Effect of the Solvent on Propolis Phenolic Profile and its Antifungal, Antioxidant, and In Vitro Cytoprotective Activity in Human Erythrocytes Under Oxidative Stress
Abstract
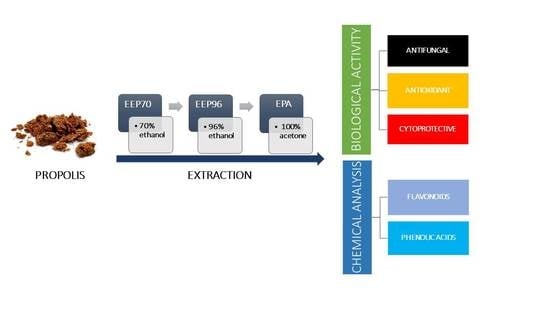
:1. Introduction
2. Results
2.1. The Concentration of Phenolic Compounds in Propolis Extracts
2.2. Antioxidant Potential, Hemolytic Potency, and Cytprotective Activity of Propolis Extracts in Human Erythrocytes
2.3. Antifungal Activity of Propolis Extracts
3. Discussion
3.1. Concentrations of Phenolic Compounds in Propolis Extracts
3.2. Antioxidant Properties and Cytoprotective Activity of Propolis Extracts in Human Erythrocytes
3.3. Antifungal Activity of Propolis Extracts
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Propolis and Preparation of Extracts
4.3. Analysis of Phenolic Comopounds in Propolis Extracts
4.4. Antioxidant Activity of Propolis Extracts
4.4.1. DPPH· Free-Radical-Scavenging Activity
4.4.2. Fe3+-Reducing Power
4.4.3. Ferrous Ion (Fe2+)-Chelating Activity
4.5. In Vitro Effects of Propolis Extracts on Human Red Blood Cells (RBCs)
4.6. Erythrocyte Shape Evaluation Using a Scanning Electron Microscope (SEM)
4.7. Antifungal Properties of Propolis Extracts
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B.; Danert, C.; Eberlin, M.N.; Falcão, S.I.; Isla, M.I.; Moreno, M.I.N.; et al. Standard methods for Apis mellifera propolis research. J. Apic. Res. 2019, 58, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve, South Portugal. Food Sci. Technol. 2014, 34, 16–23. [Google Scholar] [CrossRef] [Green Version]
- Freitas, A.S.; Cunha, A.; Cardoso, S.M.; Oliveira, R.; Almeida-Aguiar, C. Constancy of the bioactivities of propolis samples collected on the same apiary over four years. Food Res. Int. 2019, 119, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Giannopoulou, E.; Skalicka-Woźniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bęben, K.; Antosiewicz, B.; et al. Characterization and biological evaluation of propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Garedew, A.; Schmolz, E.; Lamprecht, I. Microbiological and calorimetric investigations on the antimicrobial actions of different propolis extracts: An in vitro approach. Thermochim. Acta 2004, 422, 115–124. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharmacol. Sci. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Agüero, M.B.; Svetaz, L.; Sánchez, M.; Luna, L.; Lima, B.; López, M.L.; Zacchino, S.; Palermo, J.; Wunderlin, D.; Feresin, G.E.; et al. Argentinean Andean propolis associated with the medicinal plant Larrea nitida Cav. (Zygophyllaceae). HPLC-MS and GC-MS characterization and antifungal activity. Food Chem. Toxicol. 2011, 49, 1970–1978. [Google Scholar] [CrossRef]
- Szliszka, E.; Kucharska, A.Z.; Sokół-Łętowska, A.; Mertas, A.; Czuba, Z.P.; Król, W. Chemical composition and anti-inflammatory effect of ethanolic extract of Brazilian green propolis on activated J774A.1 macrophages. Evid. Based Complement. Altern. Med. 2013, 2013, 976415. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef]
- Agüero, M.B.; Svetaz, L.; Baroni, V.; Lima, B.; Luna, L.; Zacchino, S.; Saavedra, P.; Wunderlin, D.; Feresin, G.E.; Tapia, A. Urban propolis from San Juan province (Argentina): Ethnopharmacological uses and antifungal activity against Candida and dermatophytes. Ind. Crops Prod. 2014, 57, 166–173. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Konteles, S.J.; Troullidou, E.; Mourtzinos, I.; Karathanos, V.T. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009, 116, 452–461. [Google Scholar] [CrossRef]
- Kasote, D.M.; Pawar, M.V.; Gundu, S.S.; Bhatia, R.; Nandre, V.S.; Jagtap, S.D.; Mahajan, S.G.; Kulkarni, M.V. Chemical profiling, antioxidant, and antimicrobial activities of Indian stingless bees propolis samples. J. Apic. Res. 2019, 58, 617–625. [Google Scholar] [CrossRef]
- Chen, Y.W.; Ye, S.R.; Ting, C.; Yu, Y.H. Antibacterial activity of propolins from Taiwanese green propolis. J. Food Drug Anal. 2018, 26, 761–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and functional characterization of Italian propolis obtained by different harvesting methods. J. Agric. Food Chem. 2012, 60, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Kumazawa, S.; Bonvehí, J.S.; Torres, C.; Mok-Ryeon, A.; Bermejo, F.J.O. Chemical and functional characterisation of propolis collected from East Andalusia (Southern Spain). Phytochem. Anal. 2013, 24, 608–615. [Google Scholar] [CrossRef]
- Mohammadzadeh, S.; Shariatpanahi, M.; Hamedi, M.; Ahmadkhaniha, R.; Samadi, N.; Ostad, S.N. Chemical composition, oral toxicity and antimicrobial activity of Iranian propolis. Food Chem. 2007, 103, 1097–1103. [Google Scholar] [CrossRef]
- Kasote, D.M.; Pawar, M.V.; Bhatia, R.S.; Nandre, V.S.; Gundu, S.S.; Jagtap, S.D.; Kulkarni, M.V. HPLC, NMR based chemical profiling and biological characterisation of Indian propolis. Fitoterapia 2017, 122, 52–60. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Waśkiewicz, A.; Rogoziński, T.; Ratajczak, I. The role of seasonality on the chemical composition, antioxidant activity and cytotoxicity of Polish propolis in human erythrocytes. Rev. Bras. Farmacogn. 2019, 29, 301–308. [Google Scholar] [CrossRef]
- Kędzia, B. Skład chemiczny propolisu polskiego. Cz. II. Nowe badania. Postępy Fitoter. 2009, 2, 122–128. [Google Scholar]
- Woźniak, M.; Kwaśniewska-Sip, P.; Waśkiewicz, A.; Cofta, G.; Ratajczak, I. The possibility of propolis extract application in wood protection. Forests 2020, 11, 465. [Google Scholar] [CrossRef] [Green Version]
- Silici, S.; Kutluca, S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol. 2005, 99, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Uzel, A.; Sorkun, K.; Önçaǧ, Ö.; Çoǧulu, D.; Gençay, Ö.; Salih, B. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef]
- Inmaculada González-Martín, M.; Escuredo, O.; Revilla, I.; Vivar-Quintana, A.M.; Carmen Coello, M.; Riocerezo, C.P.; Moncada, G.W. Determination of the mineral composition and toxic element contents of propolis by near infrared spectroscopy. Sensors 2015, 15, 27854–27868. [Google Scholar] [CrossRef]
- Tosic, S.; Stojanovic, G.; Mitic, S.; Pavlovic, A.; Alagic, S. Mineral composition of selected Serbian propolis samples. J. Apic. Sci. 2017, 61, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melliou, E.; Stratis, E.; Chinou, I. Volatile constituents of propolis from various regions of Greece—Antimicrobial activity. Food Chem. 2007, 103, 375–380. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Kędzia, B. Chemical content and biological activity of propolis from different world regions. Postępy Fitoter. 2006, 1, 23–35. [Google Scholar]
- Wieczyńska, A.; Wezgowiec, J.; Więckiewicz, W.; Czarny, A.; Kulbacka, J.; Nowakowska, D.; Gancarz, R.; Wilk, K.A. Antimicrobial activity, cytotoxicity and total phenolic content of different extracts of propolis from the West Pomeranian region in Poland. Acta Pol. Pharm. Drug Res. 2017, 74, 715–722. [Google Scholar]
- Pobiega, K.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Żubernik, J.; Witrowa-Rajchert, D.; Gniewosz, M. Growth biocontrol of foodborne pathogens and spoilage microorganisms of food by Polish propolis extracts. Molecules 2019, 24, 2965. [Google Scholar] [CrossRef] [Green Version]
- Dziedzic, A.; Kubina, R.; Wojtyczka, R.D.; Kabała-Dzik, A.; Tanasiewicz, M.; Morawiec, T. The antibacterial effect of ethanol extract of Polish propolis on mutans Streptococci and Lactobacilli isolated from saliva. Evid. Based Complement. Altern. Med. 2013, 2013, 681891. [Google Scholar] [CrossRef] [Green Version]
- Wezgowiec, J.; Wieczyńska, A.; Wieckiewicz, W.; Kulbacka, J.; Saczko, J.; Pachura, N.; Wieckiewicz, M.; Gancarz, R. Polish propolis—Chemical composition and biological effects in tongue cancer cells and macrophages. Molecules 2020, 25, 2426. [Google Scholar] [CrossRef]
- Woźniak, M.; Mrówczyńska, L.; Waśkiewicz, A.; Rogoziński, T.; Ratajczak, I. Phenolic profile and antioxidant activity of propolis extracts from Poland. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Woźniak, M.; Ratajczak, I.; Kwaśniewska, P.; Cofta, G.; Hołderna-Kędzia, E.; Kędzia, B.; Mazela, B. The activity of propolis extracts against selected moulds. Postępy Fitoter. 2015, 4, 205–209. [Google Scholar]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial activities of European propolis collected from various geographic origins alone and in combination with antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Molnár, S.; Mikuska, K.; Patonay, K.; Sisa, K.; Daood, H.G.; Némedi, E.; Kiss, A. Comparative studies on polyphenolic profile and antimicrobial activity of propolis samples selected from distinctive geographical areas of Hungary. Food Sci. Technol. Int. 2017, 23, 349–357. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Anastasiadou, P.; Papadopoulos, A.; Machera, K. Revisiting Greek propolis: Chromatographic analysis and antioxidant activity study. PLoS ONE 2017, 12, e0170077. [Google Scholar] [CrossRef] [Green Version]
- Gardini, S.; Bertelli, D.; Marchetti, L.; Graziosi, R.; Pinetti, D.; Plessi, M.; Marcazzan, G.L. Chemical composition of Italian propolis of different ecoregional origin. J. Apic. Res. 2018, 57, 639–647. [Google Scholar] [CrossRef]
- Narimane, S.; Demircan, E.; Salah, A.; Ozcelik, B.Ö.; Salah, R. Correlation between antioxidant activity and phenolic acids profile and content of Algerian propolis: Influence of solvent. Pak. J. Pharm. Sci. 2017, 30, 1417–1423. [Google Scholar]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottica, S.M.; Sawaya, A.C.H.F.; Eberlin, M.N.; Franco, S.L.; Zeoula, L.M.; Visentainer, J.V. Antioxidant activity and composition of propolis obtained by different methods of extraction. J. Braz. Chem. Soc. 2011, 22, 929–935. [Google Scholar] [CrossRef]
- Dos Santos, H.F.; Campos, J.F.; dos Santos, C.M.; Balestieri, J.B.P.; Silva, D.B.; Carollo, C.A.; Souza, K.D.P.; Estevinho, L.M.; Dos Santos, E.L. Chemical profile and antioxidant, anti-inflammatory, antimutagenic and antimicrobial activities of geopropolis from the stingless bee Melipona orbignyi. Int. J. Mol. Sci. 2017, 18, 953. [Google Scholar] [CrossRef]
- Touzani, S.; Al-Waili, N.; El Menyiy, N.; Filipic, B.; Pereyra, A.; El Arabi, I.; Al-Waili, W.; Lyoussi, B. Chemical analysis and antioxidant content of various propolis samples collected from different regions and their impact on antimicrobial activities. Asian Pac. J. Trop. Med. 2018, 11, 436–442. [Google Scholar] [CrossRef]
- Yang, S.Z.; Peng, L.T.; Su, X.J.; Chen, F.; Cheng, Y.J.; Fan, G.; Pan, S.Y. Bioassay-guided isolation and identification of antifungal components from propolis against Penicillium italicum. Food Chem. 2011, 127, 210–215. [Google Scholar] [CrossRef]
- Yang, H.; Dong, Y.; Du, H.; Shi, H.; Peng, Y.; Li, X. Antioxidant compounds from propolis collected in Anhui, China. Molecules 2011, 16, 3444–3455. [Google Scholar] [CrossRef]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.M.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic profiling of Portuguese propolis by LC-MS spectrometry: Uncommon propolis rich in flavonoid glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Mavri, A.; Abramovič, H.; Polak, T.; Bertoncelj, J.; Jamnik, P.; Smole Moažina, S.; Jeršek, B. Chemical properties and antioxidant and antimicrobial activities of Slovenian propolis. Chem. Biodivers. 2012, 9, 1545–1558. [Google Scholar] [CrossRef]
- Mladěnka, P.; MacÁková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef]
- Ahn, M.R.; Kumazawa, S.; Usui, Y.; Nakamura, J.; Matsuka, M.; Zhu, F.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007, 101, 1383–1392. [Google Scholar] [CrossRef]
- Malczewska-Jaskóła, K.; Jasiewicz, B.; Mrówczyńska, L. Nicotine alkaloids as antioxidant and potential protective agents against in vitro oxidative haemolysis. Chem. Biol. Interact. 2016, 243, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Jasiewicz, B.; Sierakowska, A.; Jankowski, W.; Hoffmann, M.; Piorońska, W.; Górnicka, A.; Bielawska, A.; Bielawski, K.; Mrówczyńska, L. Antioxidant and cytotoxic activity of new di- and polyamine caffeine analogues. Free Radic. Res. 2018, 52, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-contacting biomaterials: In vitro evaluation of the hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef] [PubMed]
- Grzyb, T.; Mrówczyńska, L.; Szczeszak, A.; Śniadecki, Z.; Runowski, M.; Idzikowski, B.; Lis, S. Synthesis, characterization, and cytotoxicity in human erythrocytes of multifunctional, magnetic, and luminescent nanocrystalline rare earth fluorides. J. Nanopart. Res. 2015, 17, 399. [Google Scholar] [CrossRef] [Green Version]
- Kacániová, M.; Vuković, N.; Chlebo, R.; Haščík, P.; Rovná, K.; Cubon, J.; Dzugan, M.; Pasternakiewicz, A. The antimicrobial activity of honey, bee pollen loads and beeswax from Slovakia. Arch. Biol. Sci. 2012, 64, 927–934. [Google Scholar] [CrossRef]
- Xu, X.; Pu, R.; Li, Y.; Wu, Z.; Li, C.; Miao, X.; Yang, W. Chemical compositions of propolis from China and the United States and their antimicrobial activities against Penicillium notatum. Molecules 2019, 24, 3576. [Google Scholar] [CrossRef] [Green Version]
- Quiroga, E.N.; Sampietro, D.A.; Soberón, J.R.; Sgariglia, M.A.; Vattuone, M.A. Propolis from the northwest of Argentina as a source of antifungal principles. J. Appl. Microbiol. 2006, 101, 103–110. [Google Scholar] [CrossRef]
- Peng, L.; Yang, S.; Cheng, Y.J.; Chen, F.; Pan, S.; Fan, G. Antifungal activity and action mode of pinocembrin from propolis against Penicillium italicum. Food Sci. Biotechnol. 2012, 21, 1533–1539. [Google Scholar] [CrossRef]

| Flavonoids | Concentration (mg/g of Propolis Extract) | ||
|---|---|---|---|
| EPA (Acetone) | EEP70 (70% EtOH) | EEP96 (96% EtOH) | |
| Apigenin | 9.57 a ± 0.38 | 7.96 a ± 0.47 | 6.13 b ± 0.68 |
| Chrysin | 14.62 b ± 0.55 | 18.64 a ± 0.71 | 11.41 c ± 0.73 |
| Galangin | 23.91 a ± 0.71 | 16.18 b ± 0.42 | 15.08 b ± 0.27 |
| Kaempferol | 10.63 b ± 0.39 | 8.20 c ± 0.43 | 12.15 a ± 0.40 |
| Naringenin | 0.80 a ± 0.20 | 0.86 a ± 0.23 | 0.33 a ± 0.06 |
| Pinobanksin | 3.62 a ± 0.30 | 3.10 a ± 0.24 | 3.56 a ± 0.25 |
| Pinocembrin | 30.68 b ± 0.24 | 35.89 a ± 0.95 | 26.17 c ± 0.61 |
| Quercetin | 1.42 ab ± 0.29 | 2.30 a ± 0.43 | 1.30 b ± 0.18 |
| Sum of flavonoids | 95.25 | 93.13 | 76.13 |
| Phenolic Acids | Concentration (mg/g of Propolis Extract) | ||
|---|---|---|---|
| EPA (Acetone) | EEP70 (70% EtOH) | EEP96 (96% EtOH) | |
| Caffeic acid | 2.23 a ± 0.31 | 2.54 a ± 0.37 | 2.15 a ± 0.18 |
| Coumaric acid | 9.19 a,b ± 0.55 | 7.90 b ± 0.57 | 9.56 a ± 0.32 |
| Ferulic acid | 1.63 a,b ± 0.24 | 2.14 a ± 0.24 | 1.39 b ± 0.10 |
| Syringic acid | 0.54 a ± 0.08 | 0.38 a,b ± 0.04 | 0.23 b ± 0.02 |
| Vanillic acid | 0.18 a ± 0.02 | 0.22 a ± 0.02 | nd |
| Cinnamic acid | 3.98 a ± 0.26 | 4.46 a ± 0.36 | 5.08 a ± 0.47 |
| p-Hydroxybenzoic acid | nd | 0.24 a ± 0.04 | 0.08 b ± 0.02 |
| Sum of phenolic acids | 17.75 | 17.88 | 18.49 |
| Propolis Extracts | DPPH· Free-Radical-Scavenging Activity (%) | Fe3+-Reducing Power (Ab = 700 nm) | Ferrous Ion (Fe2+)-Chelating Activity (%) |
|---|---|---|---|
| EPA | 31.25 a ± 3.73 | 1.07 a ± 0.05 | 43.33 a ± 0.94 |
| EEP70 | 29.38 a ± 4.06 | 0.96 a ± 0.06 | 43.00 a ± 1.63 |
| EEP96 | 30.44 a ± 1.83 | 1.00 a ± 0.08 | 34.00 b ± 4.32 |
| Standards | Trolox 40.22 ± 3.64 | Trolox 1.60 ± 0.05 | EDTA 77.33 ± 2.87 |
| BHT 31.67 ± 2.71 | BHT 1.27 ± 0.01 |
| Propolis Extracts | Hemolysis (%) */Dominated RBC Shape | Oxidative Hemolysis Protection (%) |
|---|---|---|
| EPA | 3.16 a ± 1.44/D | 76.80 a ± 7.37 |
| EEP70 | 2.94 a ± 1.11/D | 61.40 b ± 12.50 |
| EEP96 | 3.04 a ± 1.37/D | 76.80 a ± 7.58 |
| Standards | Trolox 2.73 ± 1.50 | Trolox 90.01 ± 6.69 |
| BHT 3.38 ± 1.86 | BHT 33.00 ± 5.57 |
| Flavonoids | DPPH· Free-Radical-Scavenging Activity | Fe3+-Reducing Power | Ferrous Ion (Fe2+)-Chelating Activity | Oxidative Hemolysis Protection |
|---|---|---|---|---|
| Apigenin | 0.9294 | 0.7719 | 0.0083 | 0.9795 |
| Chrysin | 0.5739 | 0.2944 | 0.0347 | 0.9370 |
| Galangin | 0.3893 | 0.1267 | 0.4649 | 0.7434 |
| Kaempferol | 0.5739 | 0.2944 | 0.0347 | 0.9370 |
| Pinocembrin | 0.5739 | 0.2944 | 0.0347 | 0.9370 |
| Quercetin | 0.3398 | 0.2422 | 0.1441 | 0.7630 |
| Fungal Strain | MIC (mg/mL) | |||
|---|---|---|---|---|
| EPA | EEP70 | EEP96 | 3-Iodo-2-propynylbutylcarbamate | |
| Aspergillus niger | 7.5 | 7.5 | 7.5 | 0.75 |
| Aspergillus versicolor | 2.0 | 2.0 | 2.0 | 0.75 |
| Paecilomyces variotii | 2.0 | 5.0 | 7.5 | 1.0 |
| Penicillium funiculosum | 7.5 | 5.0 | 5.0 | 1.0 |
| Trichoderma virens | 5.0 | 5.0 | 5.0 | 1.0 |
| Penicillium cyclopium | 1.0 | 1.0 | 1.5 | 0.75 |
| Aureobasidiumpullulans | 0.5 | 0.5 | 1.0 | 1.0 |
| Flavonoids | Apigenin | Chrysin | Galangin | Kaempferol | Pinocembrin | Quercetin |
|---|---|---|---|---|---|---|
| p-Value of the Wilks test | 0.0048 | 0.0000 | 0.0013 | 0.0000 | 0.0000 | 0.8934 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, M.; Mrówczyńska, L.; Kwaśniewska-Sip, P.; Waśkiewicz, A.; Nowak, P.; Ratajczak, I. Effect of the Solvent on Propolis Phenolic Profile and its Antifungal, Antioxidant, and In Vitro Cytoprotective Activity in Human Erythrocytes Under Oxidative Stress. Molecules 2020, 25, 4266. https://doi.org/10.3390/molecules25184266
Woźniak M, Mrówczyńska L, Kwaśniewska-Sip P, Waśkiewicz A, Nowak P, Ratajczak I. Effect of the Solvent on Propolis Phenolic Profile and its Antifungal, Antioxidant, and In Vitro Cytoprotective Activity in Human Erythrocytes Under Oxidative Stress. Molecules. 2020; 25(18):4266. https://doi.org/10.3390/molecules25184266
Chicago/Turabian StyleWoźniak, Magdalena, Lucyna Mrówczyńska, Patrycja Kwaśniewska-Sip, Agnieszka Waśkiewicz, Piotr Nowak, and Izabela Ratajczak. 2020. "Effect of the Solvent on Propolis Phenolic Profile and its Antifungal, Antioxidant, and In Vitro Cytoprotective Activity in Human Erythrocytes Under Oxidative Stress" Molecules 25, no. 18: 4266. https://doi.org/10.3390/molecules25184266