Comparison of the Effects of Different Food-Grade Emulsifiers on the Properties and Stability of a Casein-Maltodextrin-Soybean Oil Compound Emulsion
Abstract
:1. Introduction
2. Results and Discussion
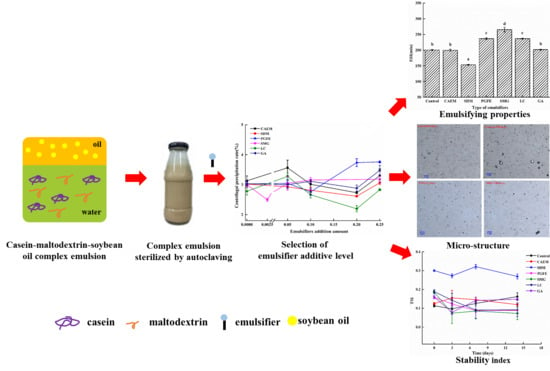
2.1. Effects of Emulsifiers on the Centrifugal Precipitation Rate (CPR) of the Emulsion
2.2. Effect of Different Emulsifiers on Emulsion Characteristics
2.2.1. Emulsifying Properties
2.2.2. Microrheological Properties
2.2.3. Zeta Potential
2.2.4. Average Particle Size
2.3. Evaluation of the Physical Stability of Different Emulsifiers in Emulsions by Turbiscan
2.3.1. ΔBS Curve of the Emulsion
2.3.2. Emulsion Stability Index
2.4. Effect of Calcium Ion Concentrations and pH on the SMG-Stabilized Emulsion
2.4.1. Calcium Ion
2.4.2. pH Values
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Emulsion
3.3. Characterization of Emulsions
3.3.1. Determination of the Centrifugal Precipitation Rate
3.3.2. Emulsifying Properties
3.3.3. Microrheological Properties
3.3.4. Microstructure Analysis
3.3.5. Zeta Potential
3.3.6. Determination of the Physical Stability of the Emulsion by Turbiscan
3.4. Influencing Factors of Emulsion Stability
3.4.1. Evaluation of Emulsion Stability Relative to Calcium Ions
3.4.2. Evaluation of Emulsion Stability Relative to the pH
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Loi, C.C.; Eyres, G.T.; Birch, E.J. Effect of mono- and diglycerides on physical properties and stability of a protein-stabilised oil-in-water emulsion. J. Food Eng. 2019, 240, 56–64. [Google Scholar] [CrossRef]
- Hu, Y.T.; Ting, Y.; Hu, J.Y.; Hsieh, S.C. Techniques and methods to study functional characteristics of emulsion systems. J. Food Drug Anal. 2017, 25, 16–26. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Hernandez Sanchez, M.D.R.; Cuvelier, M.E.; Turchiuli, C. Design of liquid emulsions to structure spray dried particles. J. Food Eng. 2015, 167, 99–105. [Google Scholar] [CrossRef]
- Arancibia, C.; Riquelme, N.; Zúñiga, R.; Matiacevich, S. Comparing the effectiveness of natural and synthetic emulsifiers on oxidative and physical stability of avocado oil-based nanoemulsions. Innov. Food Sci. Emerg. Technol. 2017, 44, 159–166. [Google Scholar] [CrossRef]
- Kralova, I.; Sjöblom, J. Surfactants used in food industry: A review. J. Dispers. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Byun, Y.K.; Hwang, S.H.; Lee, J.H. Characterization of physicochemical properties of casein mixture preparation extracted from organic milk for use as an emulsifier in organic processed foods. J. Sci. Food Agric. 2019, 99, 2375–2383. [Google Scholar] [CrossRef]
- Dalgleish, D.G. Food emulsions—Their structures and structure-forming properties. Food Hydrocoll. 2006, 20, 415–422. [Google Scholar] [CrossRef]
- Jiang, J.; Jin, Y.; Liang, X.; Piatko, M.; Campbell, S.; Lo, S.K.; Liu, Y. Synergetic interfacial adsorption of protein and low-molecular-weight emulsifiers in aerated emulsions. Food Hydrocoll. 2018, 81, 15–22. [Google Scholar] [CrossRef]
- Baby, A.R.; Santoro, D.M.; Velasco, M.V.R.; dos Reis Serra, C.H. Emulsified systems based on glyceryl monostearate and potassium cetyl phosphate: Scale-up and characterization of physical properties. Int. J. Pharm. 2008, 361, 99–103. [Google Scholar] [CrossRef]
- Dickinson, E.; Radford, S.J.; Golding, M. Stability and rheology of emulsions containing sodium caseinate: Combined effects of ionic calcium and non-ionic surfactant. Food Hydrocoll. 2003, 17, 211–220. [Google Scholar] [CrossRef]
- Jukkola, A.; Partanen, R.; Xiang, W.; Heino, A.; Rojas, O.J. Food emulsifiers based on milk fat globule membranes and their interactions with calcium and casein phosphoproteins. Food Hydrocoll. 2019, 94, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Ding, M.; Tao, N.; Wang, X.; Zhong, J. Effects of surfactant type and preparation pH on the droplets and emulsion forms of fish oil-loaded gelatin/surfactant-stabilized emulsions. Lwt 2020, 117, 108654. [Google Scholar] [CrossRef]
- Pichot, R.; Spyropoulos, F.; Norton, I.T. O/W emulsions stabilised by both low molecular weight surfactants and colloidal particles: The effect of surfactant type and concentration. J. Colloid Interface Sci. 2010, 352, 128–135. [Google Scholar] [CrossRef]
- Piorkowski, D.T.; McClements, D.J. Beverage emulsions: Recent developments in formulation, production, and applications. Food Hydrocoll. 2014, 42, 5–41. [Google Scholar] [CrossRef]
- Euston, S.E.; Singh, H.; Munro, P.A.; Dalgleish, D. Competitive adsorption between sodium caseinate and oil-soluble and water-soluble surfactants in oil-in-water emulsions. J. Food Sci. 1995, 60, 1124–1131. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Fu, X.; Huang, Q.; Zhang, B. Effect of pH and ionic strength on the emulsifying properties of two Octenylsuccinate starches in comparison with gum Arabic. Food Hydrocoll. 2018, 76, 96–102. [Google Scholar] [CrossRef]
- Ulbricht, M.; Yang, H. Porous polypropylene membranes with different carboxyl polymer brush layers for reversible protein binding via surface-initiated graft copolymerization. Chem. Mater. 2005, 17, 2622–2631. [Google Scholar] [CrossRef]
- Cheong, A.M.; Tan, K.W.; Tan, C.P.; Nyam, K.L. Kenaf (Hibiscus cannabinus L.) seed oil-in-water Pickering nanoemulsions stabilised by mixture of sodium caseinate, Tween 20 and β-cyclodextrin. Food Hydrocoll. 2016, 52, 934–941. [Google Scholar] [CrossRef]
- Mao, L.; Xu, D.; Yang, J.; Yuan, F.; Gao, Y.; Zhao, J. Effects of small and large molecule emulsifiers on the characteristics of β-carotene nanoemulsions prepared by high pressure homogenization. Food Technol. Biotechnol. 2009, 47, 336–342. [Google Scholar]
- Corredig, M.; Alexander, M. Food emulsions studied by DWS: Recent advances. Trends Food Sci. Technol. 2008, 19, 67–75. [Google Scholar] [CrossRef]
- Xu, D.; Aihemaiti, Z.; Cao, Y.; Teng, C.; Li, X. Physicochemical stability, microrheological properties and microstructure of lutein emulsions stabilized by multilayer membranes consisting of whey protein isolate, flaxseed gum and chitosan. Food Chem. 2016, 202, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, C.; Peng, T.; Zhang, W.; Zhang, J.; Liu, H.; Wu, C.; Pan, X.; Wu, C. Enhanced stability of an emulsion enriched in unsaturated fatty acids by dual natural antioxidants fortified in both the aqueous and oil phases. Food Hydrocoll. 2018, 82, 322–328. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems—A review (Part 1). Trop. J. Pharm. Res. 2013, 12, 255–264. [Google Scholar]
- Taherian, A.R.; Fustier, P.; Ramaswamy, H.S. Effects of added weighting agent and xanthan gum on stability and rheological properties of beverage cloud emulsions formulated using modified starch. J. Food Process Eng. 2007, 30, 204–224. [Google Scholar] [CrossRef]
- Roger, K. Nanoemulsification in the vicinity of phase inversion: Disruption of bicontinuous structures in oil/surfactant/water systems. Curr. Opin. Colloid Interface Sci. 2016, 25, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.M.; Oh, H.C.; Kang, I.S. Electrical charging of a conducting water droplet in a dielectric fluid on the electrode surface. J. Colloid Interface Sci. 2008, 322, 617–623. [Google Scholar] [CrossRef]
- Raikos, V.; McDonagh, A.; Ranawana, V.; Duthie, G. Processed beetroot (Beta vulgaris L.) as a natural antioxidant in mayonnaise: Effects on physical stability, texture and sensory attributes. Food Sci. Hum. Wellness 2016, 5, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Deac, A.; Zhang, G.G.Z. Assessing physical stability of colloidal dispersions using a Turbiscan optical analyzer. Mol. Pharm. 2019, 16, 877–885. [Google Scholar] [CrossRef]
- Kharat, M.; Zhang, G.; McClements, D.J. Stability of curcumin in oil-in-water emulsions: Impact of emulsifier type and concentration on chemical degradation. Food Res. Int. 2018, 111, 178–186. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Urban, T.; Nosal-Wiercińska, A.; Zarko, V.I.; Gun’ko, V.M. Comparison of stability properties of poly(acrylic acid) adsorbed on the surface of silica, alumina and mixed silica-alumina nanoparticles-Application of turbidimetry method. Cent. Eur. J. Chem. 2014, 12, 476–479. [Google Scholar] [CrossRef]
- Kaombe, D.D.; Lenes, M.; Toven, K.; Glomm, W.R. Turbiscan as a tool for studying the phase separation tendency of pyrolysis oil. Energy and Fuels 2013, 27, 1446–1452. [Google Scholar] [CrossRef]
- Munk, M.B.; Larsen, F.H.; Van Den Berg, F.W.J.; Knudsen, J.C.; Andersen, M.L. Competitive displacement of sodium caseinate by low-molecular-weight emulsifiers and the effects on emulsion texture and rheology. Langmuir 2014, 30, 8687–8696. [Google Scholar] [CrossRef] [PubMed]
- Muschiolik, G. Multiple emulsions for food use. Curr. Opin. Colloid Interface Sci. 2007, 12, 213–220. [Google Scholar] [CrossRef]
- Dickinson, E.; Eliot, C. Defining the conditions for heat-induced gelation of a caseinate-stabilized emulsion. Colloids Surfaces B Biointerfaces 2003, 29, 89–97. [Google Scholar] [CrossRef]
- Shao, P.; Ma, H.; Zhu, J.; Qiu, Q. Impact of ionic strength on physicochemical stability of o/w emulsions stabilized by Ulva fasciata polysaccharide. Food Hydrocoll. 2017, 69, 202–209. [Google Scholar] [CrossRef]
- Owens, C.; Griffin, K.; Khouryieh, H.; Williams, K. Creaming and oxidative stability of fish oil-in-water emulsions stabilized by whey protein-xanthan-locust bean complexes: Impact of pH. Food Chem. 2018, 239, 314–322. [Google Scholar] [CrossRef]
- Jensen, S.; Rolin, C.; Ipsen, R. Stabilisation of acidified skimmed milk with HM pectin. Food Hydrocoll. 2010. [Google Scholar] [CrossRef]
- Kevin, N. Pearce, and J.E.K. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar]
- Raikos, V. Encapsulation of vitamin E in edible orange oil-in-water emulsion beverages: Influence of heating temperature on physicochemical stability during chilled storage. Food Hydrocoll. 2017, 72, 155–162. [Google Scholar] [CrossRef]
Sample availability: Samples of the reagents are available from the authors. |










| Type of Emulsifier | Molecular Formula | Relative Molecular Mass (Da) | HLB | Total HLB Value | Addition Amount /% |
|---|---|---|---|---|---|
| Citric Acid Ester of Monoglyceride (CAEM) | C9H14O9 | 266 | 3.4 | 13.495 | 0, 0.0025, 0.05, 0.10, 0.20, 0.25, 0.30 |
| Saturated Distilled Monoglyceride (SDM) | C21H42O4 | 358 | 4.3 | 13.538 | |
| Polyglycerol Fatty Acid Ester (PGFE) | C24H46O6 | 430 | 5.5 | 13.793 | |
| Succinylated Monoglyceride (SMG) | C7H12O6 | 192 | 6.0 | 13.995 | |
| Lecithin (LC) | C42H80NO8P | 758 | 7.0 | 13.667 | |
| Gum Arabic (GA) | \ | 220,000 | 8.0 | 13.714 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wei, Z.-C.; Deng, Y.-Y.; Dong, H.; Zhang, Y.; Tang, X.-J.; Li, P.; Liu, G.; Zhang, M.-W. Comparison of the Effects of Different Food-Grade Emulsifiers on the Properties and Stability of a Casein-Maltodextrin-Soybean Oil Compound Emulsion. Molecules 2020, 25, 458. https://doi.org/10.3390/molecules25030458
Liu Y, Wei Z-C, Deng Y-Y, Dong H, Zhang Y, Tang X-J, Li P, Liu G, Zhang M-W. Comparison of the Effects of Different Food-Grade Emulsifiers on the Properties and Stability of a Casein-Maltodextrin-Soybean Oil Compound Emulsion. Molecules. 2020; 25(3):458. https://doi.org/10.3390/molecules25030458
Chicago/Turabian StyleLiu, Yuan, Zhen-Cheng Wei, Yuan-Yuan Deng, Hao Dong, Yan Zhang, Xiao-Jun Tang, Ping Li, Guang Liu, and Ming-Wei Zhang. 2020. "Comparison of the Effects of Different Food-Grade Emulsifiers on the Properties and Stability of a Casein-Maltodextrin-Soybean Oil Compound Emulsion" Molecules 25, no. 3: 458. https://doi.org/10.3390/molecules25030458