1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure
Abstract
:1. Introduction
2. Results and Discussion
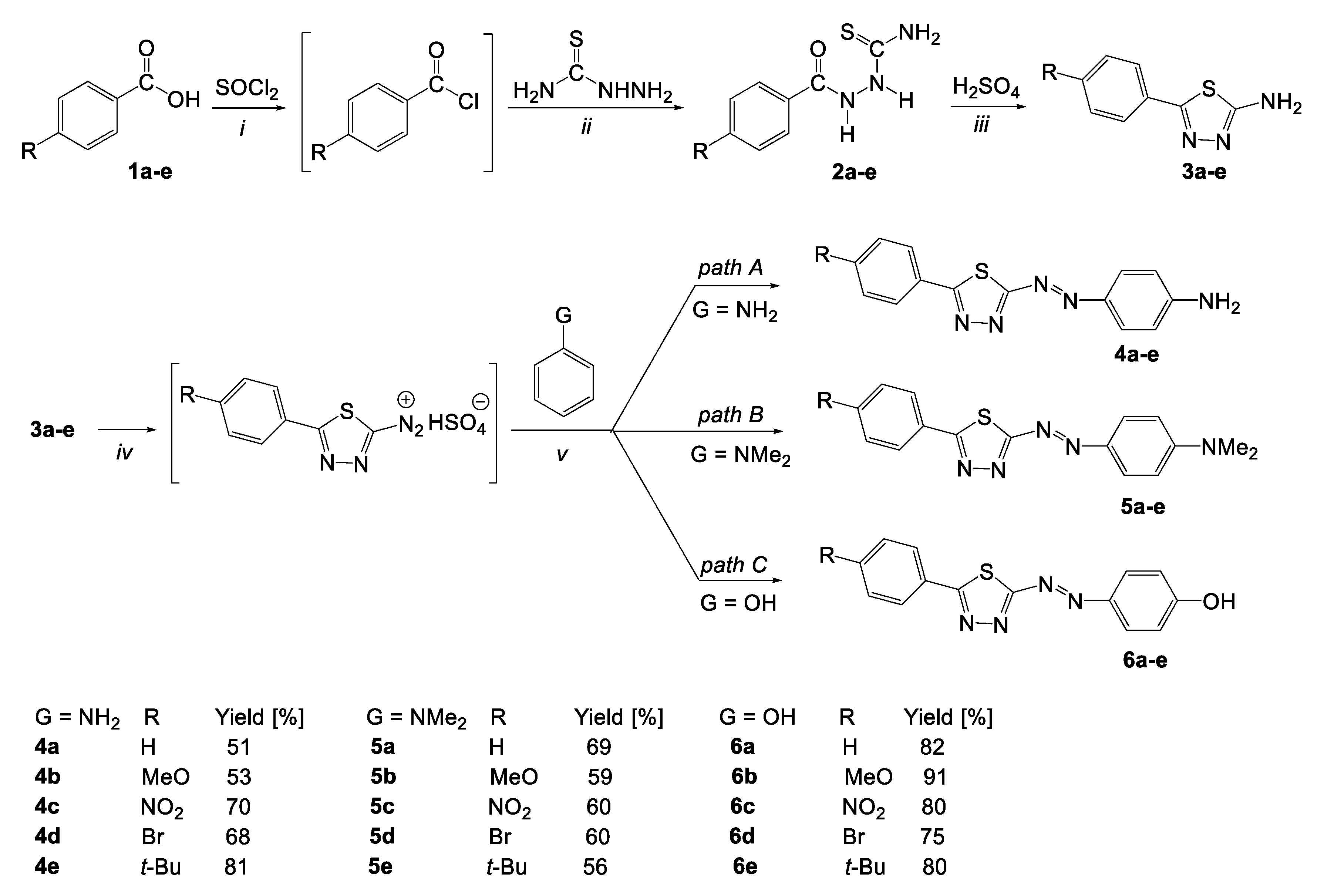
2.1. Synthesis
2.2. Spectral Characterization
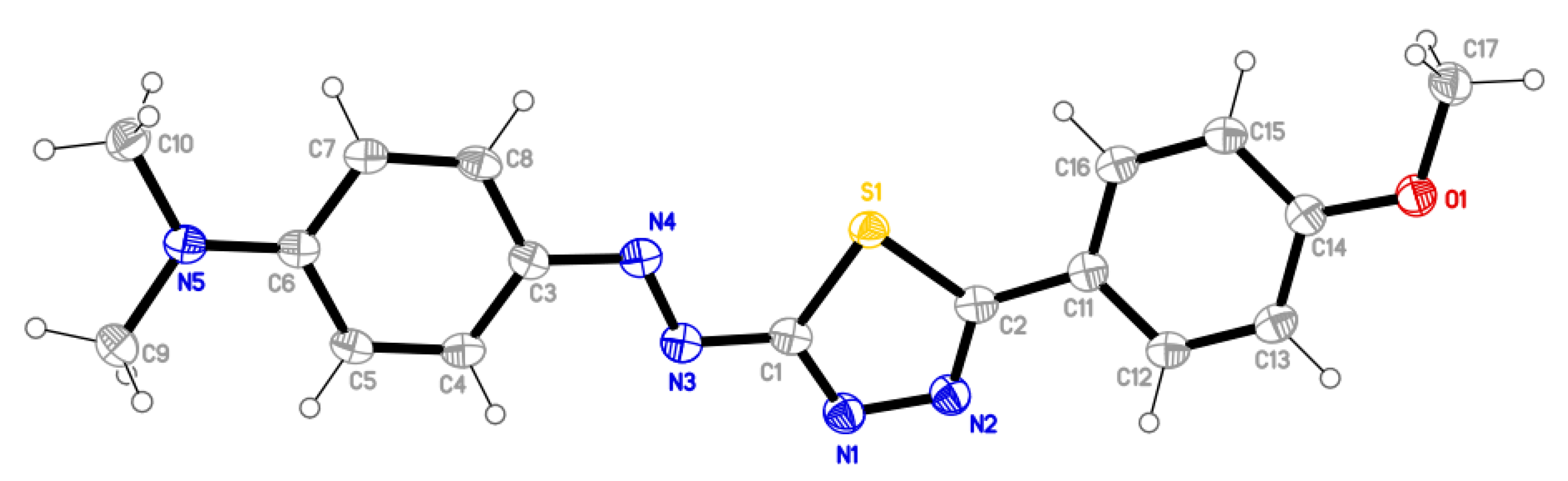
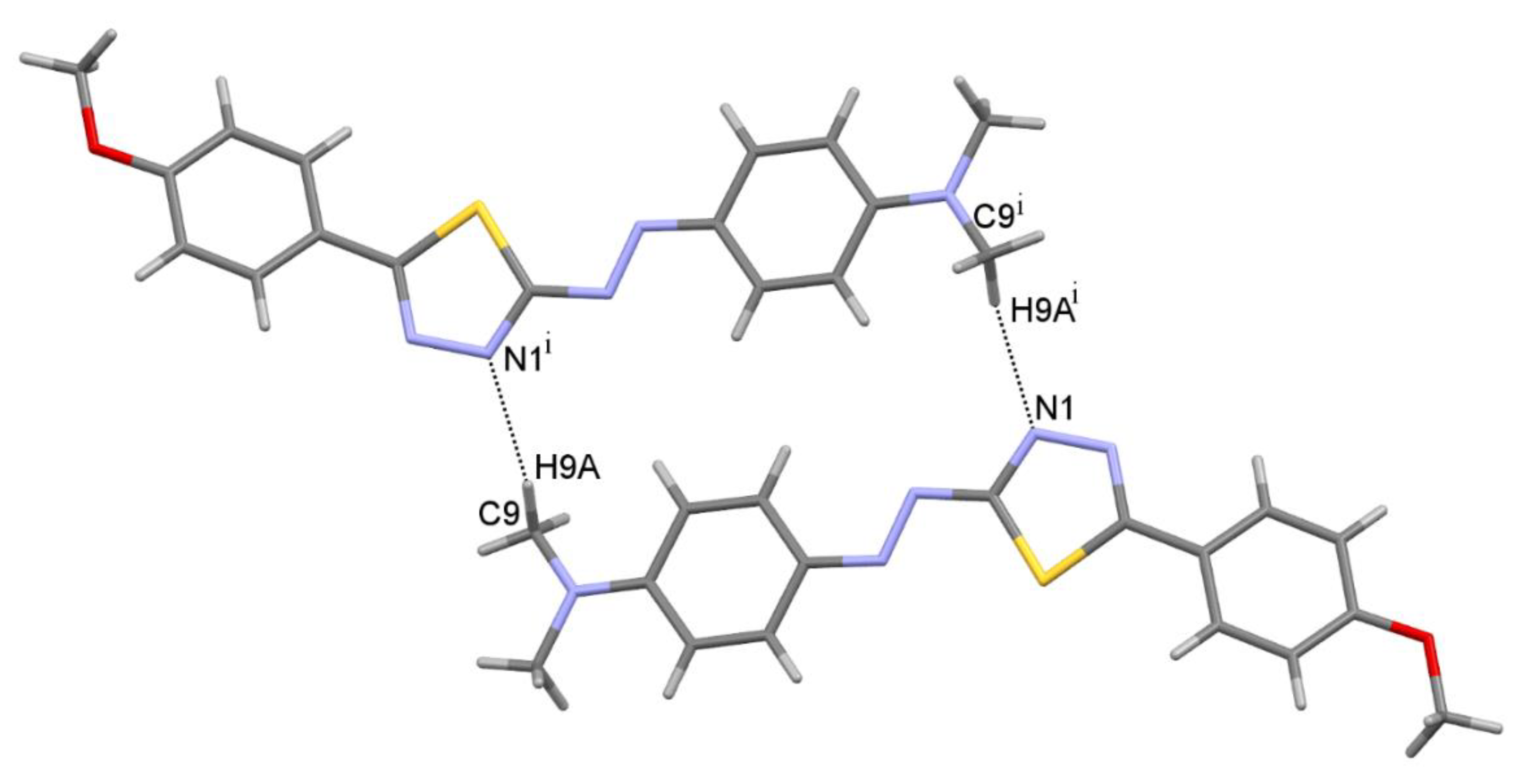
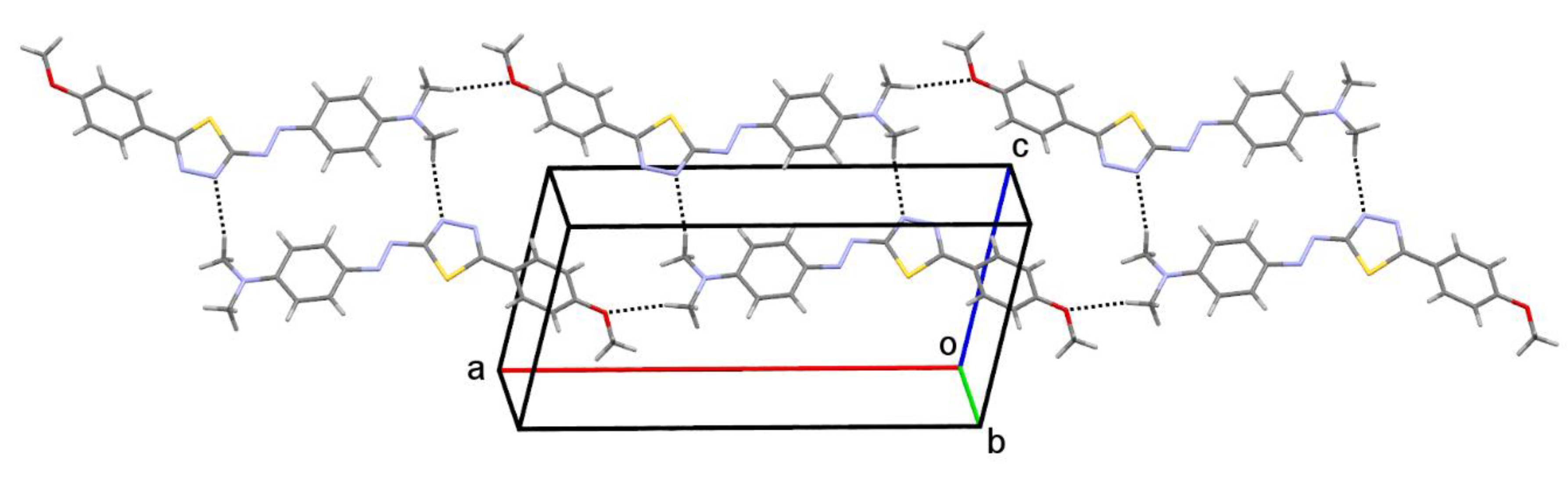
2.3. Molecular Structure
3. Experimental
3.1. General Information
3.2. Synthesis and Characterization
3.2.1. General Procedure for the Synthesis of 2-Benzoylhydrazinecarbothioamide Derivatives (2a–e)
3.2.2. General Procedure for the Synthesis of 2-Amino-1,3,4-thiadiazole Derivatives (3a–e)
3.2.3. General Procedure for the Synthesis of 2-(4-Aminophenylazo)-5-phenyl-1,3,4-thiadiazole Derivatives (4a–e)
3.2.4. General procedure for the synthesis of 2-[4-(N,N-dimethylamino)phenylazo]-5-phenyl-1,3,4-thiadiazole derivatives (5a–e)
3.2.5. General Procedure for the Synthesis of 2-(4-Hydroxyphenylazo)-5-phenyl-1,3,4-thiadiazole Derivatives (6a–e)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Koutentis, P.A.; Constantinides, C.P. 1,3,4-Thiadiazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier Science Ltd.: Oxford, UK, 2008; Volume 5, pp. 567–605. [Google Scholar]
- Hu, Y.; Li, C.; Wang, X.; Yang, Y.; Zhu, H. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Geng, J.; Liu, Y.; Yu, S.; Zhao, G. Thiadiazole—A promising structure in medicinal chemistry. Chem. Med. Chem. 2013, 8, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Karaburun, A.; Acar Cevik, U.; Osmaniye, D.; Saglik, B.; Kaya Cavusoglu, B.; Levent, S.; Ozkay, Y.; Koparal, A.; Behcet, M.; Kaplancikli, Z.A. Synthesis and evaluation of new 1,3,4-thiadiazole derivatives as potent antifungal agents. Molecules 2018, 23, 3129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gur, M.; Sener, N.; Kastas, C.A.; Ozkan, O.E.; Muglu, H.; Elmaswaria, M.A.M. Synthesis and characterization of some new heteroaromatic compounds having chirality adjacent to a 1,3,4-thiadiazole moiety and their antimicrobial activities. J. Heterocycl. Chem. 2017, 54, 3578–3590. [Google Scholar] [CrossRef]
- Hafez, H.N.; Hegab, M.I.; Ahmed-Farag, I.S.; El-Gazzar, A.B.A. A facile regioselective synthesis of novel spiro-thioxanthene and spiro-xanthene-9′,2-[1,3,4]thiadiazole derivatives as potential analgesic and anti-inflammatory agents. Bioorg. Med. Chem. 2008, 18, 4538–4543. [Google Scholar] [CrossRef]
- Altintop, M.D.; Sever, B.; Ozdemir, A.; Iglin, S.; Alti, O.; Turan-Zitouni, G.; Kaplancikli, Z.A. Synthesis and evaluation of a series of 1,3,4-thiadiazole derivatives as potential anticancer agents. Anticancer Agents Med. Chem. 2019, 18, 1606–1616. [Google Scholar] [CrossRef]
- Richwine, J.R. 2,5-Dimercapto-1,3,4-thiadiazole as a Cross-Linker for Saturated Halogen-Containing Polymers. Inventor: Hercules Incorporated Wilmington, Assignee. U.S. Patent 4288576, 8 September 1981. [Google Scholar]
- Jin, L.; Wang, G.; Li, X. Poly(2,5-dimercapto-1,3,4-thiadiazole)/sulfonated graphene composite as cathode material for rechargeable lithium batteries. J. Appl. Electrochem. 2011, 41, 377–382. [Google Scholar] [CrossRef]
- Maradiya, H.R. Monoazo disperse dyes based on 2-amino-1,3,4-thiadiazole derivatives. J. Serb. Chem. Soc. 2002, 67, 709–718. [Google Scholar] [CrossRef]
- Yan, H.; Su, H.; Tian, D.; Miao, F.; Li, H. Synthesis of triazolo-thiadiazole fluorescent organic nanoparticles as primary sensor toward Ag+ and the complex of Ag+ as secondary sensor toward cysteine. Sens. Actuators B Chem. 2011, 160, 656–661. [Google Scholar] [CrossRef]
- Hipler, F.; Fischer, R.A.; Muller, J. Matrix-isolation pyrolysis investigation of mercapto-functionalized 1,3,4-thiadiazoles: Thermal stability of thiadiazole lubricant additives. Phys. Chem. Chem. Phys. 2005, 7, 731–737. [Google Scholar] [CrossRef]
- Dawson, J.F. Developments in disperse dyes. Color. Technol. 1978, 9, 25–35. [Google Scholar] [CrossRef]
- Arcoria, A.; De Giorgi, M.R.; Fatuzzo, F.; Longo, M.L. Dyeing properties of basic azo-dyes from 2-amino thiadiazole. Dyes Pigment. 1993, 21, 67–74. [Google Scholar] [CrossRef]
- De Giorgi, M.R.; Carpignano, R.; Cerniani, A. Structure optimization in a series of thiadiazole disperse dyes using a chemometric approach. Dyes Pigment. 1998, 37, 187–196. [Google Scholar] [CrossRef]
- Zollinger, H. Azo dyes and Pigments. In Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments, 3rd ed.; Viley-VCH: Zurich, Switzerland, 2003; pp. 165–254. [Google Scholar]
- Ullrich, R.; Grewer, T. Decomposition of aromatic diazonium compounds. Thermochim. Acta 1993, 225, 201–211. [Google Scholar] [CrossRef]
- Zhao, R.; Tan, C.; Xie, Y.; Gao, C.; Liu, H.; Jiang, Y. One step synthesis of azo compounds from nitroaromatics and anilines. Tetrahedron Lett. 2011, 52, 3805–3809. [Google Scholar] [CrossRef]
- Chung, T.F.; Wu, Y.M.; Cheng, C.H. Reduction of aromatic nitro compounds by ethylenediamine. A new selective reagent for the synthesis of symmetric azo compounds. J. Org. Chem. 1984, 49, 1215–1217. [Google Scholar] [CrossRef]
- Srinivasa, G.R.; Abiraj, K.; Channe Gowda, D. Lead-catalyzed synthesis of azo compounds by ammonium acetate reduction of aromatic nitro compounds. Synth. Commun. 2003, 33, 4221–4227. [Google Scholar] [CrossRef]
- Noureldin, N.A.; Bellegarde, J.W. A novel method. The synthesis of ketones and azobenzenes using supported permanganate. Synthesis 1999, 6, 939–942. [Google Scholar] [CrossRef]
- Bhatnagar, I.; George, M.V. Oxidation with metal oxides. III. Oxidation of diamines and hydrazines with manganese dioxide. J. Org. Chem. 1968, 33, 2407–2411. [Google Scholar] [CrossRef]
- Faustino, H.; El-Shisthawy, R.M.; Reis, L.V.; Santos, P.F.; Almeida, P. 2-Nitrosobenzothiazoles: Useful synthons for new azobenzothiazole dyes. Tetrahedron Lett. 2008, 49, 6907–6909. [Google Scholar] [CrossRef]
- Tambe, S.M.; Tasaganva, R.G.; Inamdar, S.R.; Kariduraganavar, M.Y. Synthesis and characterization of nonlinear optical side-chain polyimides containing the thiadiazole chromophores. J. Appl. Pol. Sci. 2012, 125, 1049–1058. [Google Scholar] [CrossRef]
- Tomi, I.H.R.; Al-Daraji, A.H.R.; Al-Qaysi, R.R.T.; Hasson, M.M.; Al-Dulaimy, K.H.D. Synthesis, characterization and biological activities of some azo derivatives of aminothiadiazole derived from nicotinic and isonicotinic acids. Arab. J. Chem. 2014, 7, 687–694. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.T.K.; Keshavayya, J.; Rajesk, T.N.; Peethambar, S.K.; Ali, A.R.S. Synthesis, characterization, and biological activity of 5-phenyl-1,3,4-thiadiazole-2-amine incorporated azo dye derivatives. Org. Chem. Int. 2013, 370626. [Google Scholar] [CrossRef] [Green Version]
- Kedzia, A.; Kudelko, A.; Swiatkowski, M.; Kruszynski, R. Microwave-promoted synthesis of highly luminescent s-tetrazine-1,3,4-oxadiazole and s-tetrazine-1,3,4-thiadiazole hybrids. Dyes Pigment. 2020, 172, 107865. [Google Scholar] [CrossRef]
- Wróblowska, M.; Kudelko, A.; Kuźnik, N.; Łaba, K.; Łapkowski, M. Synthesis of Extended 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives in the Suzuki Cross-Coupling Reactions. J. Heterocycl. Chem. 2017, 54, 1550–1557. [Google Scholar] [CrossRef]
- Wróblowska, M.; Kudelko, A.; Łapkowski, M. Efficient Synthesis of Conjugated 1,3,4-Thiadiazole Hybrids through Palladium-Catalyzed Cross Coupling of 2,5-Bis(4-bromophenyl)-1,3,4-thiadiazole with Boronic Acids. Synlett 2015, 26, 2127–2130. [Google Scholar] [CrossRef]
- Mavrova, A.T.; Wesselinova, D.; Tsenov, Y.A.; Denkova, P. Synthesis, cytotoxity and effects of some 1,2,4-triazole and 1,3,4-thiadiazole derivatives on immunocompetent cells. Eur. J. Med. Chem. 2009, 44, 63–69. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Cowley, J.M. Scattering factors for the diffraction of electrons by crystalline solids. In International Tables for Crystallography, Volume C: Mathematical, Physical and Chemical Tables, 3rd ed.; Prince, E., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 259–262. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 1999; pp. 293–342. [Google Scholar]
- Kruszynski, R.; Sieranski, T. Can stacking interactions exist beyond the commonly accepted limits? Cryst. Growth Des. 2016, 16, 587–595. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Campaigne, E.; Selby, T.P. Thiazoles and thiadiazines. The condensation of ethyl 4-chloroacetoacetate with thiosemicarbazide. J. Heterocycl. Chem. 1978, 15, 401–411. [Google Scholar] [CrossRef]
- Plumitallo, A.; Cardia, M.C.; Distinto, S.; DeLogu, A.; Maccioni, E. Synthesis and anti-microbial activity evaluation of some new 1-benzoyl-isothiosemicarbazides. Farmaco 2004, 59, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Malbec, F.; Milcent, R.; Barbier, G. Dérivés de la dihydro-2,4 triazole-1,2,4 thione-3 et de l’amino-2 thiadiazole-1,3,4 à partir de nouvelles thiosemicarbazones d’esters. J. Heterocycl. Chem. 1984, 21, 1689–1698. [Google Scholar] [CrossRef]
- Giri, S.; Nizamuddin Srivastava, U.C. Synthesis of some N-(5-aryl/aryloxymethyl-1,3,4-thiadiazol-2-yl)glyoxylamide thiosemicarbazones as potential antiviral and antifungal agents. Agric. Biol. Chem. 1983, 47, 103–105. [Google Scholar] [CrossRef]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. Synthesis and CNS depressant activity of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008, 43, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Gibaldi, D.; Pinto, A.C.; Bozza, M.; Boechat, N. Synthesis and trypanocidal evaluation of news 5-[N-(3-(5-substituted)-1,3,4-thiadiazolyl)]amino-1-methyl-4-nitroimidazoles. Lett. Drug Des. Discov. 2006, 3, 98–101. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4a–e, 5a–e, 6a–e are available from the authors. |

| λmax (nm) | The Most Important Orbitals Involved in Electronic Transitions | Character of Transition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Calculated | ||||||||||
| 4 (G=NH2) | |||||||||||
| a | b | c | d | e | a | b | c | d | e | ||
| 237 (3.95) | 256 (4.05) | 267 (4.07) | 249 (3.90) | 248 (4.02) | 261.44 (0.0909) | 276.70 (0.1045) | 237.50 (0.0536) | 256.39 (0.0432) | 270.17 (0.2800) | (a, e)H-2→L+1 (b)H→L+2 (c)H-2→L+2 (d)H-8→L | (a, e)n(NH2)/π→π* (b)n(NH2)/n(MeO)/π→π*(C6-rings) (c)n(NH2)/n(NO2)/π→π* (d)n(Br)/π(S-ring)→π* |
| 262.74 (0.0776) | 280.67 (0.2599) | 253.28 (0.0806) | 274.45 (0.3976) | 273.29 (0.1360) | (a)H-6→L (b)H-1→L+1 (c)H-6→L+1 (d)H-2→L+1 (e) H→L+2 | (a)n(NH2)/π→π* (b)n(NH2)/n(MeO)/π→π* (c)π→π* (d)n(Br)/n(NH2)/π→π* (e)n(NH2)/π→π*(C6-rings) | |||||
| 266.87 (0.1051) | 268.23 (0.0893) | (a)H→L+3 (c)H→L+4 | (a, c)n(NH2)/π→π*(C6-rings) | ||||||||
| 272.57 (0.1083) | (a)H→L+2 | (a)n(NH2)/π→π*(C6-rings) | |||||||||
| 285 (3.72) | 286 (3.88) | 286 (3.63) | 284 (3.79) | ||||||||
| 316 (3.56) | 319 (3.78) | 321 (4.03) | 318 (3.56) | 319 (3.68) | 325.66 (0.0203) | 322.13 (0.0500) | 312.40 (0.0522) | 333.77 (0.0204) | 324.73 (0.0294) | (a, b, d, e)H→L+1 (c)H-2→L+1 | (a, c, e)n(NH2)/π→π* (b)n(NH2)/n(MeO)/π→π* (d)n(Br)/n(NH2)/π→π* |
| 344.08 (0.1372) | (c)H-2→L | (c)n(NH2)/π→π* | |||||||||
| 491 (4.52) | 491 (4.46) | 507 (4.35) | 496 (4.41) | 492 (4.47) | 383.91 (0.1281) | 428.57 (0.7203) | (b)H-1→L (c)H→L+1 | (b)n(NH2)/n(MeO)/π→π* (c)n(NH2)/π→π* | |||
| 459.54 (1.4831) | 478.70 (1.3233) | 529.34 (0.8884) | 464.44 (1.4918) | 464.82 (1.4522) | H→L | (a, c, e)n(NH2)/π→π* (b) n(NH2)/n(MeO)/π→π* (d)n(Br)/n(NH2)/π→π* | |||||
| 5 (G=NMe2) | |||||||||||
| a | b | c | d | e | a | b | c | d | e | ||
| 243 (3.96) | 256 (3.83) | 249 (3.73) | 251 (3.80) | 245 (4.06) | 264.95 (0.1359) | 278.83 (0.0592) | 241.40 (0.0442) | 256.30 (0.0430) | 270.21 (0.0655) | (a)H-2→L+1 (b, e) H-1→L+1 (c)H-2→L+2 (d)H-8→L | (a)π→π* (b) n(MeO)/n(NMe2)/π→π* (c)n(NO2)/π→π* (d)n(Br)/π→π* (e)σ(t-Bu)/n(Me2)/π→π* |
| 275.87 (0.1374) | 283.75 (0.1408) | 261.09 (0.0967) | 274.64 (0.1508) | 270.38 (0.0853) | (a)H-6→L (b)H→L+2 (c)H-6→L+1 (d)H-2→L+1 (e)H-1→L+1 | (a, c)π→π* (b)n(MeO)/n(NMe2)/π→π*(C6-ring near MeO) (d)n(Br)/n(NMe2)/π→π* (e)σ(t-Bu)/n(Me2)/π→π* | |||||
| 281.40 (0.0925) | 287.77 (0.2354) | 277.34 (0.1087) | 282.30 (0.2530) | 279.49 (0.2116) | (a)H→L+3 (b, d)H-5→L (c)H→L+4 (e)H-6→L | (a)n(NMe2)/π→π* (b)n(MeO)/π→π* (c)n(NMe2)/π→π*(C6-ring near NMe2) (d)n(Br)/π→π* (e)σ(t-Bu)/π→π* | |||||
| 281.87 (0.0869) | (e)H→L+2 | (e)n(NMe2)/π→π*(C6-rings) | |||||||||
| 293 (3.79) | 293 (3.73) | 299 (3.85) | 290 (3.61) | 295 (3.92) | |||||||
| 320 (3.68) | 334 (3.46) | 325 (3.49) | 323 (3.80) | 337.91 (0.0197) | 333.19 (0.0329) | 353.80 (0.1689) | 346.81 (0.0206) | 336.73 (0.0263) | (a, b, d, e)H→L+1 (c)H-2→L | (a, d, e)n(NMe2)/π→π* (b)n(MeO)/n(NMe2)/π→π* (c)n(NO2)/π→π* | |
| 515 (4.50) | 515 (4.25) | 530 (3.95) | 520 (4.30) | 515 (4.41) | 393.91 (0.0395) | 453.15 (0.8353) | (b)H-1→L (c)H→L+1 | (b)n(MeO)/n(NMe2)/π→π* (c)n(NMe2)/π→π* | |||
| 483.86 (1.4831) | 497.00 (1.1993) | 568.89 (0.8657) | 488.69 (1.5864) | 487.35 (1.5439) | H→L | (a, c, d, e)n(NMe2)/π→π* (b)n(MeO)/n(NMe2)/π→π* | |||||
| 6 (G=OH) | |||||||||||
| a | b | c | d | e | a | b | c | d | e | ||
| 220.52 (0.0983) | (b)H-4→L+1 | (b)π(C6-ring near OH)→π* | |||||||||
| 236 (4.00) | 231 (3.68) | 234.73 (0.0439) | 232.01 (0.0382) | (a)H→L+3 (c)H-2→L+2 | (a)n(OH)/π→π*(C6-ring near S-ring) (c)n(NO2)/(OH)/π→π* | ||||||
| 256 (3.90) | 254 (4.04) | 260 (3.72) | 245 (4.04) | 243 (4.10) | 251.23 (0.0758) | 234.51 (0.0525) | 249.20 (0.0821) | 238.98 (0.0306) | 234.61 (0.0464) | (a, e)H→L+2 (b)H-3→L+1 (c)H-8→L (d)H-4→L+1 | (a)n(OH)/π→π*(C6-ring near OH) (b)π(C6-ring near MeO)→π* (c)n(NO2)/(OH)/π→π* (d)π(C6-rings)→π* (e)σ(t-Bu)/n(OH)/π→π*(C6-ring near t-Bu) |
| 254.98 (0.1078) | 271.99 (0.1002) | 264.75 (0.0369) | 251.23 (0.0556) | 265.07 (0.2413) | (a)H-2→L+1 (b, c, e)H-6→L (d)H→L+3 | (a, c)n(OH)/π→π* (b)n(MeO)/n(OH)/π→π* (d)n(Br)/n(OH)/π→π*(C6-ring near OH) (e)σ(t-Bu)/n(OH)/π→π* | |||||
| 262.06 (0.1766) | 266.81 (0.1192) | (a)H-6→L+1 (d)H-2→L+1 | (a)n(OH)/π→π* (d)n(Br)/n(OH)/π→π* | ||||||||
| 270.78 (0.1692) | (d)H-6→L | (d)n(Br)/n(OH)/π→π* | |||||||||
| 298 (3.54) | 291 (3.74) | 290 (3.76) | 290 (3.64) | 297.41 (0.0427) | 302.67 (0.1542) | 304.17 (0.0596) | 298.30 (0.0985) | (a, b, c, e)H→L+1 | (a)n(OH)/π→π* (b)n(MeO)/n(OH)/π→π* (d)n(Br)/n(OH)/π→π* (e)σ(t-Bu)/n(OH)/π→π* | ||
| 325.44 (0.0438) | (a)H-4→L | (a)π(C6-ring near OH)→π* | |||||||||
| 310 (3.74) | 305.19 (0.1361) | (c)H-2→L+1 | (c)n(NO2)/(OH)/π→π* | ||||||||
| 405 (4.46) | 415 (4.35) | 410 (4.13) | 409 (4.28) | 409 (3.96) | 341.08 (0.0630) | (c)H-3→L | (c)π(C6-ring near OH)→π* | ||||
| 345.44 (0.0461) | 370.43 (0.3618) | 385.35 (0.2630) | 355.56 (0.0770) | 355.42 (0.1288) | (a, d, e)H-2→L (b)H-1→L (c)H-1→L+1 | (a, c)n(OH)/π→π* (b)n(MeO)/n(OH)/π→π* (d)n(Br)/n(OH)/π→π* (e)σ(t-Bu)/n(OH)/π→π* | |||||
| 432.77 (1.1904) | 471.46 (0.9677) | 458.02 (1.1983) | 439.27 (1.2728) | 444.00 (1.1983) | H→L | (a, c)n(OH)/π→π* (b)n(MeO)/n(OH)/π→π* (d)n(Br)/n(OH)/π→π* (e)σ(t-Bu)/n(OH)/π→π* | |||||
| i—j | dij [Å] | i—j—k | αijk [°] | i—j—k | αijk [°] |
|---|---|---|---|---|---|
| S1—C1 | 1.7462(14) | C1—S1—C2 | 86.22(7) | C2—C11—C16 | 122.25(13) |
| S1—C2 | 1.7366(14) | S1—C2—N2 | 114.29(11) | C5—C6—N5 | 121.16(12) |
| C1—N1 | 1.3092(18) | C2—N2—N1 | 112.80(12) | C7—C6—N5 | 121.27(12) |
| C2—N2 | 1.3124(18) | N2—N1—C1 | 112.25(12) | C6—N5—C9 | 120.76(12) |
| N1—N2 | 1.3757(17) | N1—C1—S1 | 114.42(11) | C6—N5—C10 | 120.34(12) |
| C1—N3 | 1.3821(18) | N1—C1—N3 | 120.62(13) | C9—N5—C10 | 118.16(12) |
| N3—N4 | 1.2907(16) | S1—C1—N3 | 124.91(10) | C13—C14—O1 | 115.86(12) |
| N4—C3 | 1.3851(18) | C1—N3—N4 | 111.67(11) | C15—C14—O1 | 124.38(13) |
| C2—C11 | 1.4651(19) | N3—N4—C3 | 115.29(11) | C14—O1—C17 | 117.14(11) |
| N5—C6 | 1.3520(18) | N4—C3—C4 | 125.47(12) | ||
| N5—C9 | 1.4635(18) | N4—C3—C8 | 116.30(12) | ||
| N5—C10 | 1.4606(18) | S1—C2—C11 | 122.98(10) | ||
| O1—C14 | 1.3610(17) | N2—C2—C11 | 122.55(13) | ||
| O1—C17 | 1.4346(17) | C2—C11—C12 | 118.81(13) |
| D-H•••A | d(D-H) [Å] | d(H•••A) [Å] | d(D•••A) [Å] | <(DHA) [°] | Gda(n) |
|---|---|---|---|---|---|
| C9—H9A•••N1 i | 0.98 | 2.58 | 3.5351(1) | 165.4 | R22(22) |
| C10—H10B•••O1 ii | 0.98 | 2.54 | 3.5031(1) | 166.3 | C(17) |
| C16—H16•••S1 | 0.95 | 2.86 | 3.2088(1) | 102.7 | S(5) |
| C7—H7•••Cg(C3) iii | 0.95 | 2.97 | 3.8190(1) | 148.8 | C(2) |
| R(I)•••R(J) | d(Cg•••Cg) [Å] | α [°] | β [°] | dp [Å] |
|---|---|---|---|---|
| C3•••C3 iv | 3.6217(1) | 0 | 15.5 | 3.4908 |
| C11•••C11 v | 3.5355(1) | 0 | 22.3 | 3.2703 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudelko, A.; Olesiejuk, M.; Luczynski, M.; Swiatkowski, M.; Sieranski, T.; Kruszynski, R. 1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure. Molecules 2020, 25, 2822. https://doi.org/10.3390/molecules25122822
Kudelko A, Olesiejuk M, Luczynski M, Swiatkowski M, Sieranski T, Kruszynski R. 1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure. Molecules. 2020; 25(12):2822. https://doi.org/10.3390/molecules25122822
Chicago/Turabian StyleKudelko, Agnieszka, Monika Olesiejuk, Marcin Luczynski, Marcin Swiatkowski, Tomasz Sieranski, and Rafal Kruszynski. 2020. "1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure" Molecules 25, no. 12: 2822. https://doi.org/10.3390/molecules25122822









