A New Method for In-Situ Skin Penetration Analysis by Confocal Raman Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Porcine Ear Skin
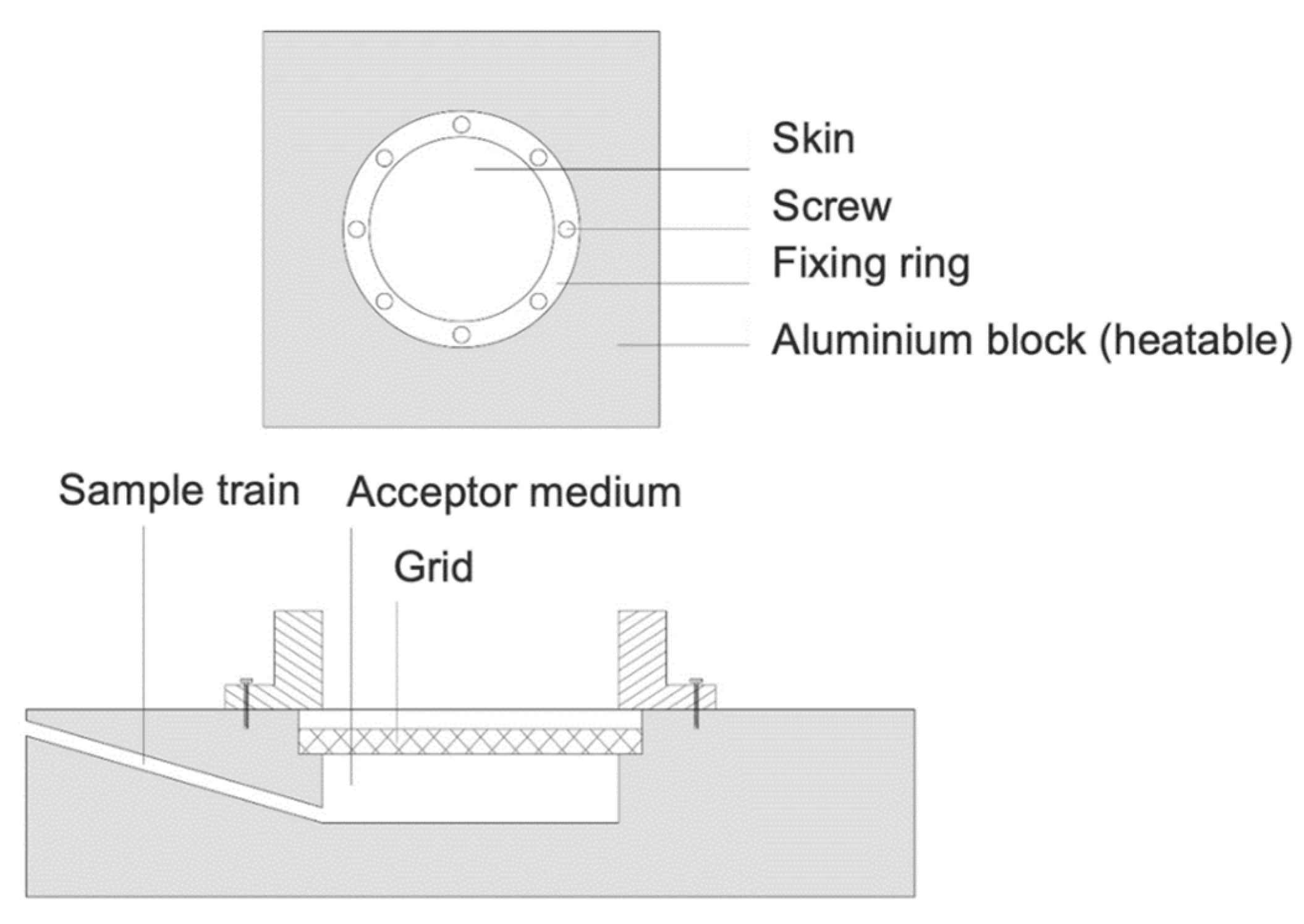
2.3. Incubation of Porcine Ear Skin in a Heatable Diffusion Cell
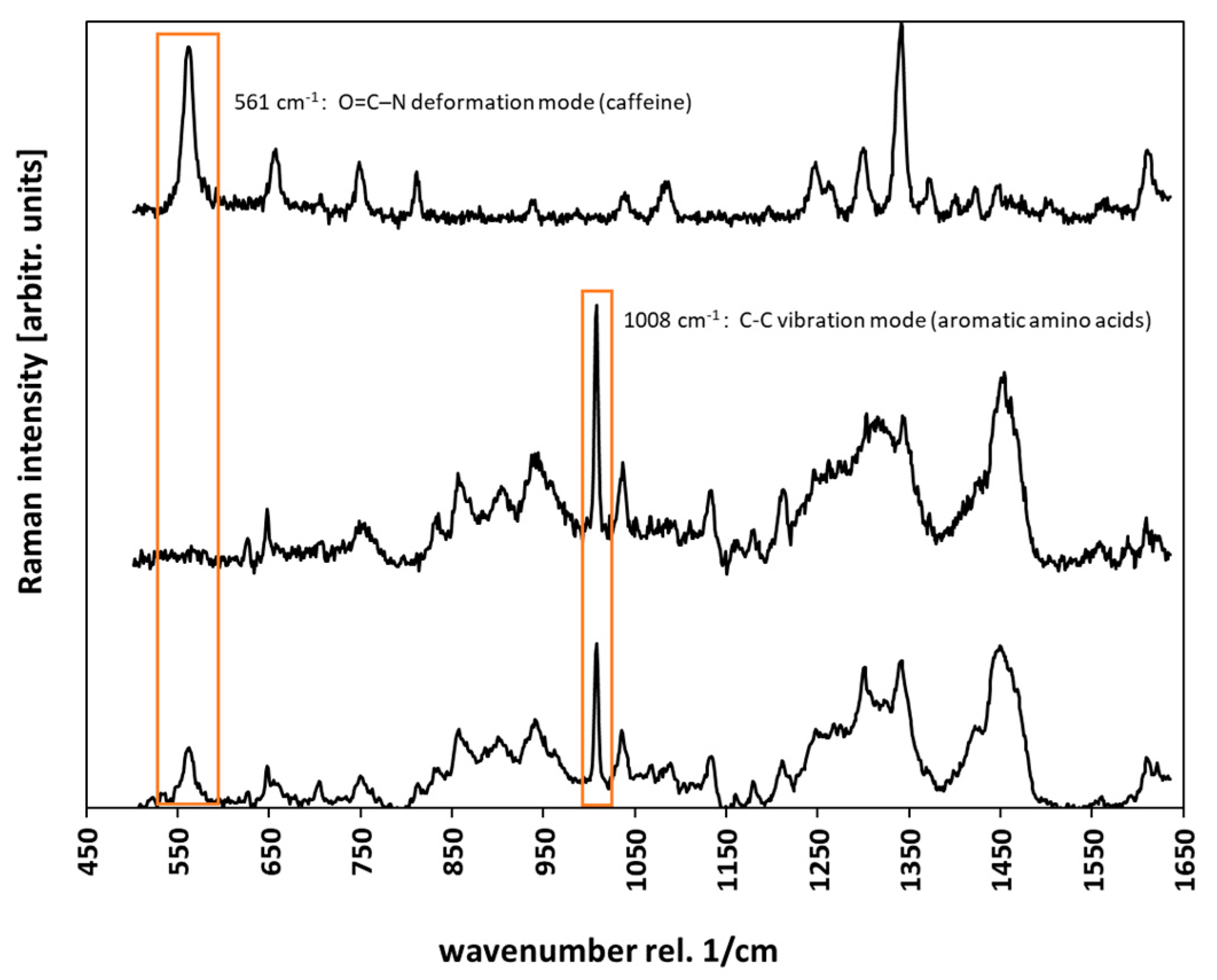
2.4. Confocal Raman Microscopy (CRM)
2.5. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouwstra, J.A.; Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 2080–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, K.; Heinrich, U.; Tronnier, H. Influence of different cosmetic formulations on the human skin barrier. Skin Pharmacol. Physiol. 2014, 27, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Norlén, L. Current understanding of skin barrier morphology. Skin Pharmacol. Physiol. 2013, 26, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Hadgraft, J. Skin the final frontier. Int. J. Pharm. 2001, 224, 1–18. [Google Scholar] [CrossRef]
- Weiser, J.R.; Saltzman, W.M. Controlled release for local delivery of drugs: Barriers and models. J. Control. Release 2014, 190, 664–673. [Google Scholar] [CrossRef] [Green Version]
- Holmgaard, R.; Benfeldt, E.; Nielsen, J.B. Percutaneous penetration - methodological considerations. Basic Clin. Pharmacol. Toxicol. 2014, 115, 101–109. [Google Scholar] [CrossRef]
- Escobar-Chavez, J.J.; Merino-Sanjuán, V.; López-Cervantes, M.; Urban-Morlan, Z.; Piñón-Segundo, E.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. The Tape-Stripping Technique as a Method for Drug Quantification in Skin. J. Pharm. Pharm. Sci. 2008, 11, 104–130. [Google Scholar] [CrossRef] [Green Version]
- Heck, R.; Lukić, M.; Savić, S.D.; Daniels, R.; Lunter, D.J. Ex vivo skin permeation and penetration of nonivamide from and in vivo skin tolerability of film-forming formulations containing porous silica. Eur. J. Pharm. Sci. 2017, 106, 34–40. [Google Scholar] [CrossRef]
- Lohan, S.B. Investigation of the cutaneous penetration behavior of dexamethasone loaded to nano-sized lipid particles by EPR spectroscopy, and confocal Raman and laser scanning microscopy. Eur. J. Pharm. Biopharm. 2017, 116, 102–110. [Google Scholar] [CrossRef]
- Gotter, B.; Faubel, W.; Neubert, R.H.H. Optical methods for measurements of skin penetration. Skin Pharmacol. Physiol. 2008, 21, 156–165. [Google Scholar] [CrossRef]
- Matousek, P. Subsurface probing in diffusely scattering media using spatially offset Raman spectroscopys. Appl. Spectrosc. 2005, 59, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Mas, J.; Forbes, L.H.; Andrews, M.R.; Dholakia, K. Depth-resolved multimodal imaging: Wavelength modulated spatially offset Raman spectroscopy with optical coherence tomography. J. Biophotonics 2018, 11, 1–7. [Google Scholar]
- Caspers, P.J. Method to quantify the in vivo skin penetration of topically applied materials based on confocal Raman spectroscopy. Transl. Biophotonics 2019, 1, 1–10. [Google Scholar]
- Lunter, D.; Daniels, R. Confocal Raman microscopic investigation of the effectiveness of penetration enhancers for procaine delivery to the skin. J. Biomed. Opt. 2014, 19, 126015. [Google Scholar] [CrossRef]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In vitro-in vivo correlation in skin permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef]
- Alonso, C. Caffeine delivery in porcine skin: A confocal Raman study. Arch. Dermatol. Res. 2018, 310, 657–664. [Google Scholar] [CrossRef]
- Tfaili, S.; Josse, G.; Angiboust, J.F.; Manfait, M.; Piot, O. Monitoring caffeine and resveratrol cutaneous permeation by confocal Raman microspectroscopy. J. Biophotonics 2014, 7, 676–681. [Google Scholar] [CrossRef]
- Potard, G.; Laugel, C.; Baillet, A.; Schaefer, H.; Marty, J.P. Quantitative HPLC analysis of sunscreens and caffeine during in vitro percutaneous penetration studies. Int. J. Pharm. 1999, 189, 249–260. [Google Scholar] [CrossRef]
- Jacobi, U. Porcine ear skin: An in vitro model for human skin. Ski. Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. Vitr. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Jung, E.C.; Maibach, H.I. Animal models for percutaneous absorption. J. Appl. Toxicol. 2015, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lunter, D.J. Confocal Raman microspectroscopy as an alternative method to investigate the extraction of lipids from stratum corneum by emulsifiers and formulations. Eur. J. Pharm. Biopharm. 2018, 127, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lunter, D.J. Systematic investigation of the effect of non-ionic emulsifiers on skin by confocal raman spectroscopy—A comprehensive lipid analysis. Pharmaceutics 2020, 12, 223. [Google Scholar] [CrossRef] [Green Version]
- Franz, T.J. Percutaneous absorption. On the relevance of in vitro data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef] [Green Version]
- Tfayli, A.; Piot, O.; Manfait, M. Confocal Raman microspectroscopy on excised human skin: Uncertainties in depth profiling and mathematical correction applied to dermatological drug permeation. J. Biophotonics 2008, 1, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Rajalahti, T.; Kvalheim, O.M. Multivariate data analysis in pharmaceutics: A tutorial review. Int. J. Pharm. 2011, 417, 280–290. [Google Scholar] [CrossRef]
- Ascencio, S.M. Confocal Raman microscopy and multivariate statistical analysis for determination of different penetration abilities of caffeine and propylene glycol applied simultaneously in a mixture on porcine skin ex vivo. Eur. J. Pharm. Biopharm. 2016, 104, 51–58. [Google Scholar] [CrossRef]
- Darvin, M.E.; Choe, C.-S.; Schleusener, J.; Lademann, J. Non-invasive depth profiling of the stratum corneum in vivo using confocal Raman microscopy considering the non-homogeneous distribution of keratin. Biomed. Opt. Express 2019, 10, 3092. [Google Scholar] [CrossRef] [Green Version]
- Franzen, L.; Selzer, D.; Fluhr, J.; Schaefer, U.F.; Windbergs, M. European Journal of Pharmaceutics and Biopharmaceutics Towards drug quantification in human skin with confocal Raman microscopy. Eur. J. Pharm. Biopharm. 2012, 84, 437–444. [Google Scholar] [CrossRef]
- Ashtikar, M.; Matthäus, C.; Schmitt, M.; Krafft, C.; Fahr, A. European Journal of Pharmaceutical Sciences Non-invasive depth profile imaging of the stratum corneum using confocal Raman microscopy: First insights into the method. Eur. J. Pharm. Sci. 2013, 50, 601–608. [Google Scholar] [CrossRef]
- Lunter, D.J. How Confocal Is Confocal Raman Microspectroscopy on the Skin? Impact of Microscope Configuration and Sample Preparation on Penetration Depth Profile. Skin Pharmacol. Physiol. 2016, 29, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Franzen, L.; Anderski, J.; Windbergs, M. Quantitative detection of caffeine in human skin by confocal Raman spectroscopy—A systematic in vitro validation study. Eur. J. Pharm. Biopharm. 2015, 95, 110–116. [Google Scholar] [CrossRef]
- Rubio, L. Barrier function of intact and impaired skin: Percutaneous penetration of caffeine and salicylic acid. Int. J. Dermatol. 2011, 50, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Pyatski, Y.; Zhang, Q.; Mendelsohn, R.; Flach, C.R. Effects of permeation enhancers on flufenamic acid delivery in Ex vivo human skin by confocal Raman microscopy. Int. J. Pharm. 2016, 505, 319–328. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |
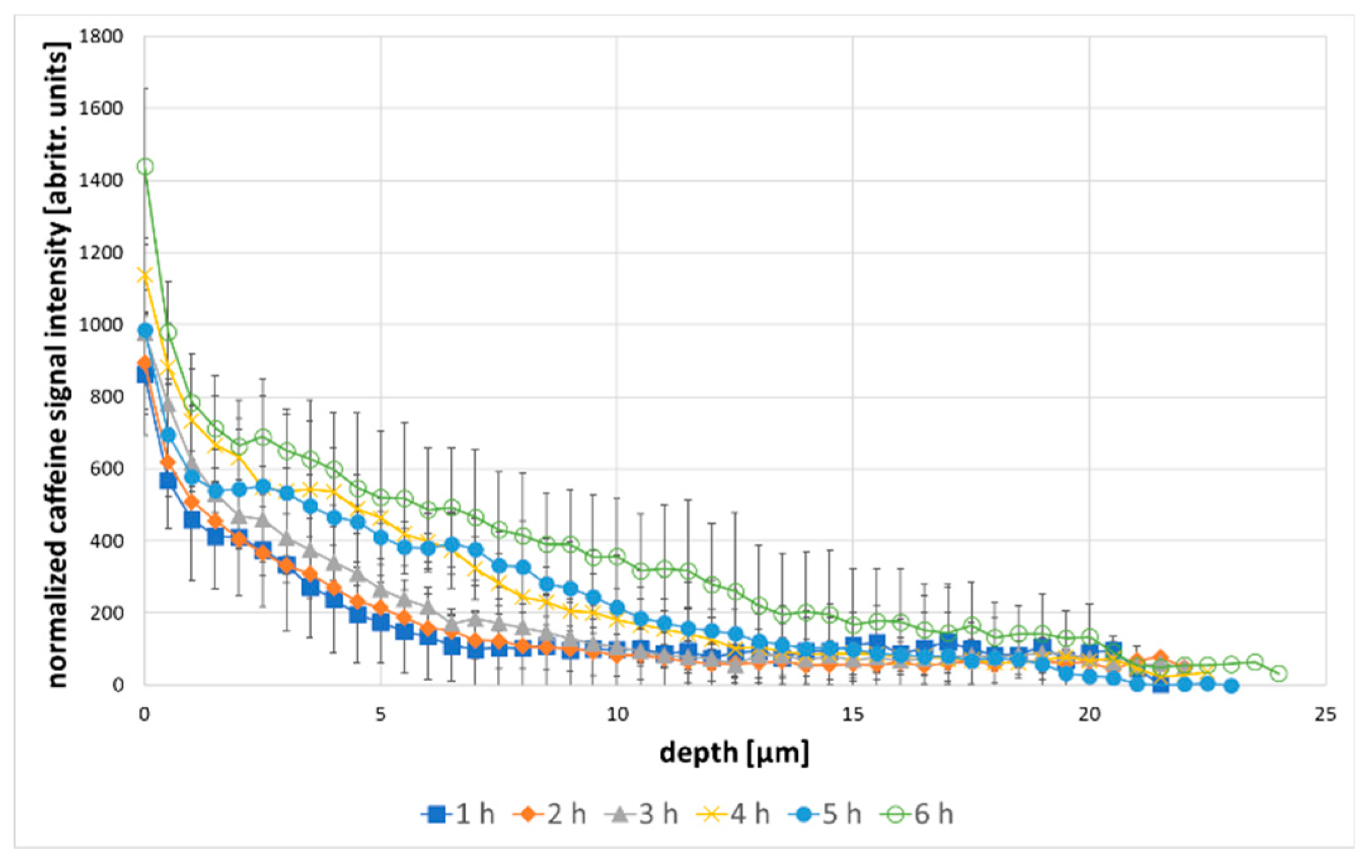

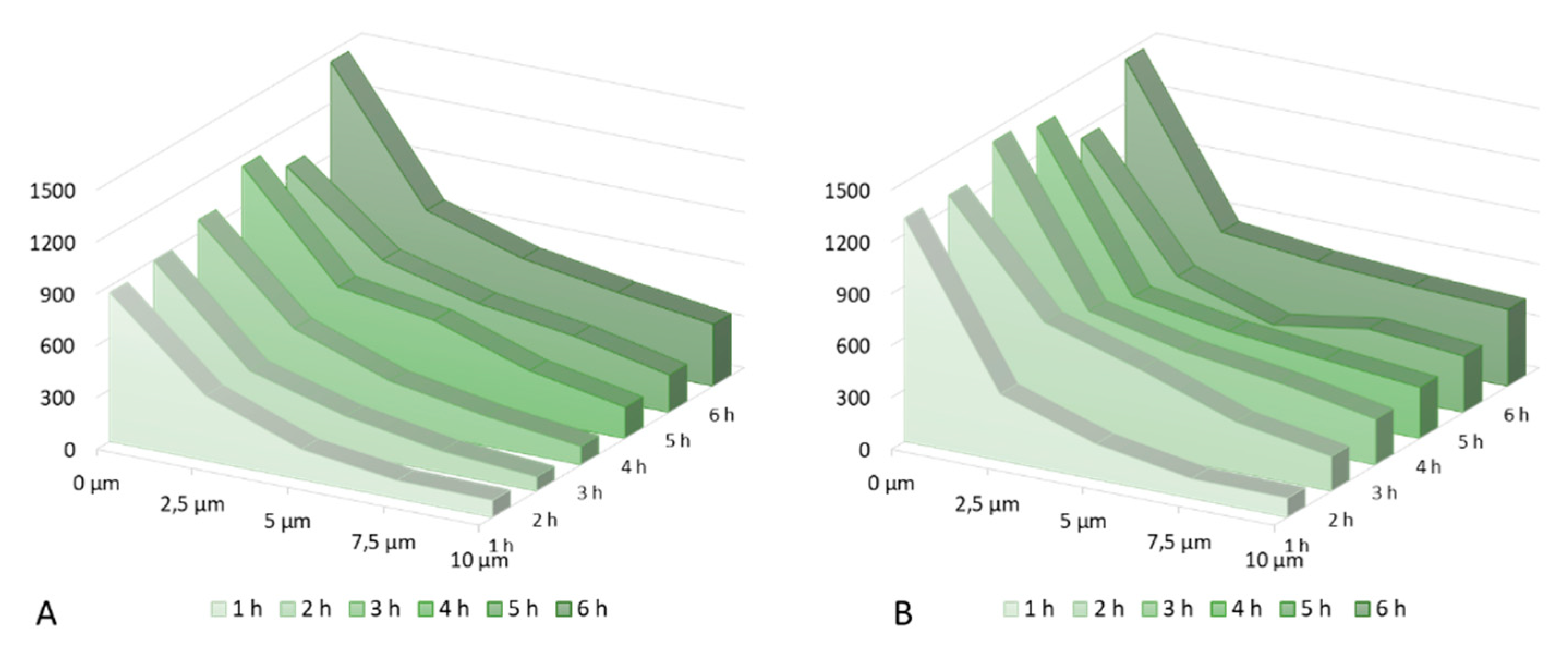
| AUC caffeine (A) [arbitr.units · µm] | AUC caffeine (B) [arbitr.units · µm] | Enhancement Ratio | |
|---|---|---|---|
| 1 h | 3499 | 4222 | 1.21 |
| 2 h | 3447 | 6841 | 1.98 |
| 3 h | 4243 | 6541 | 1.54 |
| 4 h | 5779 | 6816 | 1.18 |
| 5 h | 5509 | 6962 | 1.26 |
| 6 h | 7978 | 9636 | 1.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krombholz, R.; Lunter, D. A New Method for In-Situ Skin Penetration Analysis by Confocal Raman Microscopy. Molecules 2020, 25, 4222. https://doi.org/10.3390/molecules25184222
Krombholz R, Lunter D. A New Method for In-Situ Skin Penetration Analysis by Confocal Raman Microscopy. Molecules. 2020; 25(18):4222. https://doi.org/10.3390/molecules25184222
Chicago/Turabian StyleKrombholz, Richard, and Dominique Lunter. 2020. "A New Method for In-Situ Skin Penetration Analysis by Confocal Raman Microscopy" Molecules 25, no. 18: 4222. https://doi.org/10.3390/molecules25184222







