Self-Assembly of Low-Molecular-Weight Asymmetric Linear Triblock Terpolymers: How Low Can We Go?
Abstract
:1. Introduction
2. Materials and Methods
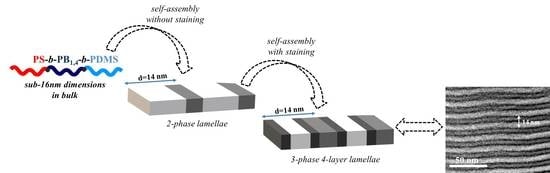
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bates, F.S.; Fredrickson, G.H. Block copolymer thermodynamics: Theory and experiment. Ann. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef]
- Bates, F.S. Polymer-polymer phase behavior. Science 1991, 251, 898–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Yoon, J.; Thomas, E.L. Enabling nanotechnology with self-assembled block copolymer patterns. Polymer 2003, 44, 6725–6760. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Gopinadhant, M.; Osuji, C.O. Directed self-assembly of block copolymer: A tutorial review of strategies for enabling nanotechnology with soft matter. Soft Matter 2014, 10, 3867–3889. [Google Scholar] [CrossRef] [PubMed]
- Borah, D.; Cummins, C.; Rasappa, S.; Senthmaraikannan, R.; Salaun, M.; Zelsmann, M.; Liontos, G.; Ntetsikas, K.; Avgeropoulos, A.; Morris, M.A. Nanopatterning via self-assembly of a lamellar-forming Polystyrene-block-Poly(dimethylsiloxane) diblock copolymer on topographical substrates fabricated by nanoimprint lithography. Nanomaterials 2018, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borah, D.; Rasappa, S.; Salaun, M.; Zelsmann, M.; Lorret, O.; Liontos, G.; Ntetsikas, K.; Avgeropoulos, A.; Morris, M.A. Soft graphoepitaxy for large area directed self-assembly of Polystyrene-block-Poly(dimethylsiloxane) block copolymer on nanopatterned POSS Substrates facricated by nanoimprint lithography. Adv. Funct. Mater. 2015, 25, 3425–3432. [Google Scholar] [CrossRef]
- Choi, P.; Fu, P.-F.; Guo, L.J. Siloxane Copolymers for Nanoimprint Lithography. Adv. Funct. Mater. 2007, 17, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.S.; Ross, C.A. Orientation-controlled self-assembled nanolithography using a polystyrene-polydimethylsiloxane block copolymer. Nano Lett. 2007, 7, 2046–2050. [Google Scholar] [CrossRef]
- Tu, K.-H.; Bai, W.; Liontos, G.; Ntetsikas, K.; Avgeropoulos, A.; Ross, C.A. Universal pattern transfer methods for metal nanostructures by block copolymer lithography. Nanotechnology 2015, 26, 375301. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.-C.; Wang, T.-C.; Ho, R.-M.; Georgopanos, P.; Avgeropoulos, A.; Thomas, E.L. Robust block copolymer mask for nanopatterning polymer films. ACS Nano 2010, 4, 2088–2094. [Google Scholar] [CrossRef]
- Chao, C.-C.; Ho, R.-M.; Georgopanos, P.; Avgeropoulos, A.; Thomas, E.L. Silicon oxy carbide nanorings from polystyrene-b-polydimethylsiloxanediblock copolymer thin films. Soft Matter 2010, 6, 3582–3587. [Google Scholar] [CrossRef]
- Tavakkoli, A. Templating three-dimensional self-assembled structures in bilayer block copolymer films. Science 2012, 336, 1294–1298. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.-Y.; Dehghan, A.; Georgopanos, P.; Avgeropoulos, A.; Shi, A.-C.; Ho, R.-M. Orienting block copolymer thin films via entropy. Macromolecules 2016, 49, 624–633. [Google Scholar] [CrossRef]
- Lee, K.; Kreider, M.; Bai, W.; Cheng, L.-C.; Dinachali, S.S.; Tu, K.-H.; Huang, T.; Ntetsikas, K.; Liontos, G.; Avgeropoulos, A.; et al. UV-solvent annealing od PDMS-majority and PS-minority PS-b-PDMS block copolymer films. Nanotechnology 2016, 27, 465301. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.A.; Hillmyer, M.A. Nanoporous membranes derived from block copolymers: From drug delivery to water filtration. ACS Nano 2010, 4, 3548–3553. [Google Scholar] [CrossRef]
- Zhang, Y.; Mulvenna, R.A.; Qu, S.; Boudouris, B.W.; Phillip, W.A. Block polymer membranes functionalized with nanoconfinement polyelectrolyte brushes achieve sub-nanometer selectivity. ACS Macro Lett. 2017, 6, 726–732. [Google Scholar] [CrossRef]
- Ahn, H.; Park, S.; Kim, S.-W.; Yoo, P.J.; Ryu, D.Y.; Russell, T.P. Nanoporous block copolymer membranes for ultrafiltration: A simple approach to size tunability. ACS Nano 2014, 8, 11745–11752. [Google Scholar] [CrossRef]
- Chang, A.B.; Bates, F.S. The ABCs of block polymers. Macromolecules 2020, 53, 2765–2768. [Google Scholar] [CrossRef]
- Gido, S.P.; Schwark, D.W.; Thomas, E.L.; Goncalves, M.D. Observation of a non-constant mean curvature interface in an ABC Triblock copolymer. Macromolecules 1993, 26, 2636–2640. [Google Scholar] [CrossRef]
- Mogi, Y.; Kotsuji, H.; Kaneko, Y.; Mori, K.; Matsushita, Y.; Noda, I. Preparation and morphology of Triblock copolymers of the ABC Type. Macromolecules 1992, 25, 5408–5411. [Google Scholar] [CrossRef]
- Mogi, Y.; Mori, K.; Matsushita, Y.; Noda, I. Tricontinuous morphology of Triblock copolymers of the ABC Type. Macromolecules 1992, 25, 5412–5415. [Google Scholar] [CrossRef]
- Mogi, Y.; Nomura, M.; Kotsuji, H.; Ohnishi, K.; Matsushita, Y.; Noda, I. Superlattice structures in morphologies of the ABC Triblock copolymers. Macromolecules 1994, 27, 6755–6760. [Google Scholar] [CrossRef]
- Arai, K.; Kotaka, T.; Kitano, Y.; Yoshimura, K. Poly(styrene-b-butadiene-b-4-vinylpyridine) three block polymers. Synthesis, characterization, morphology, and mechanical properties. Macromolecules 1980, 13, 1670–1678. [Google Scholar] [CrossRef]
- Arai, K.; Kotaka, T.; Kitano, Y.; Yoshimura, K. Synthesis and morphological behavior of a new ABC three-block polymer. Macromolecules 1980, 13, 455–457. [Google Scholar] [CrossRef]
- Matsushita, Y.; Yamada, K.; Hattori, T.; Fujimoto, T.; Sawada, Y.; Nasagawa, M.; Matsui, C. Morphologies of ABC-Type Triblock copolymers with different compositions. Macromolecules 1983, 16, 10–13. [Google Scholar] [CrossRef]
- Kudose, I.; Kotaka, T. Morphological and Viscoelastic Properties of Poly(styrene-b-butadiene-b-4-vinylpyridine) three block polymers of the ABC Type. Macromolecules 1984, 17, 2325–2332. [Google Scholar] [CrossRef]
- Mogi, Y.; Mori, K.; Kotsuji, H.; Matsushita, Y.; Noda, I. Molecular weight dependence of the lamellar domain spacing of ABC Triblock copolymers and their chain conformation in lamellar domains. Macromolecules 1993, 26, 5169–5173. [Google Scholar] [CrossRef]
- Watanabe, H.; Shimura, T.; Kotaka, T.; Tirrell, M. Synthesis, characterization, and surface structures of styrene-2-vinylpyridine-butadiene three-block polymers. Macromolecules 1993, 26, 6338–6345. [Google Scholar] [CrossRef]
- Matsushita, Y.; Tamura, M.; Noda, I. Tricontinuous double-diamond structure formed by a Styrene-Isoprene-2-Vinylpyridine Triblock copolymer. Macromolecules 1994, 27, 3680–3682. [Google Scholar] [CrossRef]
- Auschra, C.; Stadler, R. New ordered morphologies in ABC triblock copolymers. Macromolecules 1993, 26, 2171–2174. [Google Scholar] [CrossRef]
- Stadler, R.; Auschra, C.; Beckmann, J.; Krappe, U.; Voight-Martin, I.; Leibler, L. Morphology and Thermodynamics of symmetric Poly(A-block-B-block-C) Triblock copolymers. Macromolecules 1995, 28, 3080–3097. [Google Scholar] [CrossRef]
- Krappe, U.; Stadler, R.; Voight-Martin, I. Chiral Assembly in Amorphous ABC Triblock Copolymers. Formation of a Helical Morphology in Polystyrene-block-polybutadiene-block-poly(methyl methacrylate) Block Copolymers. Macromolecules 1995, 28, 4558–4561. [Google Scholar] [CrossRef]
- Balsamo, V.; von Gyldenfeldt, F.; Stadler, R. Thermal Behavior and spherulitic superstructures of SBC triblock copolymers based on polystyrene (S), polybutadiene (B) and a crystallizable poly(ε-caprolactone) (C) block. Macromol. Chem. Phys. 1996, 197, 3317–3341. [Google Scholar] [CrossRef]
- Breiner, U.; Krappe, U.; Stadler, R. Evolution of the “knitting pattern” morphology in ABC triblock copolymers. Macromol. Rapid Commun. 1996, 17, 567–575. [Google Scholar] [CrossRef]
- Jung, K.; Abetz, V.; Stadler, R. Thermodynamically Controlled Morphological Disorder in a Microphase-Separated Cylindrical Block Copolymer. Macromolecules 1996, 29, 1076–1078. [Google Scholar] [CrossRef]
- Breiner, U.; Krappe, U.; Abetz, V.; Stadler, R. Cylindrical morphologies in asymmetric ABC triblock copolymers. Macromol. Chem. Phys. 1997, 198, 1051–1083. [Google Scholar] [CrossRef]
- Giebeler, E.; Stadler, R. ABC triblock polyampholytes containing a neutral hydrophobic block, a polyacid and a polybase. Macromol. Chem. Phys. 1997, 198, 3815–3825. [Google Scholar] [CrossRef]
- Breiner, U.; Krappe, U.; Jakob, T.; Abetz, V.; Stadler, R. Spheres on spheres—A novel spherical multiphase morphology in polystyrene-block-polybutadiene-block-poly(methyl methacrylate) triblock copolymers. Polym. Bull. 1998, 40, 219–226. [Google Scholar] [CrossRef]
- Neumann, C.; Abetz, V.; Stadler, R. Phase behavior of ABC-triblock copolymers with two inherently miscible blocks. Colloid Polym. Sci. 1998, 276, 19–27. [Google Scholar] [CrossRef]
- Brinkmann, S.; Stadler, R.; Thomas, E.L. New structural Motif in Hexagonally Ordered Cylindrical Ternary (ABC) Block Copolymer Microdomains. Macromolecules 1998, 31, 6566–6572. [Google Scholar] [CrossRef]
- Breiner, U.; Krappe, U.; Thomas, E.L.; Stadler, R. Structural characterization of the “Knitting Pattern” in Polystyrene-block-poly(ethylene-co-butylene)-block-poly(methyl methacrylate) triblock copolymers. Macromolecules 1998, 31, 135–141. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hasegawa, H.; Hashimoto, T.; Ribbe, A.; Sugiyama, K.; Hirao, A.; Nakayama, S. A Study of Three-phase structures in ABC Triblock copolymers. Polym. J. 1999, 31, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Shefelbine, T.A.; Vigild, M.E.; Matsen, M.W.; Hadjuk, D.A.; Hillmyer, M.A.; Cussler, E.L.; Bates, F.S. Core-Shell gyroid morphology in a Poly(isoprene-block-styrene-block-dimethylsiloxane) Triblock copolymer. J. Am. Chem. Soc. 1999, 121, 8457–8465. [Google Scholar] [CrossRef]
- Huckstadt, H.; Gopfert, A.; Abetz, V. Influence of the block sequence on the morphological behavior of ABC triblock copolymers. Polymer 2000, 41, 9089–9094. [Google Scholar] [CrossRef]
- Bellas, V.; Iatrou, H.; Hadjichristidis, N. Controlled Anionic Polymerization of Hexamethylcyclotrisiloxane. Model Linear and Miktoarm Star Co- and Terpolymers of Dimethylsiloxane with Styrene and Isoprene. Macromolecules 2000, 33, 6993–6997. [Google Scholar] [CrossRef]
- Hückstädt, H.; Goldacker, T.; Göpfert, A.; Abetz, V. Core-Shell Double Gyroid Morphologies in ABC Triblock Copolymers with Different Chain Topologies. Macromolecules 2000, 33, 3757–3761. [Google Scholar] [CrossRef]
- Bailey, T.S.; Pham, H.D.; Bates, F.S. Morphological behavior bridging the symmetric AB and ABC States in the Poly(styrene-b-isoprene-b-ethylene oxide) TriblockCopolymer System. Macromolecules 2001, 34, 6994–7008. [Google Scholar] [CrossRef]
- Ott, H.; Abetz, V.; Altstädt, V. Morphological Studies of Poly(styrene)-block-poly(ethylene-co-butylene)-block-poly(methyl methacrylate) in a composition region of the “Knitting Pattern” morphology. Macromolecules 2001, 34, 2121–2128. [Google Scholar] [CrossRef]
- Bailey, T.; Hardy, C.; Epps, T.H.; Bates, F.S. A Noncubic Triply Periodic Network Morphology in Poly(isoprene-b-styrene-b-ethylene oxide) Triblock Copolymers. Macromolecules 2002, 35, 7007–7017. [Google Scholar] [CrossRef]
- Yamauchi, K.; Hasegawa, H.; Hashimoto, T.; Köhler, N.; Knoll, K. Synthesis and morphological studies of polyisoprene-block-polystyrene-block-poly(vinyl methyl ether) triblock terpolymer. Polymer 2002, 43, 3563–3570. [Google Scholar] [CrossRef]
- Avgeropoulos, A.; Paraskeva, S.; Hadjichristidis, N.; Thomas, E.L. Synthesis and Microphase Separation of Linear Triblock Terpolymers of Polystyrene, High 1,4-Polybutadiene, and High 3,4-Polyisoprene. Macromolecules 2002, 35, 4030–4035. [Google Scholar] [CrossRef]
- Ludwigs, S.; Böker, A.; Abetz, V.; Müller, A.H.E.; Krausch, G. Phase behavior of linear polystyrene-block-poly(2-vinylpyridine)-block-poly(tert-butyl methacrylate) triblock terpolymers. Polymer 2003, 44, 6815–6823. [Google Scholar] [CrossRef]
- Epps, T.H., III; Cochran, E.W.; Bailey, T.S.; Waletzko, R.S.; Hardy, C.M.; Bates, F.S. Ordered network phases in linear Poly(isoprene-b-styrene-b-ethylene oxide) Triblock Copolymers. Macromolecules 2004, 37, 8325–8341. [Google Scholar] [CrossRef]
- Epps, T.H., III; Bates, F.S. Effect of molecular weight on network formation in linear ABC Triblock copolymers. Macromolecules 2006, 39, 2676–2682. [Google Scholar] [CrossRef]
- Kloninger, C.; Rehahn, M. Styrene-ferrocenyldimethylsilane-methyl methacrylate Triblock copolymers: Synthesis and phase morphology. Macromol. Chem. Phys. 2007, 208, 833–840. [Google Scholar] [CrossRef]
- Jinnai, H.; Kaneko, T.; Matsunaga, K.; Abetz, C.; Abetz, V. A double helical structure formed from an amorphous, achiral ABC triblock terpolymer. Soft Matter 2009, 5, 2042–2046. [Google Scholar] [CrossRef]
- Chuang, V.P.; Gwyther, J.; Mickiewicz, R.A.; Manners, I.; Ross, C.A. Templated Self-Assembly of Square Symmetry Arrays from an ABC Triblock Terpolymer. Nano Lett. 2009, 9, 4364–4369. [Google Scholar] [CrossRef]
- Tureau, M.S.; Epps, T.H., III. Nanoscale networks in Poly[isoprene-block-styrene-block-(methyl methacrylate)] Triblock copolymers. Macromol. Rapid Commun. 2009, 30, 1751–1755. [Google Scholar] [CrossRef]
- Schacher, F.; Yuan, J.; Schoberth, H.G.; Müller, A.H.E. Synthesis, characterization, and bulk crosslinking of polybutadiene-block-poly(2-vinyl pyridine)-block-poly(tert-butyl methacrylate) block terpolymers. Polymer 2010, 51, 2021–2032. [Google Scholar] [CrossRef]
- Matsushita, Y.; Suzuki, J.; Izumi, Y.; Matsuoka, K.; Takahashi, S.; Aoyama, Y.; Mihira, T.; Takano, A. Formation of undulated lamellar structure from ABC block terpolymer blends with different chain lengths. J. Chem. Phys. 2010, 133, 194901. [Google Scholar] [CrossRef]
- Phillip, W.A.; Dorin, R.M.; Werner, J.; Hoek, E.M.V.; Wiesner, U.; Elimelech, M. Tuning structure and properties of graded triblock terpolymer-based mesoporous and hybrid films. Nano Lett. 2011, 11, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Schacher, F.H.; Sugimori, H.; Hong, S.; Jinnai, H.; Müller, A.H.E. Tetragonally Perforated Lamellae of Polybutadiene-block-poly(2-vinylpyridine)-block-poly(tert-butyl methacrylate) (BVT) Triblock Terpolymers in the Bulk: Preparation, Cross-Linking, and Dissolution. Macromolecules 2012, 45, 7956–7963. [Google Scholar] [CrossRef]
- Kumar, R.; Sides, S.W.; Goswami, M.; Sumpter, B.G.; Hong, K.; Wu, X.; Russell, T.P.; Gido, S.P.; Misichronis, K.; Rangou, S.; et al. Morphologies of ABC Triblock terpolymer melts containing Poly(Cyclohexadiene): Effects of conformational asymmetry. Lagmuir 2013, 29, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Löbling, T.I.; Hiekkataipale, P.; Hanisch, A.; Bennet, F.; Schmalz, H.; Ikkala, O.; Gröschel, A.H.; Müller, A.H.E. Bulk morphologies of polystyrene-block-polybutadiene-block-poly(tert-butyl methacrylate) triblock terpolymers. Polymer 2015, 72, 479–489. [Google Scholar] [CrossRef]
- Zapsas, G.; Moschovas, D.; Ntetsikas, K.; Rangou, S.; Lee, J.-H.; Thomas, E.L.; Zafeiropoulos, N.E.; Avgeropoulos, A. Immiscible polydiene blocks in linear copolymer and terpolymer sequences. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 1238–1246. [Google Scholar] [CrossRef]
- Asai, Y.; Takano, A.; Matsushita, Y. Creation of cylindrical morphologies with extremely large oblong unit lattices from ABC block terpolymer blends. Macromolecules 2015, 48, 1538–1542. [Google Scholar] [CrossRef]
- Asai, Y.; Takano, A.; Matsushita, Y. Asymmetric double tetragonal domain packing from ABC Triblock Terpolymer blends with chain length difference. Macromolecules 2016, 49, 6940–6946. [Google Scholar] [CrossRef]
- Ntaras, C.; Polymeropoulos, G.; Zapsas, G.; Ntetsikas, K.; Liontos, G.; Karanastasis, A.; Moschovas, D.; Rangou, S.; Stewart-Sloan, C.; Hadjichristidis, N.; et al. Synthesis, characterization and self-assembly of well-defined linear heptablockquaterpolymers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1443–1449. [Google Scholar] [CrossRef]
- Asai, Y.; Suzuki, J.; Aoyama, Y.; Nishioka, H.; Takano, A.; Matsushita, Y. Tricontinuous double diamond network structure from binary blends of ABC Triblock Terpolymers. Macromolecules 2017, 50, 5402–5411. [Google Scholar] [CrossRef]
- Ahn, S.; Kwak, J.; Choi, C.; Seo, Y.; Kim, J.K. Gyroid structures at highly asymmetric volume fractions by blending of ABC Triblock terpolymer and AB diblock copolymer. Macromolecules 2017, 50, 9008–9014. [Google Scholar] [CrossRef]
- Musteata, V.; Sutisna, B.; Polymeropoulos, G.; Avgeropoulos, A.; Meneau, F.; Peinermann, K.-V.; Hadjichristidis, N.; Nunes, S.P. Self-assembly of polystyrene-b-poly(2-vinylpyridine)-b-poly(ethylene oxide) triblock terpolymers. Eur. Polym. J. 2018, 100, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Haenelt, T.G.; Abetz, C.; Abetz, V. Morphological control over three- and four-phase superstructures in blends of asymmetric ABC and BAC Triblock Terpolymers. Macromol. Chem. Phys. 2018, 219, 1800383. [Google Scholar] [CrossRef]
- Haenelt, T.G.; Meyer, A.; Abetz, C.; Abetz, V. Planet-like nanostructures formed by an ABC Triblock Terpolymer. Macromol. Chem. Phys. 2019, 220, 1900297. [Google Scholar] [CrossRef]
- Steinhaus, A.; Chakroun, R.; Müllner, M.; Nghiem, T.-L.; Hilderbrandt, M.; Gröschel, A.H. Confinement Assembly of ABC TriblockTerpolymers for the High -Yield Synthesis of Janus Nanorings. ACS Nano 2019, 13, 6269–6278. [Google Scholar] [CrossRef] [Green Version]
- Hadjichristidis, N.; Iatrou, H.; Pispas, S.; Pitsikalis, M. Anionic polymerization: High vacuum techniques. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 3211–3234. [Google Scholar] [CrossRef]
- Uhrig, D.; Mays, J.W. Experimental Techniques in High-Vacuum Anionic Polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6179–6222. [Google Scholar] [CrossRef]
- Moschovas, D.; Manesi, G.-M.; Karydis-Messinis, A.; Zapsas, G.; Ntetsikas, K.; Zafeiropoulos, N.E.; Piryazev, A.; Thomas, E.L.; Hadjichristidis, N.; Ivanov, D.A.; et al. Alternating gyroid network structure in an abc miktoarm terpolymer comprised of polystyrene and two polydienes. Nanomaterials 2020, 10, 1497. [Google Scholar] [CrossRef]
- Fox, G.T.; Flory, P.J. The glass temperature and related properties of polystyrene. Influence of molecular weight. J. Polym. Sci. 1954, 75, 315–319. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; McEven, I.N. Molecular motions in poly(dimethyl siloxane) oligomers and polymers. Polymer 1973, 14, 423–426. [Google Scholar] [CrossRef]
- Hintermeyer, J.; Herrmann, A.; Kahlau, R.; Goiceanu, C.; Rössler, E.A. Molecular weight dependence of glassy dynamics in linear polymers revisited. Macromolecules 2008, 41, 9335–9344. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block copolymers—Designer of soft materials. Physics Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Politakos, N.; Ntoukas, E.; Avgeropoulos, A.; Krikorian, V.; Pate, B.D.; Thomas, E.L.; Hill, R.M. Strongly segregated cubic microdomain morphology consistent with the double gyroid phase in high molecular weight diblock copolymers of polystyrene and poly(dimethylsiloxane). J. Polym. Sci. Part B Polym. Phys. 2009, 47, 2419–2427. [Google Scholar] [CrossRef]
- Goldacker, T.; Abetz, V. Core-Shell cylinders and core-shell gyroid morphologies via blending of lamellar ABC Triblock and BC diblock copolymers. Macromolecules 1999, 32, 5165–5167. [Google Scholar] [CrossRef]
- Hahn, T. International tables for X-ray crystallography. In Volume A: Space Group Symmetry, 5th ed.; Springer: New York, NY, USA, 2006; p. 94. [Google Scholar]
- Sinturel, C.; Bates, F.S.; Hillmyer, M.A. High χ-Low N block polymers: How far can we go? ACS Macro Lett. 2015, 4, 1044–1050. [Google Scholar] [CrossRef]




| No. | Block Sequence | Molecular Weight Range (kg/mol) | Annealing | Observed Morphology | References |
|---|---|---|---|---|---|
| 1 | PS-b-PB1,4-b-P4VP | 74–113 | 60 °C, 14 h | Ball in Box, LAM | [23,24] |
| 2 | PS-b-PA-b-PI | 131–198 | n/a | LAM, ChC | [25] |
| 3 | PS-b-PB-b-P4VP | 65–210 | n/a | Ball in Box, LAM | [26] |
| 4 | PI-b-PS-b-P2VP | 36–279 | 120 °C, 10 days | LAM, OTDD, CYL, SPH | [20,21,22,27] |
| 5 | PS-b-PI-b-P2VP | 43 | 120 °C, 7 days | LAM, ChC | [19] |
| 6 | PS-b-P2VP-b-PB | 111–258 | n/a | n/a | [28] |
| 7 | PS-b-PI-b-P2VP | 196–201 | 120 °C, 7 days | OTDD, LAM | [29] |
| 8 | PS-b-PB1,2-b-PMMA and PS-b-PEB-b-PMMA | 225–245 and 226–248 | 85 °C, 2 days 150 °C, 2 h/4 h/6 h and 160 °C, 5 days | LAM, CR, LC, LS | [30,31] |
| 9 | PS-b-PB1,2-b-PMMA | 206–218 | 100 °C, 2 days 170 °C, 2 h/4 h/6 | ChC, CaC, HEL | [32] |
| 10 | PS-b-PB1,2-b-PCL | 105–137 | n/a | n/a | [33] |
| 11 | PS-b-PB1,2-b-PMMA and PS-b-PEB-b-PMMA | 117–245 | 160 °C, 4 h | KP, LAM, LC | [34] |
| 12 | PB1,2-b-PS-b-PMMA | 192 | 100 °C, 2 days 170 °C, 6 h | CYLT | [35] |
| 13 | PS-b-PB1,2-b-PMMA and PS-b-PEB-b-PMMA | 78–140 and 80–124 | 185 °C, 6 h and 185 °C, 2 h/4 h/6 h | ChC, HEL, CaC, uCiC, SoC | [36] |
| 14 | PS-b-PVP-b-PtBMA | 293 | n/a | LAM | [37] |
| 15 | PS-b-PB1,2-b-PMMA and PS-b-PEB-b-PMMA | 88–241 and 90–242 | 170 °C, 10 days | SoS | [38] |
| 16 | PI1,4-b-PB1,2-b-PS | 48–74 | 60 °C, 2 days 120 °C, 3 h | two-phase morphology | [39] |
| 17 | PS-b-PB1,2-b-PMMA | 215 | 120 °C, 3–5 days | LAM, HPC | [40] |
| 18 | PS-b-PB1,2-b-PMMA and PS-b-PEB-b-PMMA | 121 and 122 | 185 °C, 2–6 h | LAM, KP | [41] |
| 19 | PH-b-P2T-b-PF | 61 | n/a | LAM | [42] |
| 20 | PI1,4-b-PS-b-PDMS | 41 | n/a | CSG | [43] |
| 21 | PS-b-PB1,2-b-P2VP and PB1,2-b-PS-b-P2VP | 62–137 and 84–210 | 150 °C, 6 h | LAM, ChC, CSG | [44] |
| 22 | PS-b-PI-b-PDMS | 59 | n/a | n/a | [45] |
| 23 | PS-b-PB1,2-b-P2VP | 71 | 150 °C, 6 h | CSG | [46] |
| 24 | PS-b-PI-b-PEO | 19–30 | 80–225 °C, 0.5 h–120 h | LAM, ChC, CSG, PLS, SPL | [47] |
| 25 | PS-b-PEB-b-PMMA | 73–123 | 170 °C, 6 h | LAM, LC, KP | [48] |
| 26 | PI-b-PS-b-PEO | 13–22 | n/a | LAM, Fddd (O70) | [49] |
| 27 | PI-b-PS-b-PVME | 32 | n/a | LAM | [50] |
| 28 | PB1,4-b-PS-b-PI3,4 and PS-b-PB1,4-b-PI3,4 | 85–149 and 133 | 130 °C, 7 days | LAM, HPC | [51] |
| 29 | PS-b-P2VP-b-PtBMA | 76–140 | n/a | ChC, CSG, LAM, PL, UL | [52] |
| 30 | PI-b-PS-b-PEO | 13–25 | n/a | Q230, LAM, O70, Q214 | [53] |
| 31 | PI-b-PS-b-PEO | 19–43 | n/a | LAM, O70 | [54] |
| 32 | PS-b-PFSa-b-PMMA | 101–110 | 120 °C, 2 h 180 °C, 36 h | HEL/SoC, SoS | [55] |
| 33 | PS-b-PB-b-PMMA | 170 | 100 °C, 1 day 170 °C, 1 day | dHEL | [56] |
| 34 | PI-b-PS-b-PFSb | 82 | 150 °C, 4 days | CYLT | [57] |
| 35 | PS-b-PI-b-PMMA | 13.5–31 | n/a | LAM, Q214 | [58] |
| 36 | PB-b-P2VP-b-PtBMA | 61–165 | 50 °C, 24 h 130 °C | LAM/CSG, ChC, LAM, SoC, HELoC | [59] |
| 37 | PI-b-PS-b-P2VP | 26–150 | 150 °C, 7 days | LAM, UL | [60] |
| 38 | PI-b-PS-b-P4VP | 77 | n/a | HPC | [61] |
| 39 | PB-b-P2VP-b-PtBMA | 110 | n/a | TPL | [62] |
| 40 | PS-b-PB1,2-b-PCHD1,4 and PB1,2-b-PS-b-PCHD1,4 | 29–32 and 39 | 110 °C, 7 days | LAM, ChC | [63] |
| 41 | PS-b-PB-b-PtBMA | 57–148 | n/a | ChC, CSG, LAM, LC | [64] |
| 42 | PS-b-PB1,4-b-PI3,4 | 80–103 | 130 °C, 7 days 150 °C, 5 days | LAM, LAM/CSG | [65] |
| 43 | PI-b-PS-b-P2VP | 122–124 | 150 °C, 5 days | GS, LC | [66] |
| 44 | PI-b-PS-b-P2VP | 223–264 | 150 °C, 5 days | LS, HPC | [67] |
| 45 | PS-b-PB1,4-b-PI3,4 | 35–43 | 120 °C, 5 days | LAM | [68] |
| 46 | PI-b-PS-b-P2VP | 136–146 | 150 °C, 5 days | LAM | [69] |
| 47 | PI-b-PS-b-P2VP | 84 | 240 °C, 3 h | SPH/CYL | [70] |
| 48 | PS-b-P2VP-b-PEO | 32–161 | 130 °C, 5 days | LAM, HPC | [71] |
| 49 | PS-b-PI-b-PMMA and PI-b-PS-b-PMMA | 171 and 95–318 | 80 °C, 2 days 150 °C, 6 h | HELoC, CSG | [72] |
| 50 | PS-b-PI-b-PMMA | 171–324 | 80 °C, 2 days 150 °C, 6 h | ‘planetlike’, HELoC, SoC | [73] |
| 51 | PS-b-PB-b-PMMA | 74–202 | n/a | LAM, LS, LC | [74] |
| Sample | A-b-B-b-C | (g/mol) | (g/mol) | (g/mol) | (g/mol) | ĐSEC (b) | fA(c) | fB(c) | fC(c) | PB1,4 (c) (%) | PB1,2 (c) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PS-b-PB1,4-b-PDMS | 5.200 | 1.300 | 4.500 | 11.000 | 1.04 | 0.52 | 0.09 | 0.39 | 90 | 10 |
| 2 | 6.300 | 1.700 | 5.100 | 13.100 | 1.04 | 0.48 | 0.10 | 0.42 | 89 | 11 | |
| 3 | PB1,4-b-PS-b-PDMS | 1.900 | 6.100 | 6.000 | 14.000 | 1.04 | 0.11 | 0.42 | 0.47 | 93 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miskaki, C.; Moutsios, I.; Manesi, G.-M.; Artopoiadis, K.; Chang, C.-Y.; Bersenev, E.A.; Moschovas, D.; Ivanov, D.A.; Ho, R.-M.; Avgeropoulos, A. Self-Assembly of Low-Molecular-Weight Asymmetric Linear Triblock Terpolymers: How Low Can We Go? Molecules 2020, 25, 5527. https://doi.org/10.3390/molecules25235527
Miskaki C, Moutsios I, Manesi G-M, Artopoiadis K, Chang C-Y, Bersenev EA, Moschovas D, Ivanov DA, Ho R-M, Avgeropoulos A. Self-Assembly of Low-Molecular-Weight Asymmetric Linear Triblock Terpolymers: How Low Can We Go? Molecules. 2020; 25(23):5527. https://doi.org/10.3390/molecules25235527
Chicago/Turabian StyleMiskaki, Christina, Ioannis Moutsios, Gkreti-Maria Manesi, Konstantinos Artopoiadis, Cheng-Yen Chang, Egor A. Bersenev, Dimitrios Moschovas, Dimitri A. Ivanov, Rong-Ming Ho, and Apostolos Avgeropoulos. 2020. "Self-Assembly of Low-Molecular-Weight Asymmetric Linear Triblock Terpolymers: How Low Can We Go?" Molecules 25, no. 23: 5527. https://doi.org/10.3390/molecules25235527