Thymol Encapsulated into HP-β-Cyclodextrin as an Alternative to Synthetic Fungicides to Induce Lemon Resistance against Sour Rot Decay
Abstract
:1. Introduction
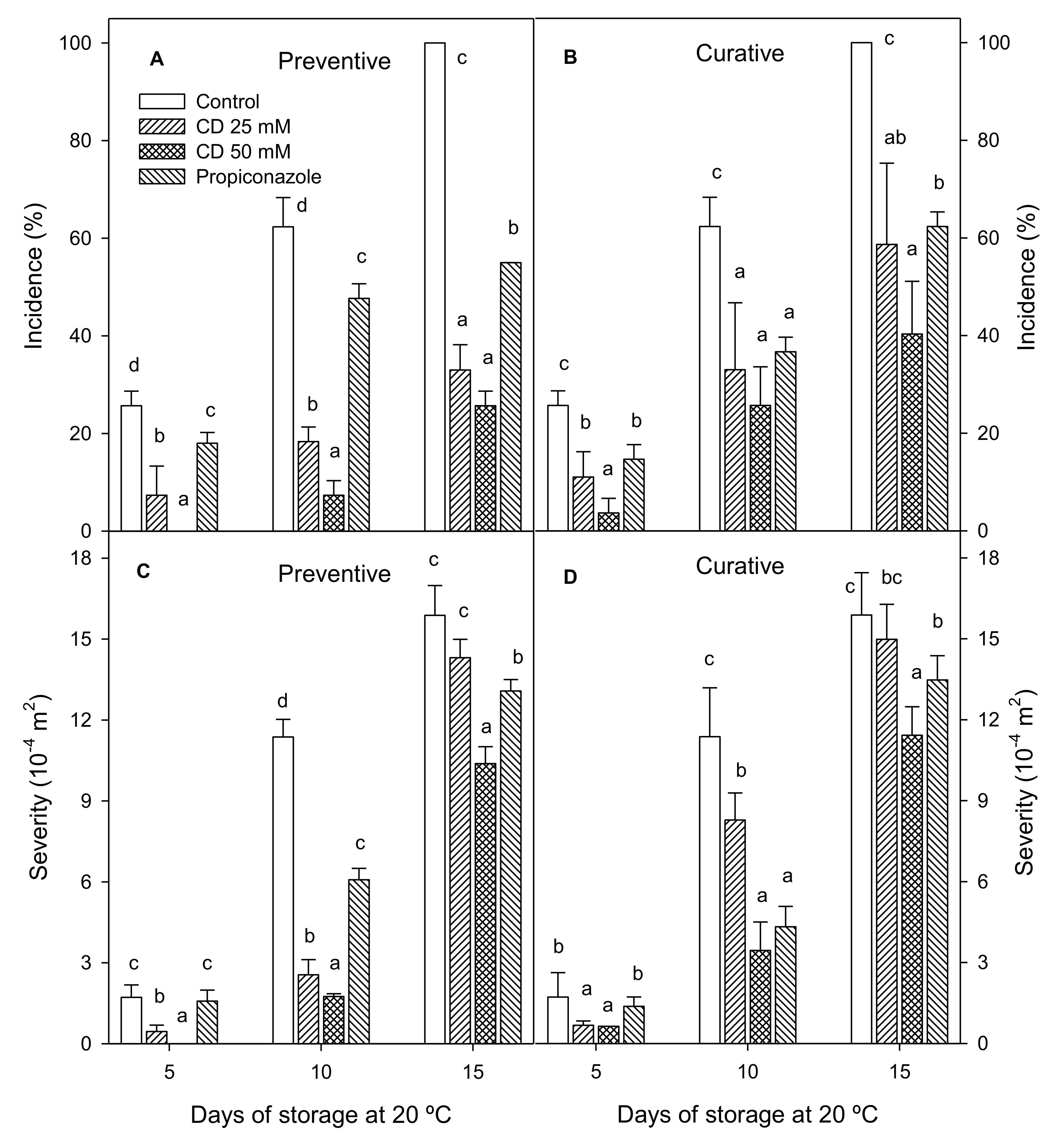
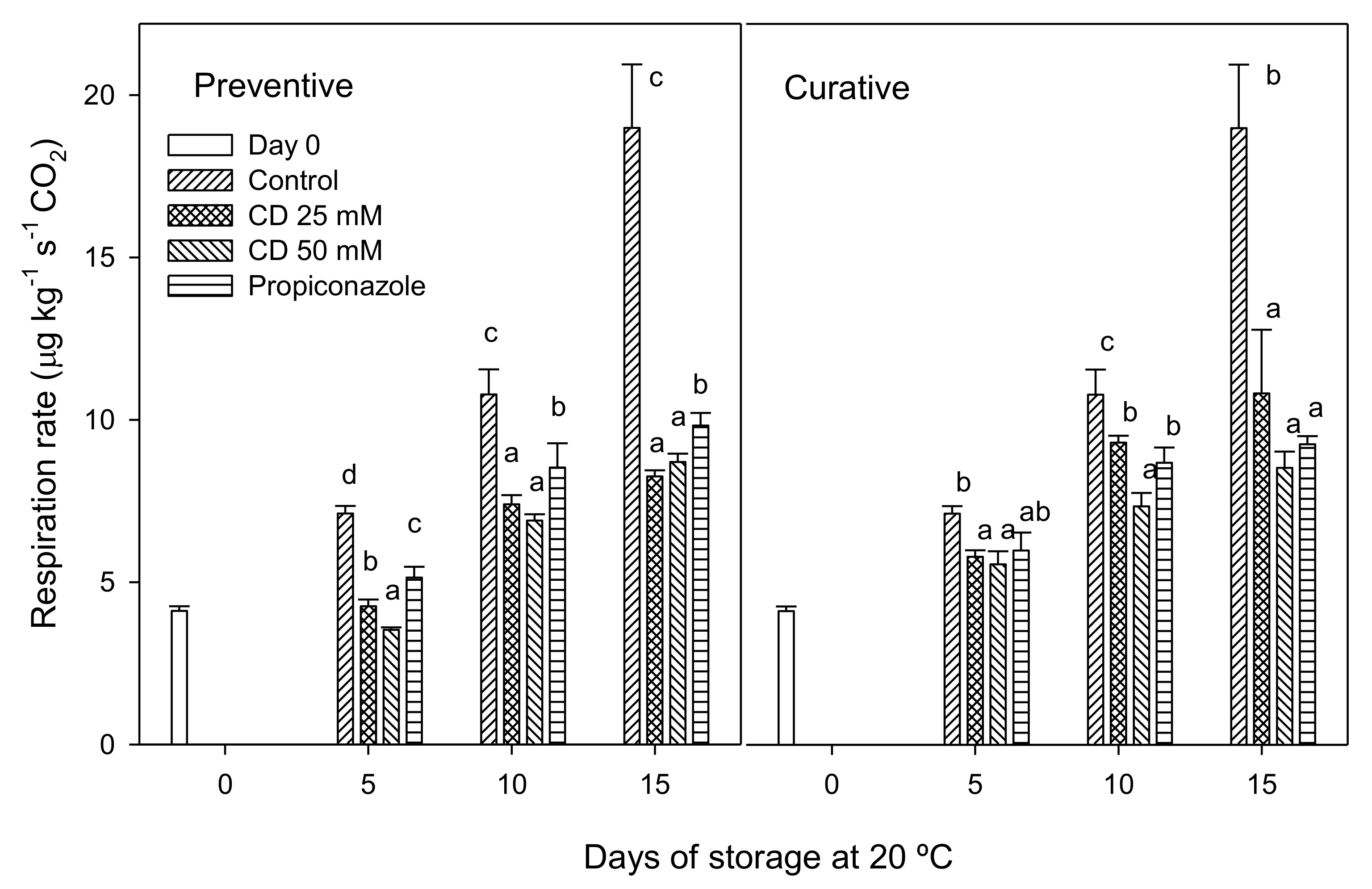
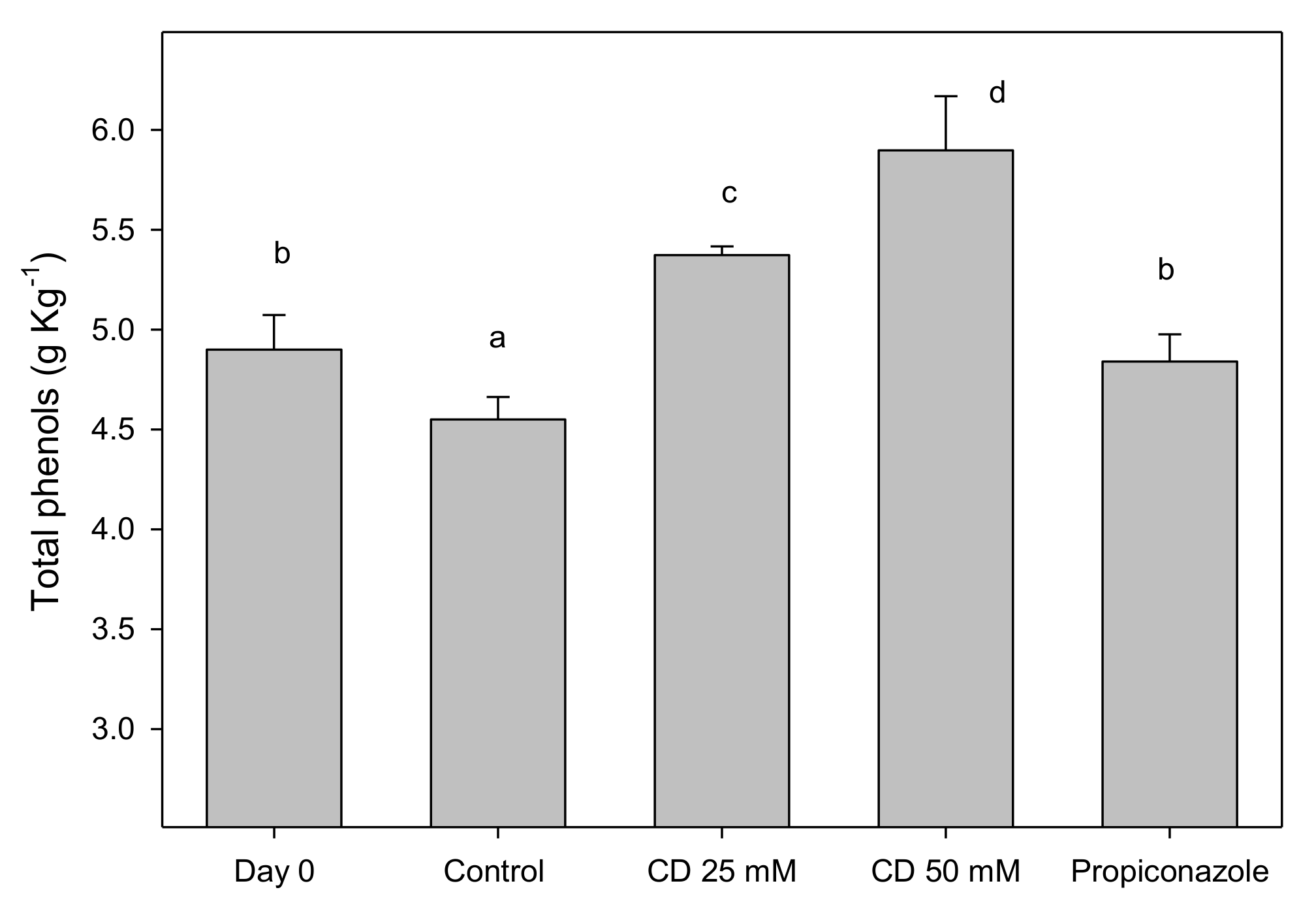
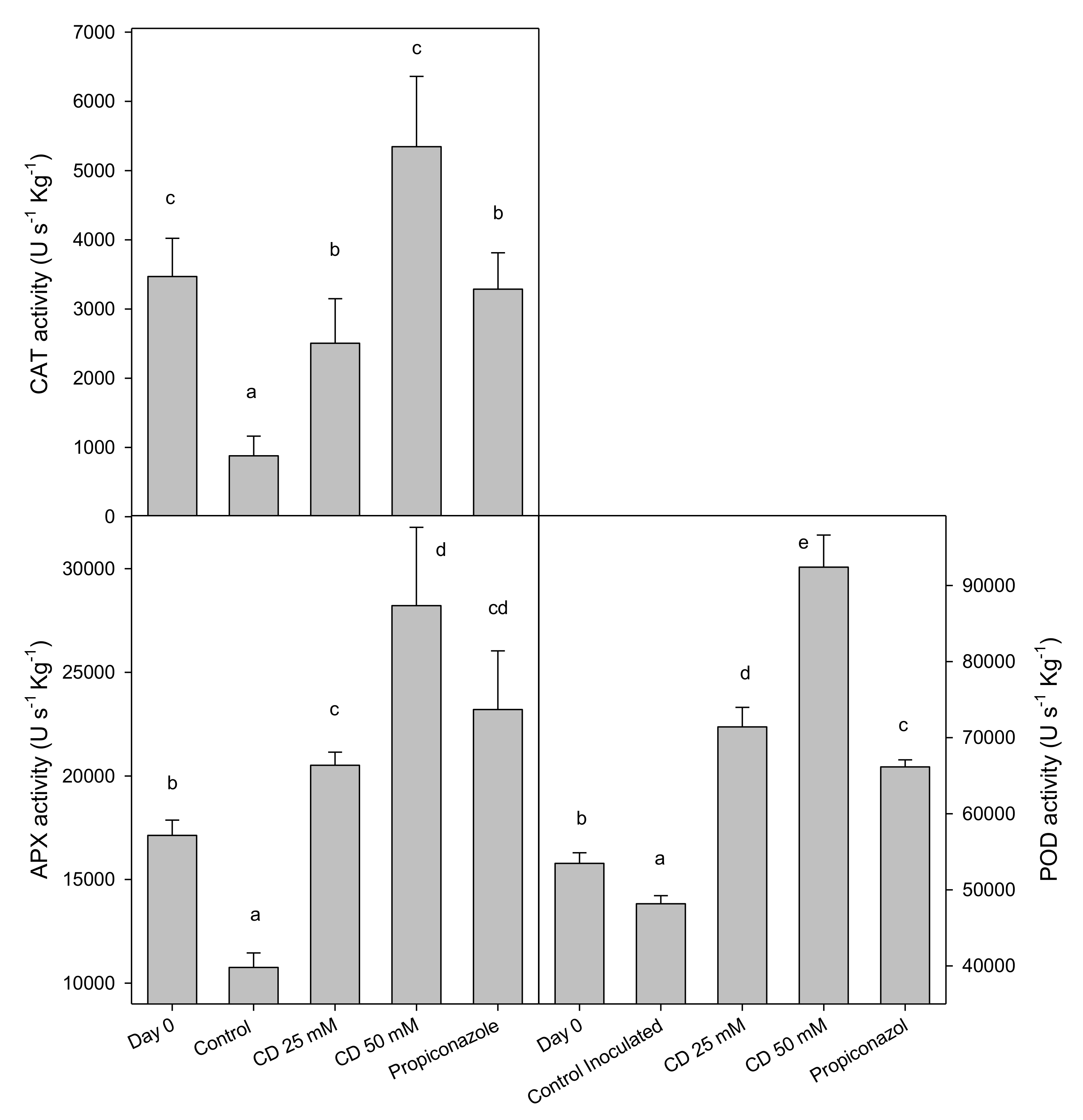
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.1.1. Thymol Encapsulation on HP-β-CD
3.1.2. Fungus
3.1.3. Fruit
3.2. Experimental Design
3.3. Disease Incidence and Severity
3.4. Fruit Weight Loss and Respiration Rate
3.5. Total Phenolic Quantification
3.6. Activity of the Antioxidant Enzymes
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smilanick, J.L.; Mansour, M.F.; Gabler, F.M.; Sorenson, D. Control of citrus postharvest green mold and sour rot by potassium sorbate combined with heat and fungicides. Postharvest Biol. Tec. 2008, 47, 226–238. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Eramus, A.; Palou, L. Citrus fruits. In Postharvest Pathology of Fresh Horticultural Produce; Palou, L., Smilanick, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 3–54. [Google Scholar] [CrossRef]
- McKay, A.H.; Förster, H.; Adaskaveg, J.E.E. Efficacy and application strategies for propiconazole as a new postharvest. Plant Dis. 2012, 96, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Fang, W.; Liu, L.; Yu, T.; Lou, B.; Zheng, X. Biological control of postharvest sour rot of citrus by two antagonistic yeasts. Lett. Appl. Microbiol. 2010, 51, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Mercier, I.; Smilanick, J.L. Control of green mold and sour rot of stored lemon by biofumigation with Muscodor albus. Biol. Control. 2005, 32, 401–407. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Rousk, J.; Bååth, E.; Bollmann, U.E.; Bester, K.; Brandt, K.K. Ecotoxicological assessment of propiconazole using soil bacterial and fungal growth assays. Appl. Soil Ecol. 2017, 115, 27–30. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Aiyabei, J.; Mlikota Gabler, F.; Doctor, J.; Sorenson, D.; Mackey, B. Quantification of the toxicity of aqueous chlorine to spores of Penicillium digitatum and Geotrichum citri-aurantii. Plant Dis. 2002, 86, 509–514. [Google Scholar] [CrossRef]
- Palou, L.; Smilanick, J.L.; Margosan, D.A. Ozone application for sanitation and control of postharvest diseases of fresh fruits and vegetables. In Recent Advances in Alternative Postharvest Technologies to Control Fungal Diseases in Fruits and Vegetables; Troncoso-Rojas, R., Tiznado-Hernández, M.E., González-León, A., Eds.; Transworld Research Network: Trivandrum, India, 2007; pp. 39–70. [Google Scholar]
- Montesinos-Herrero, C.; Del Río, M.A.; Rojas-Argudo, V.; Palou, L. Short exposure to high CO2 and O2 at curing temperature to control postharvest diseases of citrus fruits. Plant Dis. 2012, 96, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Yanez-Mendizabal, V.; Usall, J.; Viñas, I.; Casals, C.; Marın, S.; Solsona, C.; Teixido, N. Potential of a new strain of Bacillus subtilis CPA-8 to control the major postharvest diseases of fruit. Biocontrol Sci. Technol. 2011, 21, 409–426. [Google Scholar] [CrossRef]
- Ren, X.; Kong, Q.; Wang, H.; Yu, T.; Zhou, W.-W.; Zheng, X. Biocontrol of fungal decay of citrus fruit by Pichia pastoris recombinant strains expressing cecropin A. Food Chem. 2012, 131, 796–801. [Google Scholar] [CrossRef]
- Yin, C.; Liu, H.; Shan, Y.; Gupta, V.K.; Jiang, Y.; Zhang, W.; Tan, H.; Gong, L. Cytosporone B as a biological preservative: Purification, fungicidal activity and mechanism of action against Geotrichum citri-aurantii. Biomolecules 2019, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Talibi, I.; Askarne, L.; Boubaker, H.; Boudyach, E.H.; Msanda, F.; Saadi, B.; Ait Ben Oumar, A. Antifungal activity of some Moroccan plants against Geotrichum candidum, causal agent of postharvest citrus sour rot. Crop Prot. 2012, 35, 41–46. [Google Scholar] [CrossRef]
- Regnier, T.; Combrinck, S.; Veldman, W.; Du Plooy, W. Application of essential oils as multi-target fungicides for the control of Geotrichum citri-aurantii and other postharvest pathogens of citrus. Ind. Crop. Prod. 2014, 61, 151–159. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Li, Y.; Li, H.; Yu, T.; Zheng, X. Antifungal activity of thyme oil against Geotrichum citri-aurantii In Vitro and In Vivo. J. Appl. Microbiol. 2009, 107, 1450–1456. [Google Scholar] [CrossRef]
- Pérez-Alfonso, C.O.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M.; Valero, D.; Castillo, S. The effects of essential oils carvacrol and thymol on growth of Penicillium digitatum and P. italicum involved in lemon decay. Int. J. Food Microbiol. 2012, 158, 101–106. [Google Scholar] [CrossRef]
- Navarro, D.; Díaz-Mula, H.M.; Guillén, F.; Zapata, P.J.; Castillo, S.; Serrano, M.; Valero, D.; Martínez-Romero, D. Reduction of nectarine decay caused by Rhizopus stolonifer, Botrytis cinerea and Penicillium digitatum with Aloe vera gel alone or with the addition of thymol. Int. J. Food Microbiol. 2011, 151, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-López, M.I.; Mercader-Ros, M.T.; López-Miranda, S.; Pellicer, J.A.; Pérez-Garrido, A.; Pérez-Sánchez, H.; Núñez-Delicado, E.; Gabaldón, J.A. Thorough characterization and stability of HP-β-cyclodextrin thymol inclusion complexes prepared by microwave technology: A required approach to a successful application in food industry. J. Sci. Food Agr. 2019, 99, 1322–1333. [Google Scholar] [CrossRef]
- Serrano-Martínez, A.; Fortea, M.I.; Lucas-Abellán, C.; López-Miranda, S.; Mercader, M.T.; Núñez-Delicado, E.; Gabaldón, J.A. Protective effect of cyclodextrins on the quality parameters of roast preserved pepper. Food Sci. Technol. Int. 2016, 22, 565–573. [Google Scholar] [CrossRef]
- Ciobanu, A.; Landy, D.; Fourmentin, S. Complexation efficiency of cyclodextrins for volatile flavor compounds. Food Res. Int. 2013, 53, 110–114. [Google Scholar] [CrossRef]
- Lucas-Abellán, C.; Mercader-Ros, M.T.; Zafrilla, M.P.; Fortea, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. ORAC-fluorescein assay to determine the oxygen radical absorbance capacity of resveratrol complexed in cyclodextrins. J. Agric. Food Chem. 2008, 56, 2254–2259. [Google Scholar]
- Serna-Escolano, V.; Serrano, M.; Valero, D.; Rodríguez-López, M.I.; Gabaldón, J.A.; Castillo, S.; Guillén, F.; Zapata, P.J.; Martínez-Romero, D. Effect of thymol and carvacrol encapsulated in HP-β-cyclodextrin by two inclusion methods against Geotrichum citri-aurantii. J. Food Sci. 2019, 84, 1513–1521. [Google Scholar] [CrossRef]
- Rodríguez-López, M.I.; Mercader-Ros, M.T.; Pellicer, J.A.; Gómez-López, V.M.; Martínez-Romero, D.; Núñez-Delicado, E.; Gabaldón, J.A. Evaluation of monoterpene-cyclodextrin complexes as bacterial growth effective hurdles. Food Cont. 2020, 108, 106814. [Google Scholar] [CrossRef]
- Ramezanian, A.; Azadi, M.; Mostowfizadeh-Ghalamfarsa, R.; Saharkhiz, M.J. Effect of Zataria multiflora Boiss and Thymus vulgaris L. essential oils on black rot of ‘Washington Navel’ orange fruit. Postharvest Biol. Tec. 2016, 112, 152–158. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and Interactions with food matrix components. Front. Microbiol. 2012, 3, 21–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Perina, F.J.; Amaral, D.C.; Fernandes, R.S.; Labory, C.R.G.; Teixeira, G.A.; Alves, E. Thymus vulgaris essential oil and thymol against Alternaria alternata (Fr.) Keissler: Effects on growth, viability, early infection and cellular mode of action. Pest Manag. Sci. 2015, 71, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Chamorro, S.A.; Pérez-Gago, M.B.; del Río, M.A.; Palou, L. Curative and preventive activity of hydroxypropyl methylcellulose lipid edible composite coatings containing antifungal food additives to control citrus postharvest green and blue moulds. J. Agric. Food Chem. 2009, 57, 2770–2777. [Google Scholar] [CrossRef]
- Sameza, M.L.; Nguemnang Mabou, L.C.; Tchameni, S.N.; Boat Bedine, M.A.; Tchoumbougnang, F.; Jazet Dongmo, P.M.; Boyom Fekam, F. Evaluation of clove essential oil as a mycobiocide against Rhizopus stolonifer and Fusarium solani, tuber rot causing fungi in yam (Dioscorea rotundata Poir.). J. Phytopathol. 2016, 164, 433–440. [Google Scholar] [CrossRef]
- Montesinos-Herrero, C.; Palou, L. Combination of physical and low-toxicity chemical postharvest treatments for integrated disease management of citrus fruit: A review. Stewart Postharvest Rev. 2010, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Serrano, M.; Martinez-Romero, D.; Castillo, S.; Guillen, D.; Valero, M. The use of the natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage. Innov. Food Sci. Emerg. 2005, 6, 115–123. [Google Scholar] [CrossRef]
- Valverde, J.M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M.; Valero, D. Improvement of table grapes quality and safety by the combination of modified atmosphere packaging (MAP) and eugenol, menthol, or thymol. J. Agric. Food Chem. 2005, 53, 7458–7464. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ban, X.Q.; Zeng, H.; He, J.S.; Huang, B.; Wang, Y.W. Chemical composition and antifungal activity of essential oil from Cicuta virosa L. var. latisecta Celak. Int. J. Food Microbiol. 2011, 145, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Jhalegar, M.D.J.; Sharma, R.R.; Singh, S.D. In Vitro and In Vivo activity of essential oils against major postharvest pathogens of Kinnow (Citrus nobilis × C. deliciosa) mandarin. J. Food Sci. Tech. 2015, 52, 229–2237. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Romero, D.; Guillén, F.; Valverde, J.M.; Bailén, G.; Zapata, P.J.; Serrano, M.; Castillo, S.; Valero, D. Influence of carvacrol on survival of Botrytis cinerea inoculated in table grapes. Int. J. Food. Microbiol. 2007, 115, 144–148. [Google Scholar] [CrossRef]
- Jin, P.; Wang, H.; Zhang, Y.; Huang, Y.; Wang, L.; Wang, L.; Zheng, Y. UV-C enhances resistance against gray mold decay caused by Botrytis cinerea in strawberry fruit. Sci. Hortic. 2017, 225, 106–111. [Google Scholar] [CrossRef]
- Singh, R.; Rastogi, S.; Dwivedi, U.N. Phenylpropanoid metabolism in ripening fruits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 398–416. [Google Scholar] [CrossRef]
- Bill, M.; Sivakumar, D.; Korsten, L.; Thompson, A.K. The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot. 2014, 64, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Serna-Escolano, V.; Valverde, J.M.; García-Pastor, M.E.; Valero, D.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M. Pre-harvest methyl jasmonate treatments increase antioxidant systems in lemon fruit without affecting yield or other fruit quality parameters. J. Sci. Food Agr. 2019, 99, 5035–5043. [Google Scholar] [CrossRef]
- Perumal, A.B.; Sellamuthu, P.S.; Nambiar, R.B.; Sadiku, E.R. Effects of essential oil vapour treatment on the postharvest disease control and different defence responses in two mango (Mangifera indica L.) cultivars. Food Bioprocess Tech. 2017, 10, 1131–1141. [Google Scholar] [CrossRef]
- Jin, P.; Wu, X.; Xu, F.; Wang, X.; Wang, J.; Zheng, Y. Enhancing antioxidant capacity and reducing decay of Chinese bayberries by essential oils. J. Sci. Food Agric. 2012, 60, 3769–3775. [Google Scholar] [CrossRef]
- Li, H.; Sou, J.; Han, Y.; Liang, C.; Jin, M.; Zhang, Z.; Rao, J. Effects of oxalic acid treatment on lignification and related enzymes activities in ‘Huayou’ kiwifruit during cold storage. Acta Hortic. Sinica 2017, 44, 1085–1093. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds of encapsulated thymol into 2-hydroxylpropyl-beta-cyclodextrin are available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna-Escolano, V.; Serrano, M.; Valero, D.; Isabel Rodríguez-López, M.; Gabaldón, J.A.; Castillo, S.; Valverde, J.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D. Thymol Encapsulated into HP-β-Cyclodextrin as an Alternative to Synthetic Fungicides to Induce Lemon Resistance against Sour Rot Decay. Molecules 2020, 25, 4348. https://doi.org/10.3390/molecules25184348
Serna-Escolano V, Serrano M, Valero D, Isabel Rodríguez-López M, Gabaldón JA, Castillo S, Valverde JM, Zapata PJ, Guillén F, Martínez-Romero D. Thymol Encapsulated into HP-β-Cyclodextrin as an Alternative to Synthetic Fungicides to Induce Lemon Resistance against Sour Rot Decay. Molecules. 2020; 25(18):4348. https://doi.org/10.3390/molecules25184348
Chicago/Turabian StyleSerna-Escolano, Vicente, María Serrano, Daniel Valero, María Isabel Rodríguez-López, José Antonio Gabaldón, Salvador Castillo, Juan Miguel Valverde, Pedro Javier Zapata, Fabián Guillén, and Domingo Martínez-Romero. 2020. "Thymol Encapsulated into HP-β-Cyclodextrin as an Alternative to Synthetic Fungicides to Induce Lemon Resistance against Sour Rot Decay" Molecules 25, no. 18: 4348. https://doi.org/10.3390/molecules25184348









