Effect of CO2 Flow Rate on the Extraction of Astaxanthin and Fatty Acids from Haematococcus pluvialis Using Supercritical Fluid Technology
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microalgae Material and Characterization
2.2. Total Yield and Extracts Composition
2.3. Fatty Acids Composition Analysis
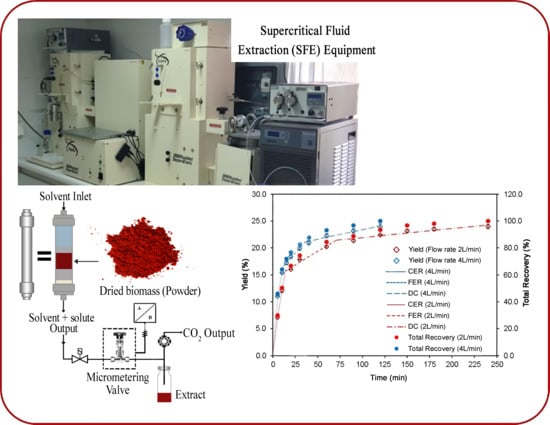
2.4. Kinetic Study
3. Material and Methods
3.1. Microalgae Material and Characterization
3.2. Supercritical Fluid Extraction (SFE)
3.3. Spline Model
3.4. Astaxanthin Quantification by High Performance Liquid Chromatography (HPLC)
3.5. Fatty Acids Composition Analysis by Gas Chromatography (GC)
3.6. Statistical Analysis of Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panis, G.; Rosales Carreon, J. Commercial astaxanthin production derived by Green alga Haematococcus pluvialis: A microalgae process model and techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, J.; Wang, J.; Liu, T. Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresour. Technol. 2014, 158, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Muhr, A.; Braunegg, G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–53. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M.T. Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: From lab to pilot scale. J. Clean. Prod. 2014, 61, 332–344. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A. High-value products from microalgae-their development and commercialization. J. Appl. Phycol. 2013, 23, 743–756. [Google Scholar] [CrossRef]
- Shah, M.R.; Liang, Y.; Chang, J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial Products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [Green Version]
- Brendler, T.; Williamson, E.M. Astaxanthin: How much is too much? A safety review. Phyther. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef]
- Osterlie, M.; Bjerkeng, B.; Liaaen-Jensen, S. Accumulation of astaxanthin all-E, 9Z and 13Z geometrical isomers and 3 and 3’ RS optical isomers in rainbow trout (Oncorhynchus mykiss) is selective. J. Nutr. 1999, 129, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, K.; Rawat, A.; Global Market Insights, Inc. Astaxanthin Market to exceed $800 mn by 2026. 2019. Available online: https://www.gminsights.com/pressrelease/astaxanthin-market (accessed on 12 November 2020).
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Attalage, R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: Astaxanthin from H. pluvialis and β-carotene from D. salina. Food Chem. 2019, 277, 128–134. [Google Scholar] [CrossRef]
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein and fatty acids from Haematococcus pluvialis Microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagen, C.; Siegmund, S.; Braune, W. Ultrastructural and chemical chances in the cell Wall of Haematococcus pluvialis (Volvocales Chlorophyta) during aplanospore formation. Eur. J. Phycol. 2011, 37, 217–226. [Google Scholar] [CrossRef]
- Mendiola, J.A.; Jaime, L.; Santoyo, S.; Reglero, G.; Cifuentes, A.; Ibañez, E.; Señoráns, F.J. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007, 102, 1357–1367. [Google Scholar] [CrossRef]
- Jokić, S.; Jerković, I.; Rajić, M.; Aladić, K.; Bilić, M.; Vidović, S. SC-CO2 extraction of Vitex agnus-castus L. fruits: The influence of pressure, temperature and water presoaking on the yield and GC–MS profiles of the extracts in comparison to the essential oil composition. J. Supercrit. Fluids 2017, 123, 50–57. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [Green Version]
- Careri, M.; Furlattini, L.; Mangia, A.; Musci, M.; Anklam, E.; Theobald, A.; von Holst, C. Supercritical fluid extraction for liquid chromatographic determination of carotenoids in Spirulina Pacifica algae: A chemometric approach. J. Chromatogr. A 2001, 912, 61–71. [Google Scholar] [CrossRef]
- Vardanega, R.; Nogueira, G.C.; Nascimento, C.D.O.; Faria-Machado, A.F.; Meireles, M.A.A. Selective extraction of bioactive compounds from annatto seeds by sequential supercritical CO2 process. J. Supercrit. Fluids 2019, 150, 122–127. [Google Scholar] [CrossRef]
- Sodeifian, G.; Ardestani, N.S.; Sajadian, S.A.; Moghadamian, K. Properties of Portulaca oleracea seed oil via supercritical fluid extraction: Experimental and optimization. J. Supercrit. Fluids 2018, 135, 34–44. [Google Scholar] [CrossRef]
- Casella, P.; Rimauro, J.; Iovine, A.; Mehariya, S.; Musmarra, D.; Molino, A. Characterization of Extracts from Haematococcus pluvialis Red Phase by using Accelerated Solvent Extraction. Chem. Eng. Trans. 2019, 74, 1417–1422. [Google Scholar] [CrossRef]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of astaxanthin from Haematococcus pluvialis using supercritical CO2 and ethanol as entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Valderrama, J.O.; Perrut, M.; Majewski, W. Extraction of astaxantine and phycocyanine from microalgae with supercritical carbon dioxide. J. Chem. Eng. Data 2003, 48, 827–830. [Google Scholar] [CrossRef]
- Pan, J.L.; Wang, H.M.; Chen, C.Y.; Chang, J.S. Extraction of astaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier. Eng. Life Sci. 2012, 12, 638–647. [Google Scholar] [CrossRef]
- Thana, P.; Machmudah, S.; Goto, M.; Sasaki, M.; Pavasant, P.; Shotipruk, A. Response surface methodology to supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2008, 99, 3110–3115. [Google Scholar] [CrossRef]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibañez, E.; del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Krichnavaruk, S.; Shotipruk, A.; Goto, M.; Pavasant, P. Supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis with vegetable oils as co-solvent. Bioresour. Technol. 2008, 99, 5556–5560. [Google Scholar] [CrossRef]
- Perrut, M. Supercritical fluid applications: Industrial developments and economic issues. Ind. Eng. Chem. Res. 2000, 39, 4531–4535. [Google Scholar] [CrossRef]
- Cheng, X.; Qi, Z.; Burdyny, T.; Kong, T.; Sinton, D. Low pressure supercritical CO2 extraction of astaxanthin from Haematococcus pluvialis demonstrated on a microfluidic chip. Bioresour. Technol. 2018, 250, 481–485. [Google Scholar] [CrossRef]
- Ma, N.; Long, X.; Liu, J.; Chang, G.; Deng, D.; Cheng, Y.; Wu, X. Defatted Haematococcus pluvialis meal can enhance the coloration of adult Chinese mitten crab Eriocheir sinensis. Aquaculture 2019, 510, 371–379. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of Astaxanthin and Lutein from Microalga Haematococcus pluvialis in the Red Phase Using CO2 Supercritical Fluid Extraction Technology with Ethanol as Co-Solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otero, P.; Saha, S.K.; Mc Gushin, J.; Moane, S.; Barron, J.; Murray, P. Identification of optimum fatty acid extraction methods for different microalgae Phaeodactylum tricornutum and Haematococcus pluvialis for food and biodiesel applications. Anal. Bioanal. Chem. 2017, 409, 4659–4667. [Google Scholar] [CrossRef] [PubMed]
- Cerón, M.C.; García-Malea, M.C.; Rivas, J.; Acien, F.G.; Fernández, J.M.; del Río, E.; Guerrero, M.G.; Molina, E. Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl. Microbiol. Biotechnol. 2007, 75, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Solana, M.; Rizza, C.S.; Bertucco, A. Exploiting microalgae as a source of essential fatty acids by supercritical fluid extraction of lipids: Comparison between Scenedesmus obliquus, Chlorella protothecoides and Nannochloropsis salina. J. Supercrit. Fluids 2014, 92, 311–318. [Google Scholar] [CrossRef]
- Reyes, F.A.; Sielfeld, C.S.; del Valle, J.M. Effect of high-pressure compaction on supercritical CO2 extraction of astaxanthin from Haematococcus pluvialis. J. Food Eng. 2016, 189, 123–134. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Uquiche, E.L. Particle size effects on supercritical CO2 extraction of oil-containing seeds. J. Am. Oil Chem. Soc. 2002, 79, 1261–1266. [Google Scholar] [CrossRef]
- Snyder, J.M.; Friederich, J.P.; Christianson, D.D. Effect of moisture and particle Size on the extractability of oils from seeds with supercritical CO2. J. Am. Oil Chem. Soc. 1984, 61, 1851–1856. [Google Scholar] [CrossRef]
- Priyanka, S.K. Influence of operating parameters on supercritical fluid extraction of essential oil from turmeric root. J. Clean. Prod. 2018, 188, 816–824. [Google Scholar] [CrossRef]
- Chang, L.; Jong, T.; Huang, H.; Nien, Y.; Chang, C.J. Supercritical carbon dioxide extraction of turmeric oil from Curcuma longa Linn. and purification of turmerones. Sep. Purif. Technol. 2006, 47, 119–125. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Tachaprutinun, A.; Udomsup, T.; Luadthong, C.; Wanichwecharungruang, S. Preventing the thermal degradation of astaxanthin through nanoencapsulation. Int. J. Pharm. 2009, 374, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Zhang, C.; Zhang, L.; Liu, J. Changes of carotenoids contents and analysis of astaxanthin geometrical isomerization in Haematococcus pluvialis under outdoor high light conditions. Aquac. Res. 2020, 51, 770–778. [Google Scholar] [CrossRef]
- Yuan, J.P.; Chen, F. Kinetics for the reversible isomerization reaction of trans-astaxanthin. Food Chem. 2001, 73, 131–137. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Das, U.N. Arachidonic acid in health and disease with focus on hypertension and diabetes mellitus: A review. J. Adv. Res. 2018, 11, 43–55. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Espinosa, C.; Paredes, A.; Palma, J.; Jaime, C.; Vílchez, C.; Cerezal, P. Determining the Potential of Haematococcus pluvialis Oleoresin as a Rich Source of Antioxidants. Molecules 2019, 24, 4073. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.B.; Gupta, A.K.; Fedacko, J.; Juneja, L.R.; Jarcuska, P.; Pella, D. Chapter 40: Effects of Diet and Nutrients on Epigenetic and Genetic Expressions. In The Role of Functional Food Security in Global Health; Singh, R.B., Watson, R.R., Takahashi, T., Eds.; Academic Press: London, UK; Elsevier-Inc.: Amsterdam, The Netherlands, 2019; pp. 681–708. ISBN 978-0-12-813148-0. [Google Scholar]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Bishop, K.S.; Ferguson, L.R. The Interaction between epigenetics, nutrition and the development of cancer. Nutrients 2015, 7, 922–947. [Google Scholar] [CrossRef] [Green Version]
- Mathers, J.C.; Strathdee, G.; Relton, C.L. Induction of epigenetic alterations by dietary and other environmental factors. Adv. Genet. 2010, 71, 3–39. [Google Scholar] [CrossRef]
- Del Valle, J.M.; de la Fuente, J.C.; Uquiche, E. A refined equation for predicting the solubility of vegetable oils in high-pressure CO2. J. Supercrit. Fluids 2012, 67, 60–70. [Google Scholar] [CrossRef]
- Fábryová, T.; Tůmová, L.; da Silva, D.C.; Pereira, D.M.; Andrade, P.B.; Valentão, P.; Hrouzek, P.; Kopecký, J.; Cheel, J. Isolation of astaxanthin monoesters from the microalgae Haematococcus pluvialis by high performance countercurrent chromatography (HPCCC) combined with high performance liquid chromatography (HPLC). Algal Res. 2020, 49, 101947. [Google Scholar] [CrossRef]
- Holtin, K.; Kuehnle, M.; Rehbein, J.; Schuler, P.; Nicholson, G.; Albert, K. Determination of astaxanthin and astaxanthin esters in the microalgae Haematococcus pluvialis by LC-(APCI)MS and characterization of predominant carotenoid isomers by NMR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Salinas, F.; Vardanega, R.; Espinosa-Álvarez, C.; Jiménez, D.; Bugueño-Muñoz, W.; Ruiz-Domínguez, M.C.; Meireles, M.A.A.; Cerezal-Mezquita, P. Supercritical fluid extraction of chañar oil: Global yield, kinetics and oil characterization. J. Supercrit. Fluids 2020, 161, 10484. [Google Scholar] [CrossRef]
- Meireles, M.A.A. Extraction of bioactive compounds from Latin American plants. In Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds; Martinez, J., Ed.; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 243–274. [Google Scholar]
- Jesús, S.P.; Calheiros, M.N.; Hense, H.; Meireles, M.A.A. A Simplified Model to Describe the Kinetic Behavior of Supercritical Fluid Extraction from a Rice Bran Oil Byproduct. Food Public. Health 2013, 3, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- ASAE Standards American. Method of Determining and Expressing Fineness of Feed Materials by Sieving; ANSI/ASAE S319.3; ASAE: St. Joseph, MI, USA, 2003. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC: Rockville, Maryland, 2012. [Google Scholar]
- Cerezal-Mezquita, P.; Barragan-Huerta, B.E.; Palma-Ramírez, J.C.; Ortíz-Hinojosa, C.P. Stability of astaxanthin in yogurt used to simulate apricot color, under refrigeration. Food Sci. Technol. 2014, 34, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.-P.; Chen, F. Chromatographic Separation and Purification of trans -Astaxanthin from the Extracts of Haematococcus pluvialis. J. Agric. Food Chem. 1998, 46, 3371–3375. [Google Scholar] [CrossRef]
- Britton, G. Example 9 Astaxanthin. In Carotenoids, Volume 1A: Isolation and Analysis; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Verlag: Basel, Switzerland, 1995; pp. 273–276. [Google Scholar]
- Medina-Pérez, E.; Ruiz-Domínguez, M.; Morales-Espinoza, J.; Cerezal-Mezquita, P. Evaluación del perfil de ácidos grasos de Isochrysis galbana mediante el uso de métodos ácidos y alcalinos de transesterificación. Inf. Técnico 2019, 83, 66–75. [Google Scholar] [CrossRef]







| Component | Content (%, d.b.) | ||
|---|---|---|---|
| This Study | Other Studies | ||
| [32] | [33] | ||
| Protein | 30.9 ± 0.4 | 27.0 | 25.7 |
| Lipids | 28.6 ± 0.3 | 39.7 | 2.6 |
| Ash | 13.0 ± 2.0 | 2.8 | 4.0 |
| Moisture | 3.8 ± 0.4 | 7.1 | 7.0 |
| Carbohydrates | 27.3 | 28.1 | 54.5 |
| SFE Condition | CO2 Density (g/mL) | Content (mg Astaxanthin/g Extract) | Yield (mg Astaxanthin/g Biomass) | Recovery (%) |
|---|---|---|---|---|
| 40 °C/40 MPa | 0.956 | 59 ± 3 c | 11.6 ± 0.4 c | 49.7 |
| 40 °C/50 MPa | 0.991 | 68 ± 5 b | 13.9 ± 0.8 b | 59.6 |
| 50 °C/40 MPa | 0.923 | 68 ± 7 b c | 13.2 ± 0.9 b | 56.4 |
| 50 °C/50 MPa | 0.962 | 84 ± 6 a | 19 ± 1 a | 80.0 |
| Fatty Acids | T (°C) | 40 | 50 | ||
|---|---|---|---|---|---|
| P (MPa) | 40 | 50 | 40 | 50 | |
| C14:0-Myristic acid | 2.83 ± 0.02 a | 2.9 ± 0.8 a | 3 ± 1 a | 2 ± 1 a | |
| C16:0-Palmitic acid | 95 ± 4 a | 92 ± 13 a | 110 ± 15 a | 96 ± 13 a | |
| C16:1-Palmitoleic acid | 3 ± 1 a | 2.8 ± 0.2 a | 3.5 ± 0.1 a | 2.9 ± 0.6 a | |
| C18:0-Stearic acid | 7.3 ± 0.7 a | 6.7 ± 0.3 a | 7 ± 2 a | 7.6 ± 0.5 a | |
| C18:1n9c-Oleic acid | 98 ± 2 a | 93 ± 17 a | 108 ± 15 a | 91 ± 15 a | |
| C18:2n6c-Linoleic acid (LA) | 132 ± 1 a | 126 ± 17 a | 145 ± 20 a | 131 ± 15 a | |
| C18:3n6-ɣ-Linolenic acid (GLA) | 2.7 ± 0.3 b | 2 ± 1 b c | 6 ± 1 a | 1.2 ± 0.6 c | |
| C18:3n3–α-Linolenic acid (ALA) | 155 ± 4 a | 147 ± 24 a | 170 ± 21 a | 149 ± 23 a | |
| C20:4n6-Arachidonic acid (ARA) | 6 ± 2 a | 5.0 ± 0.2 a | 9 ± 3 a | 5 ± 2 a | |
| C20:5n3-Eicosapentaenoic acid (EPA) | 5.2 ± 0.7 a | 4.4 ± 0.3 a | 5 ± 2 a | 4 ± 2 a | |
| SFA—Saturated Fatty acids | 105 ± 4 a | 101 ± 13 a | 121 ± 18 a | 106 ± 15 a | |
| MUFA-Monosaturated Fatty acids | 101.8 ± 0.7 a | 96 ± 17 a | 112 ± 15 a | 94 ± 16 a | |
| PUFA-Polyunsaturated Fatty acids | 300 ± 3 a | 285 ± 42 a | 336 ± 45 a | 290 ± 43 a | |
| Total FA (mg/g extract) | 507 ± 9 a | 482 ± 72 a | 568 ± 78 a | 490 ± 73 a | |
| Total FA Yield (% g/g biomass) | 10.0 ± 0.9 a | 9.9 ± 0.7 a | 11.4 ± 0.7 a | 10.7 ± 0.6 a | |
| Omega 6/Omega 3 | 0.86 | 0.87 | 0.88 | 0.89 | |
| Parameters | Stages of the Overall Extraction Curve | |||||
|---|---|---|---|---|---|---|
| CO2 Flow Rate (2 L/min) | CO2 Flow Rate (4 L/min) | |||||
| CER | FER | DC | CER | FER | DC | |
| Time (min.) | 13.71 | 70.98 | 240.0 | 11.56 | 36.57 | 120.0 |
| Accumulated extract (%) | 15.76 | 21.44 | 24.28 | 16.70 | 21.16 | 24.10 |
| Total extract recovery (%) | 55.68 | 86.08 | 100.0 | 66.57 | 86.09 | 100.0 |
| M (g extract/min) | 0.039 | 5.0 × 10−3 | 7.9 × 10−4 | 0.056 | 7.4 × 10−3 | 1.6 × 10−3 |
| Y (mg extract/g biomass) | 135.50 | 70.80 | 33.39 | 159.37 | 46.87 | 33.21 |
| Y (mg astaxanthin/g biomass) | 2.78 | 10.45 | 10.58 | 6.75 | 8.50 | 9.42 |
| Total Recovery of astaxanthin (%) | 11.91 | 56.72 | 100.0 | 28.95 | 65.43 | 100.0 |
| Y* (g extract/g CO2) | 1.1 × 10−2 | 1.4 × 10−3 | 2.2 × 10−4 | 7.7 × 10−3 | 1.0 × 10−3 | 2.2 × 10−4 |
| R2 | 0.9904 | 1.0000 | 1.0000 | 0.9436 | 1.0000 | 1.0000 |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa Álvarez, C.; Vardanega, R.; Salinas-Fuentes, F.; Palma Ramírez, J.; Bugueño Muñoz, W.; Jiménez-Rondón, D.; Meireles, M.A.A.; Cerezal Mezquita, P.; Ruiz-Domínguez, M.C. Effect of CO2 Flow Rate on the Extraction of Astaxanthin and Fatty Acids from Haematococcus pluvialis Using Supercritical Fluid Technology. Molecules 2020, 25, 6044. https://doi.org/10.3390/molecules25246044
Espinosa Álvarez C, Vardanega R, Salinas-Fuentes F, Palma Ramírez J, Bugueño Muñoz W, Jiménez-Rondón D, Meireles MAA, Cerezal Mezquita P, Ruiz-Domínguez MC. Effect of CO2 Flow Rate on the Extraction of Astaxanthin and Fatty Acids from Haematococcus pluvialis Using Supercritical Fluid Technology. Molecules. 2020; 25(24):6044. https://doi.org/10.3390/molecules25246044
Chicago/Turabian StyleEspinosa Álvarez, Carolina, Renata Vardanega, Francisca Salinas-Fuentes, Jenifer Palma Ramírez, Waldo Bugueño Muñoz, Diana Jiménez-Rondón, M. Angela A. Meireles, Pedro Cerezal Mezquita, and Mari Carmen Ruiz-Domínguez. 2020. "Effect of CO2 Flow Rate on the Extraction of Astaxanthin and Fatty Acids from Haematococcus pluvialis Using Supercritical Fluid Technology" Molecules 25, no. 24: 6044. https://doi.org/10.3390/molecules25246044