DPM-Cu(II)-TPY-ssDNA | 1.01 | 0.30 | From 1 to 20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malecka, K.; Menon, S.; Palla, G.; Kumar, K.G.; Daniels, M.; Dehaen, W.; Radecka, H.; Radecki, J. Redox-Active Monolayers Self-Assembled on Gold Electrodes—Effect of Their Structures on Electrochemical Parameters and DNA Sensing Ability. Molecules 2020, 25, 607. https://doi.org/10.3390/molecules25030607
Malecka K, Menon S, Palla G, Kumar KG, Daniels M, Dehaen W, Radecka H, Radecki J. Redox-Active Monolayers Self-Assembled on Gold Electrodes—Effect of Their Structures on Electrochemical Parameters and DNA Sensing Ability. Molecules. 2020; 25(3):607. https://doi.org/10.3390/molecules25030607
Chicago/Turabian StyleMalecka, Kamila, Shalini Menon, Gopal Palla, Krishnapillai Girish Kumar, Mathias Daniels, Wim Dehaen, Hanna Radecka, and Jerzy Radecki. 2020. "Redox-Active Monolayers Self-Assembled on Gold Electrodes—Effect of Their Structures on Electrochemical Parameters and DNA Sensing Ability" Molecules 25, no. 3: 607. https://doi.org/10.3390/molecules25030607