Gamma Aminobutyric Acid-Enriched Fermented Oyster (Crassostrea gigas) Increases the Length of the Growth Plate on the Proximal Tibia Bone in Sprague-Dawley Rats
Abstract
:1. Introduction
2. Results
2.1. Fermented Oyster (FO) Administration Has No Toxicity
2.2. FO Increased the Expression of Hepatic Insulin-Like Growth Factor-1 (IGF-1) and Insulin-Like Growth Factor Binding Protein-3 (IGFBP-3)
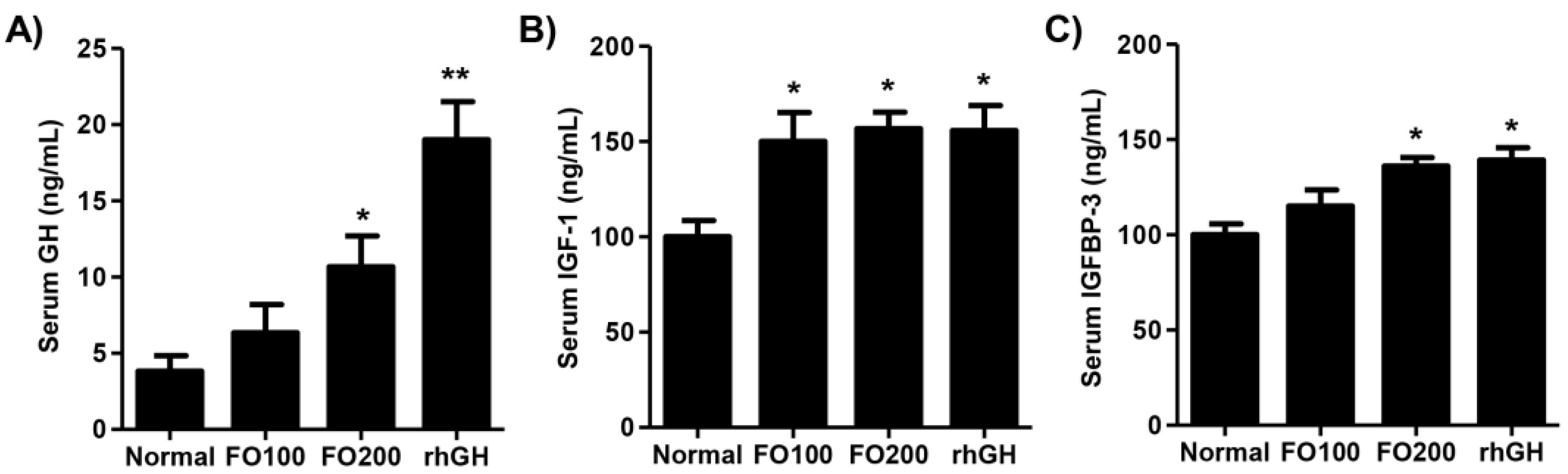
2.3. FO Induced Circulating Levels of Growth Hormone (GH), IGF-1, and IGFBP-3
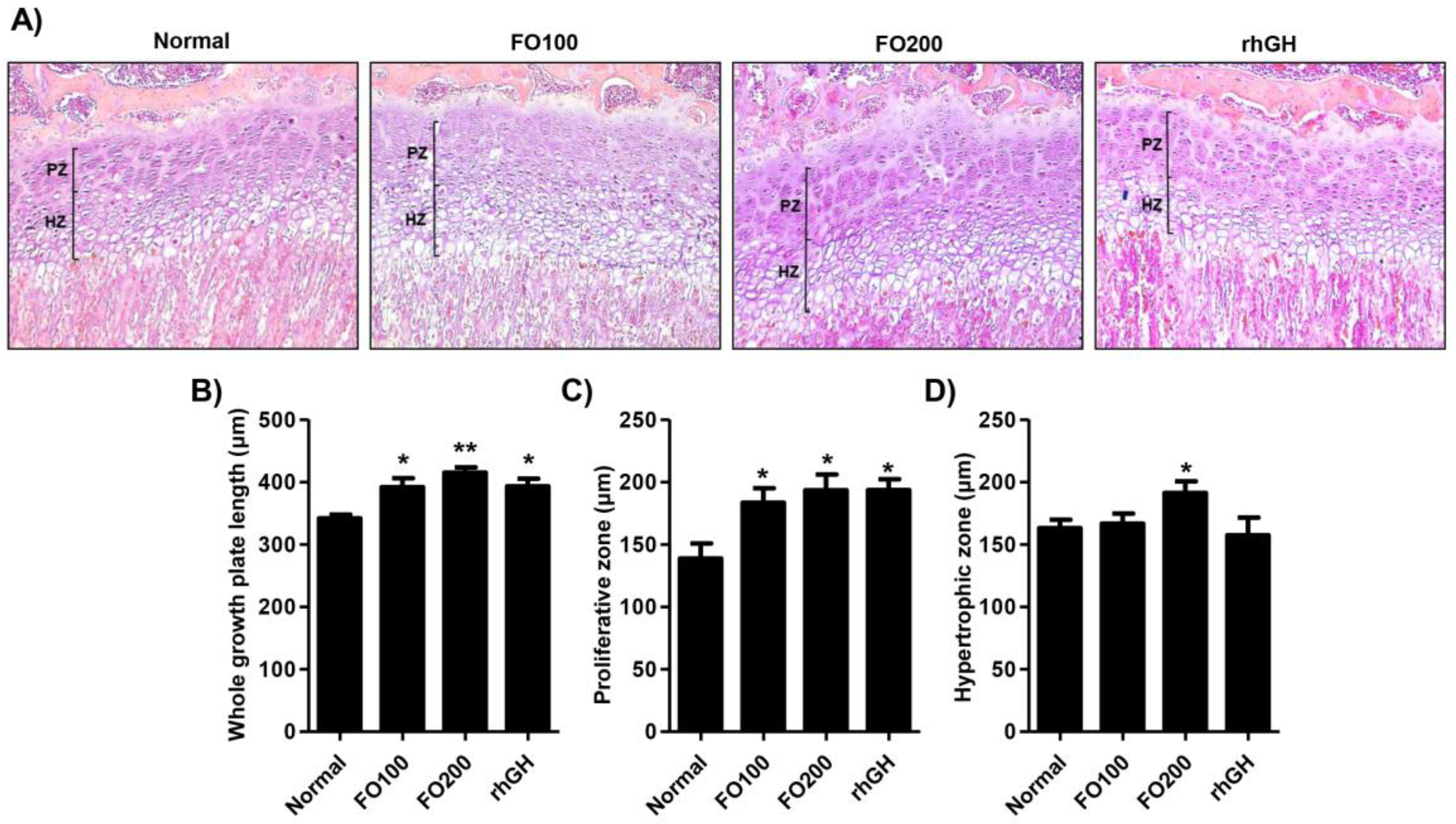
2.4. FO Increased the Height of the Proximal Tibial Growth Plate
2.5. FO Enhanced the Expression of Bone Morphogenetic Proteins in the Proximal Tibial Growth Plate
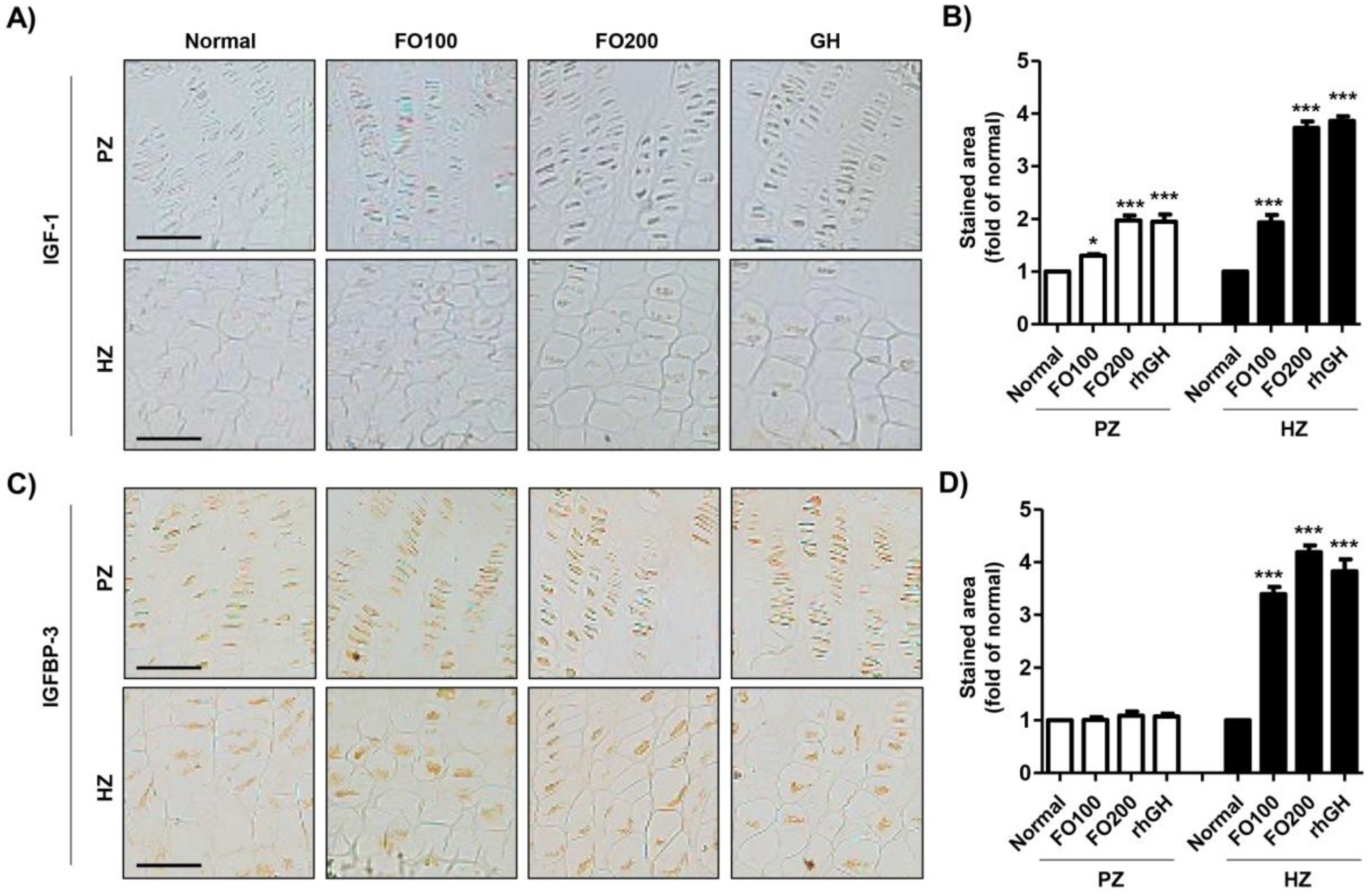
2.6. FO Promoted the Expression of IGF-1 and IGFBP in the Proximal Tibial Growth Plate
3. Discussion
4. Materials and Methods
4.1. Preparation of Fermented Oyster
4.2. Animal Study
4.3. Collection of Blood and Tissue Samples
4.4. Hematological and Biochemical Analysis
4.5. Western Blotting Analysis
4.6. Enzyme-Linked Immunosorbent Assay
4.7. Histochemical Analysis
4.8. Immunohistochemistry
4.9. Statistical Analysis
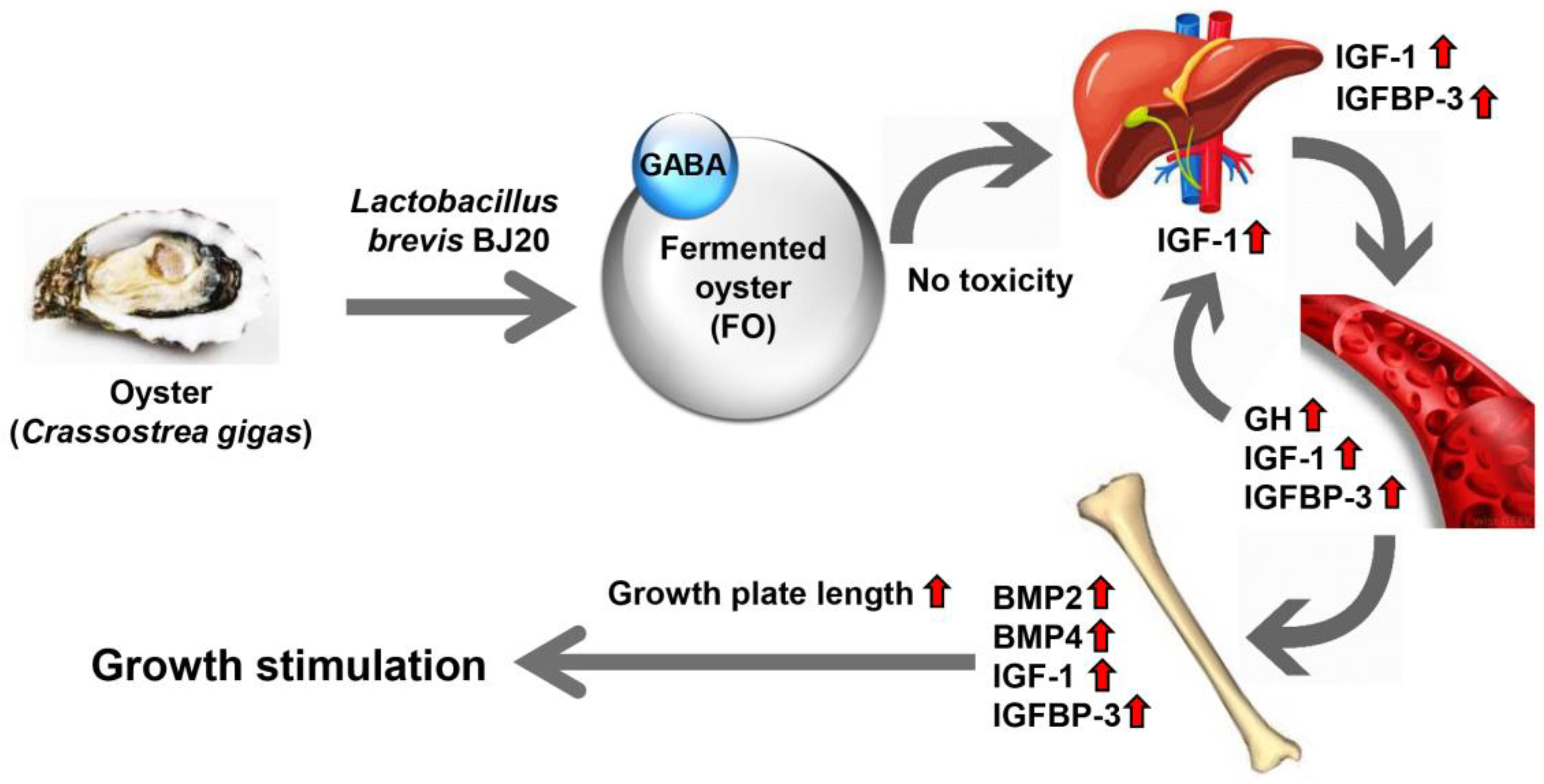
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, S.; Tu, M.; Liu, H.; An, Y.; Du, M.; Zhu, B. A novel heptapeptide derived from Crassostrea gigas shows anticoagulant activity by targeting for thrombin active domain. Food Chem. 2020, 334, 127507. [Google Scholar] [CrossRef]
- Bang, J.S.; Choung, S.Y. Inhibitory effect of oyster hydrolysate on wrinkle formation against UVB irradiation in human dermal fibroblast via MAPK/AP-1 and TGFβ/Smad pathway. J. Photochem. Photobiol. B 2020, 209, 111946. [Google Scholar] [CrossRef]
- Moon, P.D.; Kim, M.H.; Lim, H.S.; Oh, H.A.; Nam, S.Y.; Han, N.R.; Kim, M.J.; Jeong, H.J.; Kim, H.M. Taurine, a major amino acid of oyster, enhances linear bone growth in a mouse model of protein malnutrition. Biofactors 2015, 41, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, W.; Han, Z.; Liu, S.; Yang, W.; Li, M.; Wang, L.; Song, L. The dicer from oyster Crassostrea gigas functions as an intracellular recognition molecule and effector in anti-viral immunity. Fish. Shellfish Immunol. 2019, 95, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Lee, J.R.; Kwon, Y.K.; Jo, M.J.; Park, S.J.; Kim, S.C.; Lee, H.S.; Ku, S.K. Ostreae testa prevent ovariectomy-induced bone loss in mice by osteoblast activations. J. Ethnopharmacol. 2007, 114, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.V.; Silva, T.S.; Cordeiro, O.D.; Cavaco, S.I.; Simes, D.C. Identification of proteins with potential osteogenic activity present in the water-soluble matrix proteins from Crassostrea gigas nacre using a proteomic approach. Sci. World J. 2012, 2012, 765909. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Pallela, R. Medicinal foods from marine animals: Current status and prospects. Adv. Food Nutr. Res. 2012, 65, 1–9. [Google Scholar] [PubMed]
- Lin, S.; Hao, G.; Lai, D.; Tian, Y.; Long, M.; Lai, F.; Xiong, Y.; Ji, C.; Zang, Y. Effect of oyster meat preload on postmeal glycemic control in healthy young adults. J. Am. Coll. Nutr. 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Visessanguan, W.; Benjakul, S.; Riebroy, S.; Thepkasikul, P. Changes in composition and functional properties of proteins and their contributions to Nham characteristics. Food Chem. 2004, 66, 579–588. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Jung, W.-K.; Kim, S.-K. Amino acid changes in fermented oyster (Crassostrea gigas) sauce with different fermentation periods. Food Chem. 2005, 91, 15–18. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, J.-Y.; Jung, W.-K.; Park, P.-J.; Kim, S.-K. Isolation of angiotensin I converting enzyme (ACE) inhibitor from fermented oyster sauce. Crassostrea gigas. Food Chem. 2005, 90, 809–814. [Google Scholar] [CrossRef]
- Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Kim, S.Y.; Woo, M.; Keum, Y.S.; Noh, J.S.; Park, J.H.; Lee, B.J.; et al. Effect of fermented oyster extract on growth promotion in Sprague-Dawley rats. Integr. Med. Res. 2020, 9, 100412. [Google Scholar] [CrossRef] [PubMed]
- Ihn, H.J.; Kim, J.A.; Lim, S.; Nam, S.H.; Hwang, S.H.; Lim, J.; Kim, G.Y.; Choi, Y.H.; Jeon, Y.J.; Lee, B.J.; et al. Fermented oyster extract prevents ovariectomy-induced bone loss and suppresses osteoclastogenesis. Nutrients 2019, 11, 1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molagoda, I.M.N.; Karunarathne, W.A.H.M.; Choi, Y.H.; Park, E.K.; Jeon, Y.J.; Lee, B.J.; Kang, C.H.; Kim, G.Y. Fermented oyster extract promotes osteoblast differentiation by activating the Wnt/β-Catenin signaling pathway, leading to bone formation. Biomolecules 2019, 9, 711. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.W.; Choi, S.H.; Han, M.H.; Kim, G.Y.; Park, C.; Hong, S.H.; Lee, B.-J.; Park, E.K.; Kim, S.O.; Leem, S.-H.; et al. Protective effects of fermented oyster extract against RANKL-induced osteoclastogenesis through scavenging ROS generation in RAW 264.7 cells. Int. J. Mol. Sci. 2019, 20, 1439. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Bian, L.; Mo, C.; Shen, H.; Zhao, L.J.; Su, K.J.; Kukula, M.; Lee, J.T.; Armstrong, D.W.; Recker, R.; et al. Quantification of aminobutyric acids and their clinical applications as biomarkers for osteoporosis. Commun. Biol. 2020, 3, 39. [Google Scholar] [CrossRef]
- Bown, A.W.; Shelp, B.J. The metabolism and functions of c-aminobutyric acid. Plant. Physiology. 1997, 115, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Ueno, H. Enzymatic and structural aspects on glutamate decarboxylase. J. Mol. Catal. B Enzym. 2000, 10, 67–79. [Google Scholar] [CrossRef]
- Lu, X.; Xie, C.; Gu, Z. Isolation of γ-aminobutyric acidproducing bacteria and optimization of fermentative medium. Biochem. Eng. J. 2008, 41, 48–52. [Google Scholar] [CrossRef]
- Jeng, K.C.; Chen, C.S.; Fang, Y.P.; Hou, R.C.W.; Chen, Y.S. Effect of microbial fermentation on content of statin, GABA, and polyphenols in Pu-Erh tea. J. Agric. Food Chem. 2007, 55, 8787–8792. [Google Scholar] [CrossRef]
- Dhakal, R.; Bajpai, V.K.; Baek, K.H. Production of gaba (γ-Aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, H.; Furuya, Y.; Endo, Y.; Fujimoto, K. Effect of gamma-aminobutyric acid-enriched tempeh-like fermented soybean (GABA-Tempeh) on the blood pressure of spontaneously hypertensive rats. Biosci Biotechnol. Biochem. 2003, 67, 1806–1808. [Google Scholar] [CrossRef] [PubMed]
- Park, N.H.; Lee, S.J.; Mechesso, A.F.; Boby, N.; Yixian, Q.; Yoon, W.K.; Lee, S.P.; Lee, J.S.; Park, S.C. Hepatoprotective effects of gamma-aminobutyric acid-enriched fermented Hovenia dulcis extract on ethanol-induced liver injury in mice. BMC Complement. Med. Ther. 2020, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Sokovic Bajic, S.; Djokic, J.; Dinic, M.; Veljovic, K.; Golic, N.; Mihajlovic, S.; Tolinacki, M. GABA-producing natural dairy isolate from Artisanal Zlatar Cheese attenuates gut inflammation and strengthens gut epithelial barrier in vitro. Front. Microbiol. 2019, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Si, X.; Zhou, Z.; Strappe, P.; Blanchard, C. Wheat bran with enriched gamma-aminobutyric acid attenuates glucose intolerance and hyperinsulinemia induced by a high-fat diet. Food Funct. 2018, 9, 2820–2828. [Google Scholar] [CrossRef]
- Watanabe, M.; Maemura, K.; Kanbara, K.; Tamayama, T.; Hayasaki, H. GABA and GABA receptors in the central nervous system and other organs. Int. Rev. Cytol. 2002, 213, 1–47. [Google Scholar]
- Muhammad, S.I.; Maznah, I.; Mahmud, R.; Zuki, A.B.; Imam, M.U. Upregulation of genes related to bone formation by γ-amino butyric acid and γ-oryzanol in germinated brown rice is via the activation of GABAB-receptors and reduction of serum IL-6 in rats. Clin. Interv. Aging 2013, 8, 1259–1271. [Google Scholar] [CrossRef] [Green Version]
- Racagni, G.; Apud, J.A.; Cocchi, D.; Locatelli, V.; Muller, E.E. GABAergic control of anterior pituitary hormone secretion. Life Sci. 1982, 31, 823–838. [Google Scholar] [CrossRef]
- Nilsson, O.; Marino, R.; De Luca, F.; Phillip, M.; Baron, J. Endocrine regulation of the growth plate. Horm. Res. Paediatr. 2005, 64, 157–165. [Google Scholar] [CrossRef]
- Esposito, S.; Leonardi, A.; Lanciotti, L.; Cofini, M.; Muzi, G.; Penta, L. Vitamin D and growth hormone in children: A review of the current scientific knowledge. J. Transl. Med. 2019, 17, 87. [Google Scholar] [CrossRef] [Green Version]
- Betts, P. Which children should receive growth hormone treatment. Long term side effects possible with high doses. Arch. Dis. Child. 2000, 83, 176–178. [Google Scholar] [PubMed]
- Svensson, J.; Lall, S.; Dickson, S.L.; Bengtsson, B.A.; Rømer, J.; Ahnfelt-Rønne, I.; Ohlsson, C.; Jansson, J.O. Effects of growth hormone and its secretagogues on bone. Endocrine 2001, 14, 63–66. [Google Scholar] [CrossRef]
- Olney, R.C. Regulation of bone mass by growth hormone. Med. Pediatr. Oncol. 2003, 41, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.; Sävendahl, L.; De Luca, F.; Dauber, A.; Phillip, M.; Wit, J.M.; Nilsson, O. Short and tall stature: A new paradigm emerges. Nat. Rev. Endocrinol. 2015, 11, 735–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binoux, M.; Hossenlopp, P. Insulin-like growth factor (IGF) and IGF-binding proteins: Comparison of human serum and lymph. J. Clin. Endocrinol. Metab. 1988, 67, 509–514. [Google Scholar] [CrossRef]
- Spagnoli, A.; Rosenfeld, R.G. The mechanisms by which growth hormone brings about growth. The relative contributions of growth hormone and insulin-like growth factors. Endocrinol. Metab. Clin. North. Am. 1996, 25, 615–631. [Google Scholar] [CrossRef]
- Baker, J.; Liu, J.P.; Robertson, E.J.; Efstratiadis, A. Role of insulinlike growth factors in embryonic and postnatal growth. Cell 1993, 75, 73–82. [Google Scholar] [CrossRef]
- Roith, D.L.; Bondy, C.; Yakar, S.; Liu, J.L.; Butler, A. The somatomedin hypothesis: 2001. Endocr. Rev. 2001, 22, 53–74. [Google Scholar] [CrossRef]
- Firth, S.M.; Baxter, R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef]
- Barrios, V.; Argente, J.; Munoz, M.T.; Pozo, J.; Chowen, J.A.; Hernandez, M. Diagnostic interest of acid-labile subunit measurement in relationship to other components of the igf system in pediatric patients with growth or eating disorders. Eur. J. Endocrinol. 2001, 144, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, R.G. Is growth hormone deficiency a viable diagnosis? J. Clin. Endocrinol. MeTab. 1997, 82, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Van der Kaay, D.C.; Hendriks, A.E.; Ester, W.A.; Leunissen, R.W.; Willemsen, R.H.; de Kort, S.W.; Paquette, J.R.; Hokken-Koelega, A.C.; Deal, C.L. Genetic and epigenetic variability in the gene for IGFBP-3 (IGFBP3): Correlation with serum IGFBP-3 levels and growth in short children born small for gestational age. Growth Horm. IGF Res. 2009, 19, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, Y.S.; Song, J.; Kim, H.S.; Lee, H.J.; Guo, H.; Kim, H. Effects of Phlomis umbrosa root on longitudinal bone growth rate in adolescent female rats. Molecules 2016, 21, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.Y.; Kim, J.Y.; Lim, D.; Lee, D.; Kim, Y.; Chang, G.T.; Choi, H.Y.; Kim, H. Skeletal growth and IGF levels in rats after HT042 treatment. Phytother. Res. 2012, 26, 1771–1778. [Google Scholar] [CrossRef]
- Nishiyama, K.; Sugimoto, T.; Kaji, H.; Kanatani, M.; Kobayashi, T.; Chihara, K. Stimulatory effect of growth hormone on bone resorption and osteoclast differentiation. Endocrinology 1996, 137, 35–41. [Google Scholar] [CrossRef]
- Weise, M.; De-Levi, S.; Barnes, K.M.; Gafni, R.I.; Abad, V.; Baron, J. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc. Natl. Acad. Sci. USA 2001, 98, 6871–6876. [Google Scholar] [CrossRef] [Green Version]
- Schenk, R.K.; Hunziker, E.B. Rickets; Glorieux, F.H., Ed.; Nestle Nutrition Workshop Series; Raven: New York, NY, USA, 1991; pp. 63–76. [Google Scholar]
- Ballock, R.T.; O’Keefe, R.J. The biology of the growth plate. J. Bone Joint Surg. Am. 2003, 85, 715–726. [Google Scholar] [CrossRef]
- Abad, V.; Meyers, J.L.; Weise, M.; Gafni, R.I.; Barnes, K.M.; Nilsson, O.; Bacher, J.D.; Baron, J. The role of the resting zone in growth plate chondrogenesis. Endocrinology 2002, 143, 1851–1857. [Google Scholar] [CrossRef]
- Stokes, I.A.; Clark, K.C.; Farnum, C.E.; Aronsson, D.D. Alterations in the growth plate associated with growth modulation by sustained compression or distraction. Bone 2007, 41, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Hallett, S.A.; Ono, W.; Ono, N. Growth plate chondrocytes: Skeletal development, growth and beyond. Int. J. Mol. Sci. 2019, 20, 6009. [Google Scholar] [CrossRef] [Green Version]
- Storm, E.E.; Huynh, T.V.; Copeland, N.G.; Jenkins, N.A.; Kingsley, D.M.; Lee, S.J. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature 1994, 368, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Zhang, M.; Xie, R.; Wang, M.; Jin, H.; Hou, W.; Tang, D.; Harris, S.E.; Mishina, Y.; O’Keefe, R.J.; et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J. Cell Sci. 2011, 124, 3428–3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006, 38, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF- and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Lee, B.J.; Kim, J.S.; Kang, Y.M.; Lim, J.H.; Kim, Y.M.; Lee, M.S.; Jeong, M.H.; Ahn, C.B.; Je, J.Y. Antioxidant activityand γ-aminobutyric acid (GABA) content in sea tangle fermented by Lactobacillus brevis BJ20 isolated from traditional fermented foods. Food Chem. 2010, 122, 271–276. [Google Scholar] [CrossRef]
- Seo, M.S.; Kim, Y.I.; Kang, K.K.; Oh, S.K.; Sung, S.E.; Jung, Y.S.; Cho, J.Y.; Song, H.; Hwang, D.Y.; Park, S.J.; et al. Comparison study of the response with botulinum toxin muscle injection in the ICR mice from three different sources. Lab. Anim. Res. 2019, 35, 11. [Google Scholar] [CrossRef]
- Xian, C.J.; Cool, J.C.; van Gangelen, J.; Foster, B.K.; Howarth, G.S. Effect of etoposide and cyclophosphamide acute chemotherapy on growth plate and metaphyseal bone in rats. Cancer Biol. Ther. 2007, 6, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, D.; Pandey, A.; Adhikary, S.; Ahmad, N.; Bhatia, C.; Bhambhani, S.; Trivedi, P.K.; Trivedi, R. Genetically engineered flavonol enriched tomato fruit modulates chondrogenesis to increase bone length in growing animals. Sci. Rep. 2016, 6, 21668. [Google Scholar] [CrossRef] [Green Version]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of FO are available from the authors. |


| Parameter (Units) 2 | Group 1 | |||
|---|---|---|---|---|
| Normal | FO100 | FO200 | rhGH | |
| RBC (106/μL) | 6.83 ± 0.42 | 6.77 ± 0.40 | 6.81 ± 0.37 | 6.84 ± 0.33 |
| WBC (103/μL) | 3.44 ± 0.38 | 3.52 ± 0.59 | 3.68 ± 0.64 | 3.15 ± 0.76 |
| Hematocrit (%) | 50.11 ± 2.32 | 49.74 ± 2.08 | 51.00 ± 1.93 | 49.93 ± 2.15 |
| Hemoglobin (g/dL) | 14.55 ± 0.74 | 14.36 ± 0.49 | 14.64 ± 0.63 | 14.37 ± 0.58 |
| MCV (fL) | 72.68 ± 1.83 | 72.33 ± 1.54 | 73.41 ± 1.92 | 73.51 ± 1.75 |
| MCH (pg) | 21.42 ± 0.53 | 21.04 ± 0.44 | 20.79 ± 0.67 | 21.28 ± 0.53 |
| MCHC (g/dL) | 28.97 ± 0.36 | 28.35 ± 0.42 | 28.75 ± 0.39 | 28.72 ± 0.35 |
| Platelet (103/μL) | 1217.49 ± 236.81 | 1334.52 ± 201.57 | 1294.63 ± 194.68 | 1315.44 ± 186.37 |
| ALT (U/L) | 13.72 ± 1.02 | 13.44 ± 0.85 | 12.96 ± 0.78 | 13.35 ± 0.89 |
| AST (U/L) | 24.38 ± 2.72 | 21.06 ± 2.47 | 22.41 ± 3.11 | 20.85 ± 3.26 |
| BUN (mg/dL) | 19.15 ± 2.34 | 20.27 ± 2.75 | 18.89 ± 3.06 | 18.12 ± 2.51 |
| Creatinine (mg/dL) | 0.29 ± 0.04 | 0.29 ± 0.04 | 0.29 ± 0.02 | 0.28 ± 0.04 |
| Calcium (mg/dL) | 12.84 ± 0.65 | 12.37 ± 0.39 | 12.27 ± 0.41 | 12.33 ± 0.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Hwangbo, H.; Ji, S.Y.; Kim, M.Y.; Kim, S.Y.; Kim, D.H.; Hong, S.H.; Lee, S.J.; Assefa, F.; Kim, G.-Y.; et al. Gamma Aminobutyric Acid-Enriched Fermented Oyster (Crassostrea gigas) Increases the Length of the Growth Plate on the Proximal Tibia Bone in Sprague-Dawley Rats. Molecules 2020, 25, 4375. https://doi.org/10.3390/molecules25194375
Lee H, Hwangbo H, Ji SY, Kim MY, Kim SY, Kim DH, Hong SH, Lee SJ, Assefa F, Kim G-Y, et al. Gamma Aminobutyric Acid-Enriched Fermented Oyster (Crassostrea gigas) Increases the Length of the Growth Plate on the Proximal Tibia Bone in Sprague-Dawley Rats. Molecules. 2020; 25(19):4375. https://doi.org/10.3390/molecules25194375
Chicago/Turabian StyleLee, Hyesook, Hyun Hwangbo, Seon Yeong Ji, Min Yeong Kim, So Young Kim, Da Hye Kim, Su Hyun Hong, Su Jeong Lee, Freshet Assefa, Gi-Young Kim, and et al. 2020. "Gamma Aminobutyric Acid-Enriched Fermented Oyster (Crassostrea gigas) Increases the Length of the Growth Plate on the Proximal Tibia Bone in Sprague-Dawley Rats" Molecules 25, no. 19: 4375. https://doi.org/10.3390/molecules25194375
APA StyleLee, H., Hwangbo, H., Ji, S. Y., Kim, M. Y., Kim, S. Y., Kim, D. H., Hong, S. H., Lee, S. J., Assefa, F., Kim, G.-Y., Park, E. K., Park, J.-H., Lee, B.-J., Jeon, Y.-J., & Choi, Y. H. (2020). Gamma Aminobutyric Acid-Enriched Fermented Oyster (Crassostrea gigas) Increases the Length of the Growth Plate on the Proximal Tibia Bone in Sprague-Dawley Rats. Molecules, 25(19), 4375. https://doi.org/10.3390/molecules25194375








