Methotrexate Gold Nanocarriers: Loading and Release Study: Its Activity in Colon and Lung Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of AuNPs and AuNP–MTX Conjugates
2.1.1. UV-Vis Spectroscopy
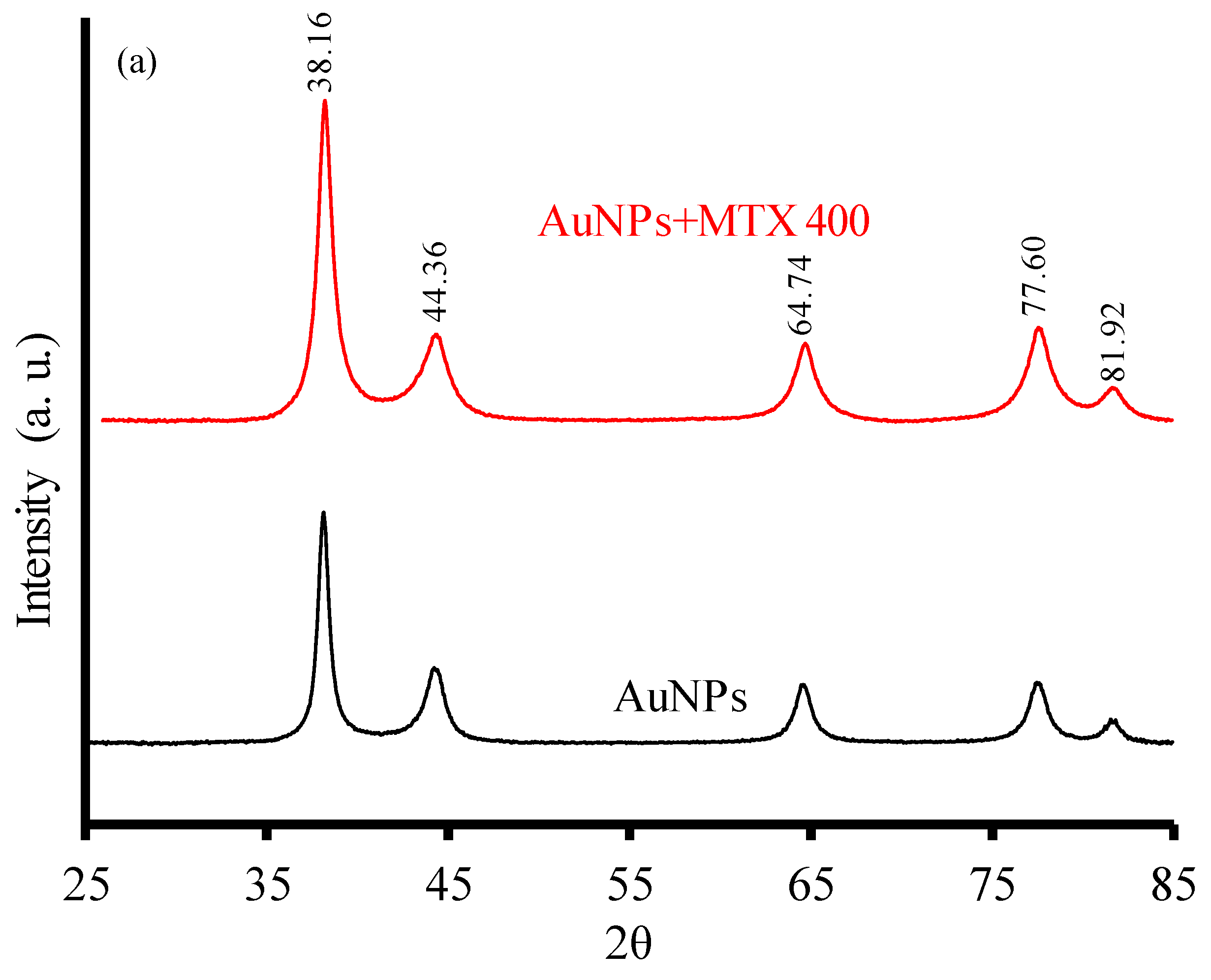
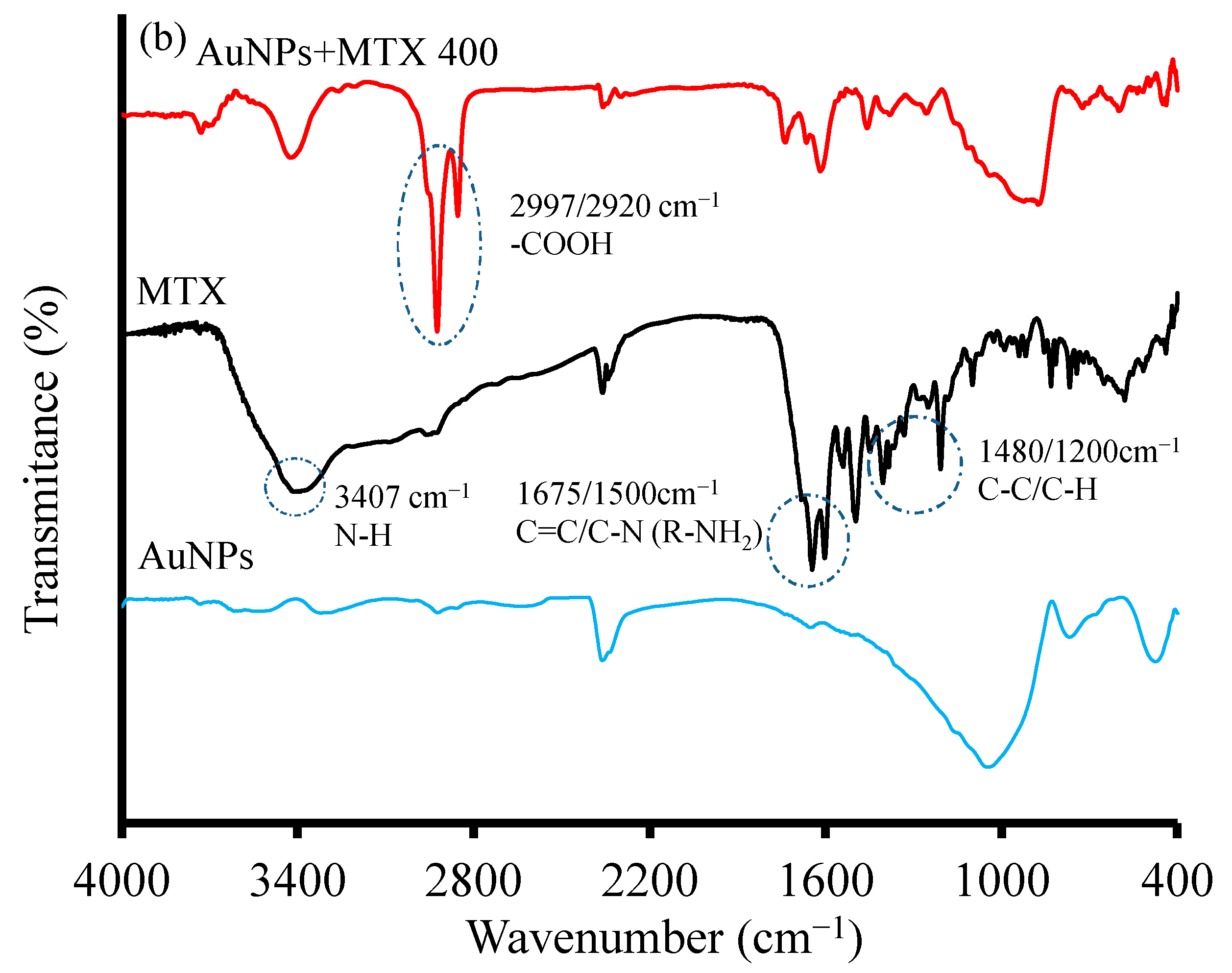
2.1.2. X-ray Diffraction and FTIR Analysis
2.1.3. HRTEM Microscopy
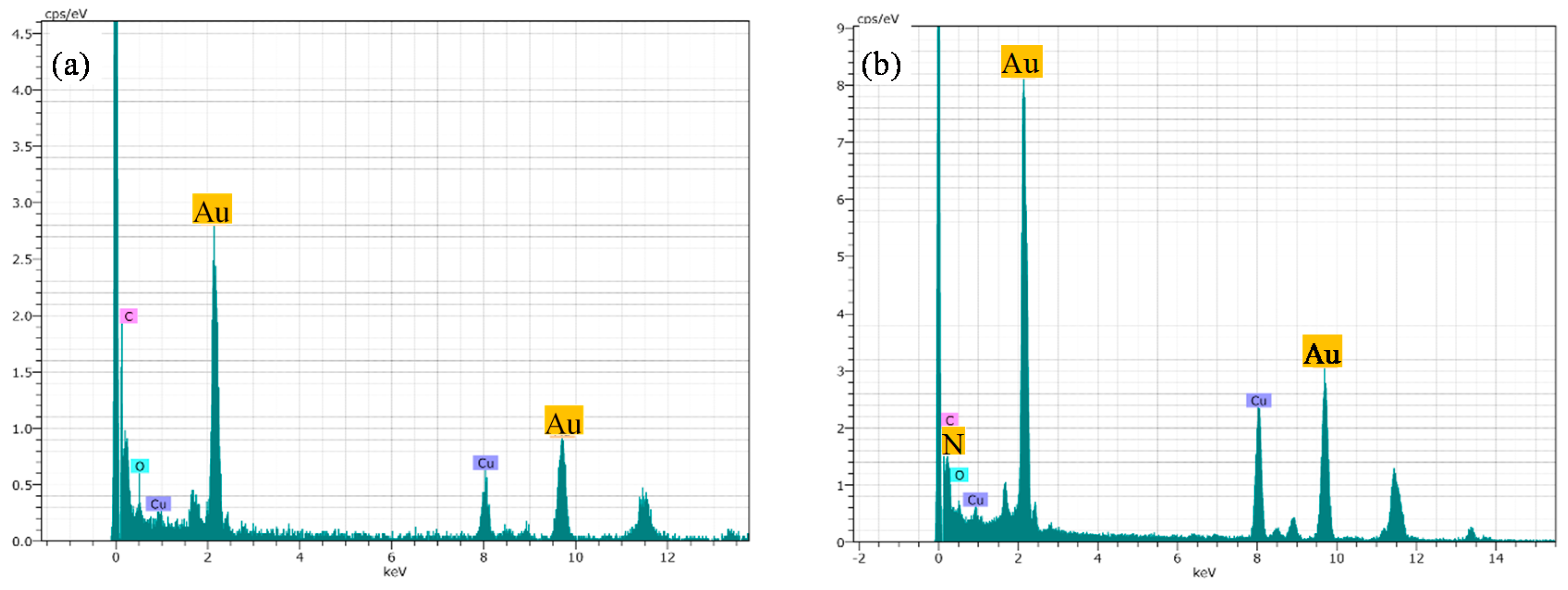
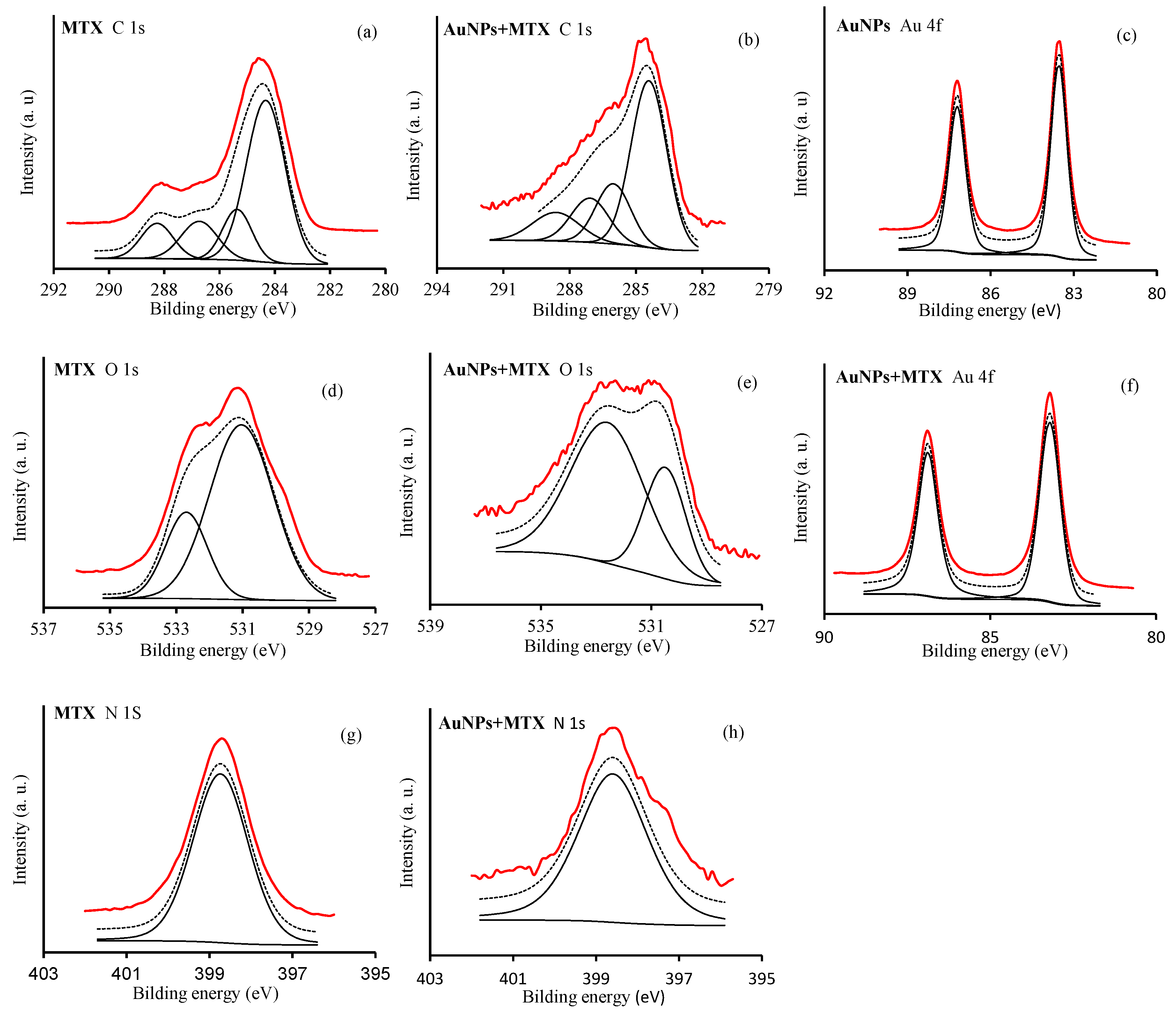
2.1.4. X-ray Photoelectron Spectroscopy (XPS)
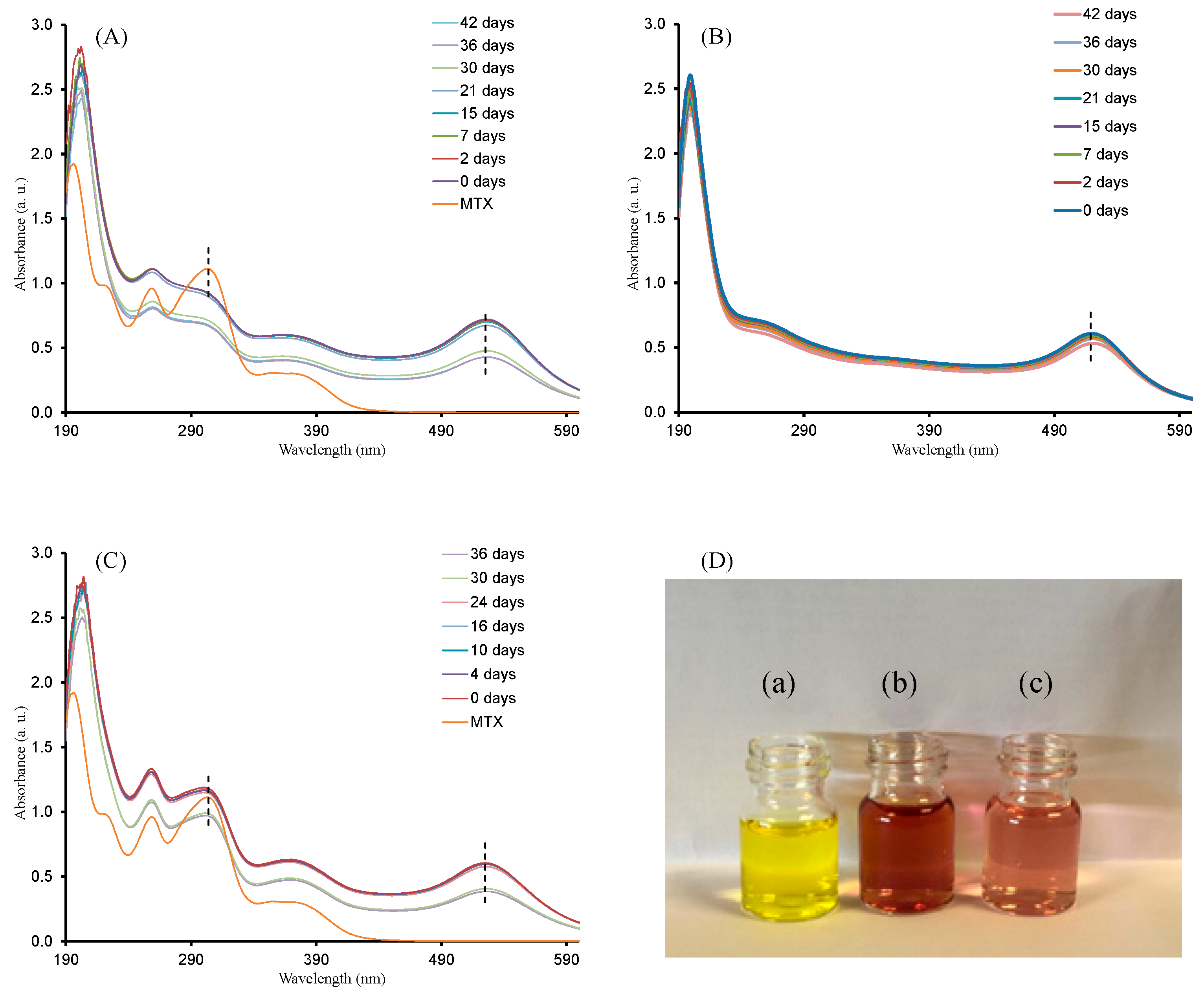
2.2. Stability Analysis of AuNP–MTX Conjugates
2.3. Quantification and Drug Release Study
2.4. Kinetic Modeling
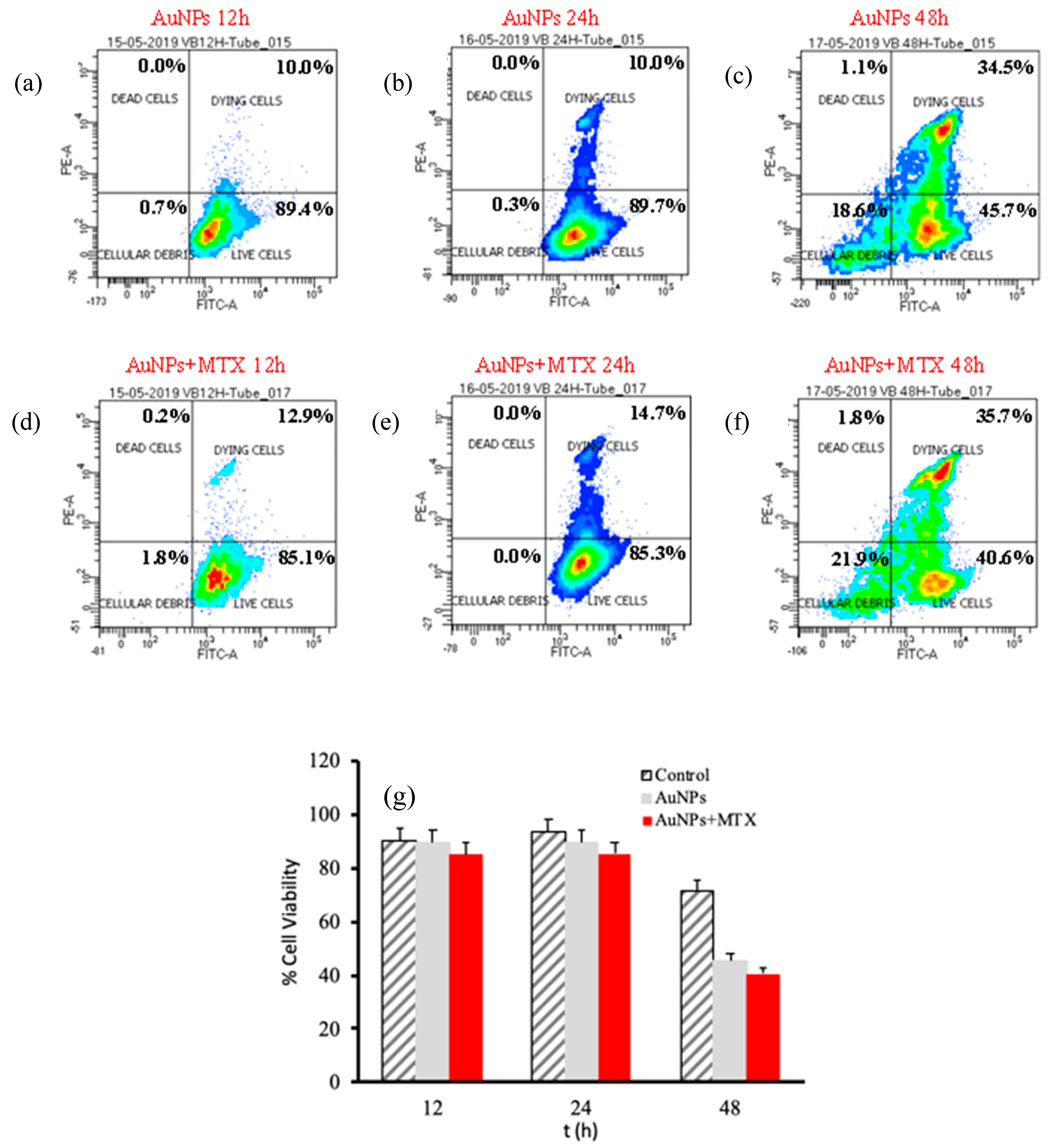
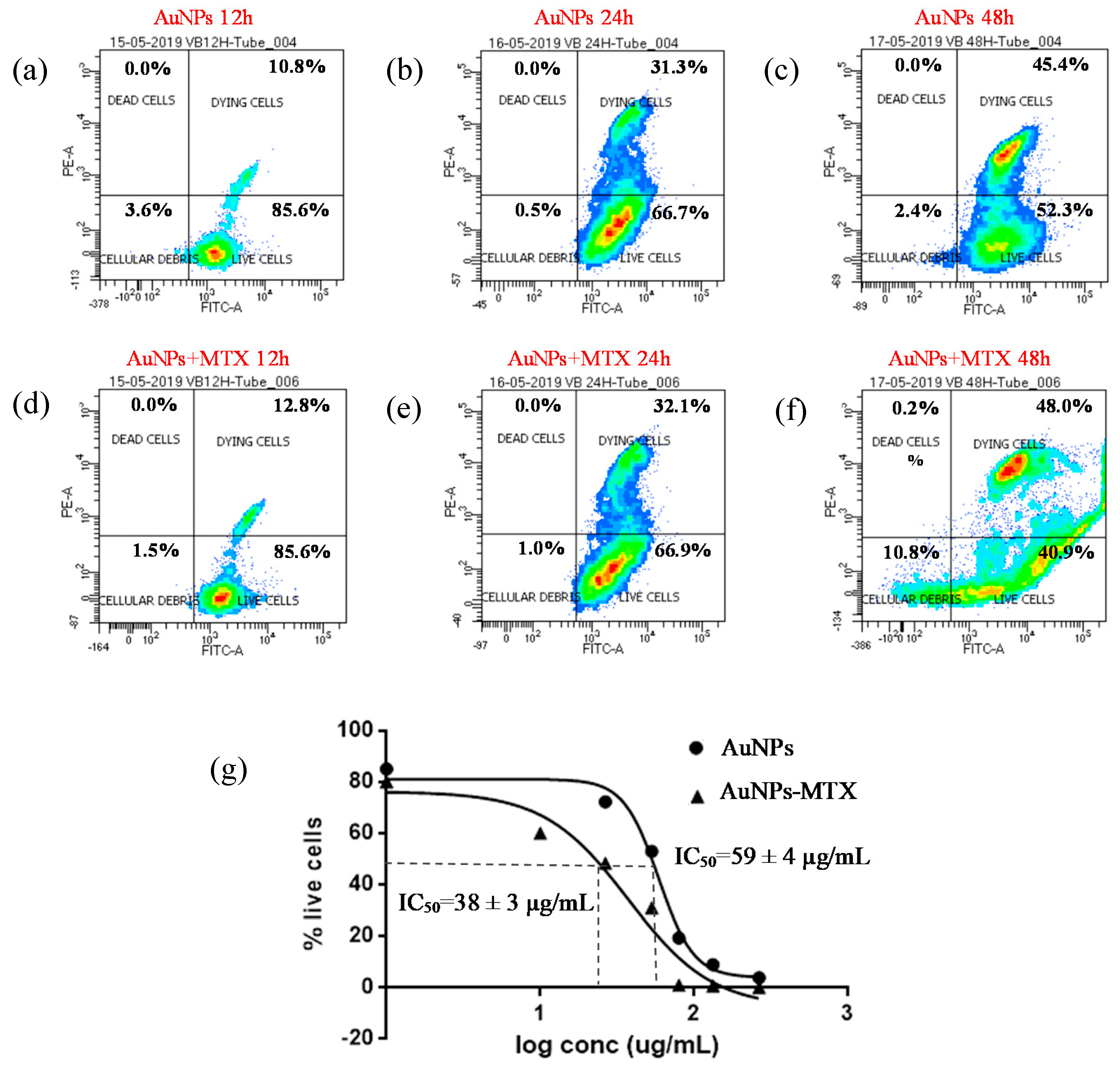
2.5. In Vitro Anticancer Activity
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of Gold Nanoparticles (AuNPs)
3.3. Synthesis of Gold Nanoparticles with Various MTX Contents (AuNP–MTX)
3.4. Characterization of AuNPs and AuNP–MTX Conjugates
3.5. Short- and Long-Term Stability Test
3.6. Drug Loading Capacity
3.7. pH-Dependent Drug Release Study
3.8. Cytotoxicity and Anticancer Effect
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Disclosure for Cell Culture Experiments
References
- Han, G.; Ghosh, P.; Rotello, V.M. Functionalized gold nanoparticles for drug delivery. NanoMed 2007, 2, 113–123. [Google Scholar] [CrossRef]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef]
- Dhar, S.; Liu, Z.; Thomale, J.; Dai, H.; Lippard, S.J. Targeted single-wall carbon nanotube-mediated Pt(IV) prodrug delivery using folate as a homing device. J. Am. Chem. Soc. 2008, 130, 11467–11476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, N.; Sun, C.; Fichtenholtz, A.; Gunn, J.; Fang, C.; Zhang, M. Methotrexate-immobilized poly(ethylene glycol) magnetic nanoparticles for MR imaging and drug delivery. Small 2006, 2, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, W.A. The clinical pharmacology of methotrexate: New applications of an old drug. Cancer 1978, 41, 36–51. [Google Scholar] [CrossRef]
- Young, K.L.; Xu, C.; Xie, J.; Sun, S. Conjugating methotrexate to magnetite (Fe3O4) nanoparticles via trichloro-s-triazine. J. Mater. Chem. 2009, 19, 6400–6406. [Google Scholar] [CrossRef] [PubMed]
- Bessar, H.; Venditti, I.; Benassi, L.; Vaschieri, C.; Azzoni, P.; Pellacani, G.; Magnoni, C.; Botti, E.; Casagrande, V.; Federici, M.; et al. Functionalized gold nanoparticles for topical delivery of methotrexate for the possible treatment of psoriasis. Colloids Surf. B Biointerfaces 2016, 141, 141–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-H.; Tsai, C.-Y.; Huang, P.-Y.; Chang, M.-Y.; Cheng, P.-C.; Chou, C.-H.; Chen, D.-H.; Wang, C.-R.; Shiau, A.-L.; Wu, C.-L. Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Mol. Pharm. 2007, 4, 713–722. [Google Scholar] [CrossRef]
- Dey, S.; Sherly, M.C.D.; Rekha, M.R.; Sreenivasan, K. Alginate stabilized gold nanoparticle as multidrug carrier: Evaluation of cellular interactions and hemolytic potential. Carbohydr. Polym. 2016, 136, 71–80. [Google Scholar] [CrossRef]
- Murawala, P.; Tirmale, A.; Shiras, A.; Prasad, B.L.V. In situ synthesized BSA capped gold nanoparticles: Effective carrier of anticancer drug methotrexate to MCF-7 breast cancer cells. Mater. Sci. Eng. C 2014, 34, 158–167. [Google Scholar] [CrossRef]
- Tran, N.T.T.; Wang, T.-H.; Lin, C.-Y.; Tai, Y. Synthesis of methotrexate-conjugated gold nanoparticles with enhanced cancer therapeutic effect. Biochem. Eng. J. 2013, 78, 175–180. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Zhao, X.-F.; Ju, X.-H.; Wang, Y.; Wang, L.; Li, S.-P.; Li, X.-D. Novel morphology change of Au-Methotrexate conjugates: From nanochains to discrete nanoparticles. Int. J. Pharm. 2016, 515, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Ahmad, M.Z.; Ahmad, F.J.; Storm, G.; Kok, R.J. Gold nanoparticles in theragnostic oncology: Current state-of-the-art. Expert Opin. Drug Deliv. 2012, 9, 1225–1243. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. NanoMed 2007, 2, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef] [Green Version]
- Podsiadlo, P.; Sinani, V.A.; Bahng, J.H.; Kam, N.W.S.; Lee, J.; Kotov, N.A. Gold nanoparticles enhance the anti-leukemia action of a 6-mercaptopurine chemotherapeutic agent. Langmuir 2008, 24, 568–574. [Google Scholar] [CrossRef]
- Ulbrich, K.; Etrych, T.; Chytil, P.; Pechar, M.; Jelinkova, M.; Rihova, B. Polymeric anticancer drugs with pH-controlled activation. Int. J. Pharm. 2004, 277, 63–72. [Google Scholar] [CrossRef]
- Hong, R.; Han, G.; Fernández, J.M.; Kim, B.; Forbes, N.S.; Rotello, V.M. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 2006, 128, 1078–1079. [Google Scholar] [CrossRef]
- Hostetler, M.J.; Templeton, A.C.; Murray, R.W. Dynamics of place-exchange reactions on monolayer-protected gold cluster molecules. Langmuir 1999, 15, 3782–3789. [Google Scholar] [CrossRef]
- Moaseri, E.; Bollinger, J.A.; Changalvaie, B.; Johnson, L.; Schroer, J.; Johnston, K.P.; Truskett, T.M. Reversible self-assembly of glutathione-coated gold nanoparticle clusters via pH-tunable interactions. Langmuir 2017, 33, 12244–12253. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Tai, Y.; Imae, T. Biomodulation approach for gold nanoparticles: Synthesis of anisotropic to luminescent particles. Chem. Asian J. 2010, 5, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-J.; Zhang, P.-H.; Zheng, F.; Chen, H.; Chen, W.; Ding, Y.; Xia, X.-H. Preliminary quality criteria of citrate-protected gold nanoparticles for medicinal applications. ACS Appl. Nano Mater. 2018, 1, 2120–2128. [Google Scholar] [CrossRef]
- Liang, M.; Lin, I.-C.; Whittaker, M.R.; Minchin, R.F.; Monteiro, M.J.; Toth, I. Cellular uptake of densely packed polymer coatings on gold nanoparticles. ACS Nano 2010, 4, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Ayyappan, S.; Sundaraganesan, N.; Aroulmoji, V.; Murano, E.; Sebastian, S. Molecular structure, vibrational spectra and DFT molecular orbital calculations (TD-DFT and NMR) of the antiproliferative drug Methotrexate. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2010, 77, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Chandni, K.; Andhariya, N.; Pandey, O.P.; Chudasama, B. A growth kinetic study of ultrafine monodispersed silver nanoparticles. RSC Adv. 2012, 3, 1127–1136. [Google Scholar] [CrossRef]
- Alexander, M.R.; Payan, S.; Duc, T.M. Interfacial interactions of plasma-polymerized acrylic acid and an oxidized aluminium surface investigated using XPS, FTIR and poly (acrylic acid) as a model compound. Surf. Interface Anal. 1998, 26, 961–973. [Google Scholar] [CrossRef]
- Frydman, E.; Cohen, H.; Maoz, R.; Sagiv, J. Monolayer damage in XPS measurements as evaluated by independent methods. Langmuir 1997, 13, 5089–5106. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Davaran, S.; Danafar, H.; Manjili, H.K. Methotrexate-conjugated L-lysine coated iron oxide magnetic nanoparticles for inhibition of MCF-7 breast cancer cells. Drug Dev. Ind. Pharm. 2018, 44, 886–894. [Google Scholar] [CrossRef]
- Asokan, A.; Cho, M.J. Exploitation of intracellular pH gradients in the cellular delivery of macromolecules. J. Pharm. Sci. 2002, 91, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Gerweck, L.E.; Vijayappa, S.; Kozin, S. Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol. Cancer Ther. 2006, 5, 1275–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominska, M.; Dykxhoorn, D.M. Breaking down the barriers: siRNA delivery and endosome escape. J. Cell Sci. 2010, 123, 1183–1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Gokhale, A. Achieving zero-order release kinetics using multi-step diffusion-based drug delivery. Pharm. Technol. 2014, 26, 38–42. [Google Scholar]
- Soe, Z.C.; Poudel, B.K.; Nguyen, H.T.; Thapa, R.K.; Ou, W.; Gautam, M.; Poudel, K.; Jin, S.G.; Jeong, J.-H.; Ku, S.K.; et al. Folate-targeted nanostructured chitosan/chondroitin sulfate complex carriers for enhanced delivery of bortezomib to colorectal cancer cells. Asian J. Pharm. Sci. 2019, 14, 40–51. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, Z.; Jia, K.; Zhang, W.; Dang, M. Rabdosia rubescens Linn: Green synthesis of gold nanoparticles and their anticancer effects against human lung cancer cells A549. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2171–2178. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.M.; Attardi, L.D. The role of apoptosis in cancer development and treatment response. Nat. Rev. Cancer 2005, 5, 231–237. [Google Scholar] [CrossRef]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: Size focusing versus Ostwald ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.-A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Y.; Di, D.; Zhang, X.; Wang, D.; Zhao, Q.; Wang, S. Gold nanoparticle-gated mesoporous silica as redox-triggered drug delivery for chemo-photothermal synergistic therapy. J. Colloid Interface Sci. 2017, 508, 323–331. [Google Scholar] [CrossRef] [PubMed]




| Model | First Order | Zero Order | Hixson–Crowell | |||
|---|---|---|---|---|---|---|
| r2 | kH (h−1) | r2 | kH (h) | r2 | kH (h) | |
| Free MTX | 0.9885 | 0.9111 | 0.9609 | 0.9009 | 0.946 | 0.013 |
| AuNP–MTX200 | 0.8857 | 0.0025 | 0.9116 | 0.0034 | 0.8952 | 0.00006 |
| AuNP–MTX300 | 0.9409 | 0.0035 | 0.9757 | 0.004 | 0.9553 | 0.00008 |
| AuNP–MTX400 | 0.8428 | 0.0112 | 0.9801 | 0.006 | 0.9594 | 0.0002 |
| AuNP–MTX400 pH | 0.933 | 0.0128 | 0.8632 | 0.0066 | 0.9559 | 0.0002 |
| (MTX) IC50 | HTC-116 | (MTX) IC50 | A-549 | ||||
|---|---|---|---|---|---|---|---|
| Time | mM | µg/mL | Time | mM | µg/mL | ||
| 12 h | 2.3 ± 0.2 | 1051 ± 105 | 12 h | - | - | ||
| 24 h | 0.37 ± 0.04 | 169 ± 17 | 24 h | - | - | ||
| 48 h | 0.15 ± 0.02 | 70 ± 7 | 48 h | 0.10 ± 0.01 | 45 ± 4 | ||
| Ratio Au/MTX/TCS | |||||||
|---|---|---|---|---|---|---|---|
| HAuCl4 | MTX | TCS | HAuCl4 | MTX | TCS | pH | |
| mmol | |||||||
| AuNPs | 0.025 | - | 0.55 | 1 | - | 0.045 | 8.77 |
| AuNP–MTX200 | 0.025 | 0.0020 | 0.55 | 1 | 0.2 | 0.045 | 7.47 |
| AuNP–MTX300 | 0.025 | 0.0030 | 0.55 | 1 | 0.3 | 0.045 | 7.65 |
| AuNP–MTX400 | 0.025 | 0.0040 | 0.55 | 1 | 0.4 | 0.045 | 7.72 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-González, B.; Rozalen, M.; Fernández-Perales, M.; Álvarez, M.A.; Sánchez-Polo, M. Methotrexate Gold Nanocarriers: Loading and Release Study: Its Activity in Colon and Lung Cancer Cells. Molecules 2020, 25, 6049. https://doi.org/10.3390/molecules25246049
Álvarez-González B, Rozalen M, Fernández-Perales M, Álvarez MA, Sánchez-Polo M. Methotrexate Gold Nanocarriers: Loading and Release Study: Its Activity in Colon and Lung Cancer Cells. Molecules. 2020; 25(24):6049. https://doi.org/10.3390/molecules25246049
Chicago/Turabian StyleÁlvarez-González, Beatriz, Marisa Rozalen, María Fernández-Perales, Miguel A. Álvarez, and Manuel Sánchez-Polo. 2020. "Methotrexate Gold Nanocarriers: Loading and Release Study: Its Activity in Colon and Lung Cancer Cells" Molecules 25, no. 24: 6049. https://doi.org/10.3390/molecules25246049






