Mutagenesis and Adaptation of the Psychrotrophic Fungus Chrysosporium pannorum A-1 as a Method for Improving β-pinene Bioconversion
Abstract
:1. Introduction
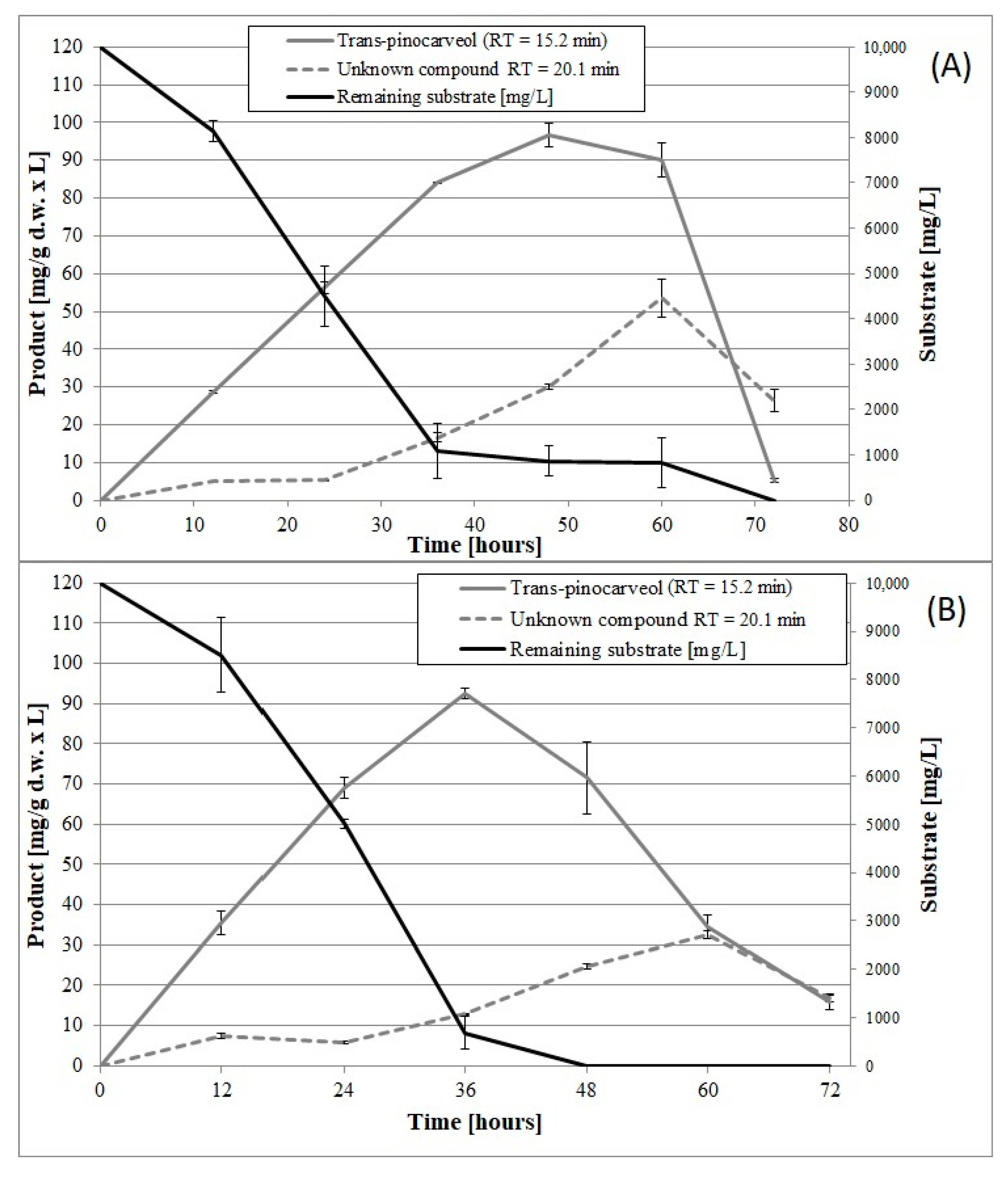
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Fungus and Media
3.3. Induction, Adaptation, and Selection of Mutants
3.4. General Metabolic Activity (GMA) Assay
3.5. Operation at Shake Flask Scale
3.6. Effect of Hydrogen Peroxide Concentration on the Metabolic Activity of the Parental and the Mutant Strain of C. pannorum A-1
3.7. Biotransformation Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vespermann, K.A.C.; Paulino, B.N.; Barcelos, M.C.S.; Pessôa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of α- and β-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses, Completely Revised and Enlarged Edition, 5th ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2006. [Google Scholar]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Trytek, M.; Paduch, R.; Pięt, M.; Kozieł, A.; Kandefer-Szerszeń, M.; Szajnecki, Ł.; Gromada, A. Biological activity of oxygenated pinene derivatives on human colon normal and carcinoma cells. Flavour Fragr. J. 2018, 33, 428–437. [Google Scholar] [CrossRef]
- Viana, A.F.S.C.; Lopes, M.T.P.; Oliveira, F.T.B.; Nunes, P.I.G.; Santos, V.G.; Braga, A.D.; Silva, A.C.A.; Sousa, D.P.; Viana, D.A.; Rao, V.S.; et al. (-)-Myrtenol accelerates healing of acetic-induced gastric ulcers in rats and human gastric adenocarcinoma cells. Eur. J. Pharmacol. 2019, 854, 139–148. [Google Scholar] [CrossRef] [PubMed]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; De Sousa, D.P. A Review on anti-inflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Hollmann, F.; Arends, I.W.; Buehler, K.; Schallmey, A.; Bühler, B. Enzyme-mediated oxidations for the chemist. Green Chem. 2011, 13, 226–265. [Google Scholar] [CrossRef]
- Sales, A.; Paulino, B.N.; Pastore, G.M.; Bicas, J.L. Biogeneration of aroma compounds. Curr. Opin. Food Sci. 2018, 19, 77–84. [Google Scholar] [CrossRef]
- Molina, G.; Pessôa, M.G.; Bicas, J.L.; Fontanille, P.; Larroche, C.; Pastore, G.M. Optimization of limonene biotransformation for the production of bulk amounts of α-terpineol. Bioresour. Technol. 2019, 294, 122180. [Google Scholar] [CrossRef]
- Molbase: Chemical B2B E-Commerce Platform. 2018. Available online: https://www.molbase.com (accessed on 3 October 2018).
- De Oliveira Felipe, L.; De Oliveira, A.M.; Bicas, J.L. Bioaromas—Perspectives for sustainable development. Trends Food Sci. Technol. 2017, 62, 141–153. [Google Scholar] [CrossRef]
- Gavrilov, V.V.; Startseva, V.A.; Nikitina, L.E.; Lodochnikova, O.A.; Gnezdilov, O.I.; Lisovskaya, S.A.; Clushko, N.I.; Klimovitskii, E.N. Synthesis and antifungal activity of sulfides, sulfoxides, and sulfones based on (1S)-(-)-β-pinene. Pharm. Chem. J. 2010, 44, 126–129. [Google Scholar] [CrossRef]
- Silva, A.C.R.; Lopes, P.M.; Azevedo, M.M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman-Gutierrez, S.L.; Gomez-Cansino, R.; Garcia-Zebadua, J.C.; Jimenez-Perez, N.C.; Reyes-Chilpa, R. Antidepressant activity of Litsea glaucescens essential oil: Identification of β-pinene and linalool as active principles. J. Ethnopharmacol. 2012, 143, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Gutierrez, S.L.; Bollina-Jaime, H.; Gomez-Cansino, R.; Reyes-Chilpa, R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015, 128, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Rahman, A.; Choi, U.K.; Youn, S.J.; Kang, S.C. Inhibitory parameters of the essential oil and various extracts of Metasequoia glyptostroboides Miki ex Hu to reduce food spoilage and food-borne pathogens. Food Chem. 2007, 105, 1061–1066. [Google Scholar] [CrossRef]
- Tavares, C.S.; Martins, A.; Faleiro, M.L.; Miguel, M.M.; Duarte, L.C.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Bioproducts from forest biomass: Essential oils and hydrolates from wastes of Cupressus lusitanica Mill. and Cistus ladanifer L. Ind. Crop. Prod. 2020, 144, 112034. [Google Scholar] [CrossRef]
- Drosopoulou, E.; Vlastos, D.; Efthimiou, I.; Kyrizaki, P.; Tsamadou, S.; Anagnostopoulou, M.; Kofidou, D.; Gavriilidis, M.; Mademtzoglou, D.; Mavragani-Tsipidou, P. In vitro and in vivo evaluation of the genotoxic and antigenetoxic potential of the major Chios mastic water constituents. Sci. Rep. 2018, 8, 12200. [Google Scholar] [CrossRef]
- Rozenbaum, H.F.; Patitucci, M.L.; Antunes, O.A.C.; Pereira, N. Production of aromas and fragrances through microbial oxidation of monoterpenes. Braz. J. Chem. Eng. 2006, 23, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Kim, S.H.; Hong, C.Y.; Kim, H.Y.; Ryu, S.H.; Choi, I.G. Biotransformation of (-)-α-pinene by whole cells of white rot fungi, Ceriporia sp. ZLY-2010 and Stereum hirsutum. Mycobiology 2015, 43, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, R.; Lu, D.; Ma, S.; Yan, Y.; Li, W. A quick isolation method for mutants with high lipid yield in oleaginous yeast. World J. Microbiol. Biotechnol. 2009, 25, 921–925. [Google Scholar] [CrossRef]
- Harper, M.; Lee, C.J. Genome-wide analysis of mutagenesis bias and context sensitivity of N-methyl-N-nitro-N-nitrosoguanidine (NTG). Mutat. Res. 2012, 731, 64–67. [Google Scholar] [CrossRef]
- Wakai, S.; Arazoe, T.; Ogino, C.; Kondo, A. Future insights in fungal metabolic engineering. Bioresour. Technol. 2017, 245, 1314–1326. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V. Genetic engineering of filamentous fungi—Progress, obstacles and future trends. Biotechnol. Adv. 2008, 26, 177–185. [Google Scholar] [CrossRef]
- Nakane, A.; Takamatsu, H.; Oguro, A.; Sodaie, Y.; Nakamura, K.; Yamane, K. Acquisition of azide-resistance by elevated SecA ATPase activity confers azide-resistance upon cell growth and protein translocation in Bacillus subtilis. Microbiology 1995, 141, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skowronek, M.; Fiedurek, J. Selection of biochemical mutants of Aspergillus niger resistant to some abiotic stresses with increased inulinase production. J. Appl. Microbiol. 2003, 95, 686–692. [Google Scholar] [CrossRef]
- Fiedurek, J.; Trytek, M.; Szczodrak, J. Strain improvement of industrially important microorganisms based on resistance to toxic metabolites and abiotic stresses. J. Basic Microbiol. 2017, 57, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Fiedurek, J.; Trytek, M.; Skowronek, M. Strategies for improving the efficiency of bioprocesses involving toxic metabolites. Curr. Org. Chem. 2012, 16, 2946–2960. [Google Scholar] [CrossRef]
- Kirimura, K.; Sarangbin, S.; Rugsaseel, S.; Usami, S. Citric acid production by 2-deoxyglucose-resistant mutant strains of Aspergillus niger. Appl. Microbiol. Biotechnol. 1992, 36, 573–577. [Google Scholar] [CrossRef]
- Aono, R.; Inoue, A. Organic solvent tolerance in microorganisms. In Microbial Life in Extreme Environments; Horikoshi, K., Grant, W.D., Eds.; Wiley-Liss Inc.: New York, NY, USA, 1998; pp. 287–310. [Google Scholar]
- Meyer, D.; Buehler, B.; Schmid, A. Process and catalyst design objectives for specific redox biocatalysis. Adv. Appl. Microbiol. 2006, 59, 53–91. [Google Scholar]
- Doukyu, N.; Ishikawa, K.; Watanabe, R.; Ogino, H. Improvement in organic solvent tolerance by double disruptions of proV and marR genes in Escherichia coli. J. Appl. Microbiol. 2012, 112, 464–474. [Google Scholar] [CrossRef]
- Asako, H.; Nakajima, H.; Kobayashi, K.; Kobayashi, M. Organic solvent tolerance and antibiotic resistance increased by overexpression of marA in Escherichia coli. Appl. Environ. Microbiol. 1997, 63, 1428–1433. [Google Scholar] [CrossRef] [Green Version]
- Volmer, J.; Neumann, C.; Bühler, B.; Schmid, A. Engineering of Pseudomonas taiwanensis VLB120 for constitutive solvent tolerance and increased specific styrene epoxidation activity. Appl. Environ. Microbiol. 2014, 80, 6539–6548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, N.; Ozato, N.; Matsui, K.; Kuroda, K. ABC transporters and cell wall proteins involved in organic solvent tolerance in Saccharomyces cerevisiae. J. Biotechnol. 2013, 165, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.K.; Bergholz, T.M. Variation in growth and evaluation of cross-protection in Listeria monocytogenes under salt and bile stress. J. Appl. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.N.; Xu, M.; Ren, J.N.; Dong, M.; Yang, Z.Y.; Pan, S.Y.; Fan, G. Optimisation of α-terpineol production by limonene biotransformation using Penicillium digitatum DSM 62840. J. Sci. Food Agric. 2016, 96, 954–961. [Google Scholar] [CrossRef]
- Rottava, I.; Cortina, P.F.; Grando, C.E.; Colla, A.R.S.; Martello, E.; Cansian, R.L.; Toniazzo, G.; Treichel, H.; Antunes, O.A.C.; Oestreicher, E.G.; et al. Isolation and screening of microorganisms for R-(+)-limonene and (−)-β-pinene biotransformation. Appl. Biochem. Biotechnol. 2010, 162, 719–732. [Google Scholar] [CrossRef]
- Santos, P.M.; Sá-Correia, I. Adaptation to β-myrcene catabolism in Pseudomonas sp. M1: An expression proteomics analysis. Proteomics 2009, 9, 5101–5111. [Google Scholar] [CrossRef]
- Kakuk, B.; Kovács, K.L.; Szuhaj, M.; Rákhely, G.; Bagi, Z. Adaptation of continuous biogas reactors operating under wet fermentation conditions to dry conditions with corn stover as substrate. Anaerobe 2017, 46, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Van Hamme, J.D.; Singh, A.; Ward, O.P. Recent advances in petroleum microbiology. Microbiol. Mol. Biol. Rev. 2003, 67, 503–549. [Google Scholar] [CrossRef] [Green Version]
- Park, J.B.; Bühler, B.; Panke, S.; Witholt, B.; Schmid, A. Carbon metabolism and product inhibition determine the epoxidation efficiency of solvent-tolerant Pseudomonas sp. strain VLB120DC. Biotechnol. Bioeng. 2007, 98, 1219–1229. [Google Scholar] [CrossRef]
- Trytek, M.; Jędrzejewski, K.; Fiedurek, J. Bioconversion of α-pinene by a novel cold-adapted fungus Chrysosporium pannorum. J. Ind. Microbiol. Biotechnol. 2015, 42, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Trytek, M.; Fiedurek, J.; Gromada, A. Effect of some abiotic stresses on the biotransformation of α-pinene by a psychrotrophic Chrysosporium pannorum. Biochem. Eng. J. 2016, 112, 86–93. [Google Scholar] [CrossRef]
- Agrawal, R.; Deepika, N.U.A.; Joseph, R. Strain improvement of Aspergillus sp. and Penicillium sp. by induced mutation for biotransformation of α-pinene to verbenol. Biotechnol. Bioeng. 1999, 63, 249–252. [Google Scholar] [CrossRef]
- Lloyd, D.; Morrell, S.; Carlsen, H.N.; Degn, H.; James, P.E.; Rowlands, C.C. Effects of growth with ethanol on fermentation and membrane fluidity of Saccharomyces cerevisiae. Yeast 1993, 9, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, N.; Santos, P.M.; Heipieper, H.J. Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in Acinetobacter calcoaceticus. FEMS Microbiol. Lett. 2003, 220, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Neumann, G.; Kabelitz, N.; Zehnsdorf, A.; Miltner, A.; Lippold, H.; Meyer, D.; Schmid, A.; Heipieper, H.J. Prediction of the adaptability of Pseudomonas putida DOT-T1E to a second phase of a solvent for economically sound two-phase biotransformations. Appl. Environ. Microbiol. 2005, 71, 6606–6612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortiz-Zamora, O.; Cortes-García, R.; Ramírez-Lepe, M.; Gómez-Rodríguez, J.; Aguilar-Uscanga, M.G. Isolation and selection of ethanol-resistant and osmotolerant yeasts from regional agricultural sources in Mexico. J. Food Process. Eng. 2008, 32, 775–786. [Google Scholar] [CrossRef]
- Loffler, C.; Eberlein, C.; Mausezahl, I.; Kappelmeyer, U.; Heipieper, H.J. Physiological evidence for the presence of a cis-trans isomerase of unsaturated fatty acids in Methylococcus capsulatus Bath to adapt to the presence of toxic organic compounds. FEMS Microbiol. Lett. 2010, 308, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Naether, D.J.; Slawtschew, S.; Stasik, S.; Engel, M.; Olzog, M.; Wick, L.Y.; Timmis, K.N.; Heipieper, H.J. Adaptation of the hydrocarbonoclastic bacterium Alcanivorax borkumensis SK2 to alkanes and toxic organic compounds: A physiological and transcriptomic approach. Appl. Environ. Microbiol. 2013, 79, 4282–4293. [Google Scholar] [CrossRef] [Green Version]
- Stanley, D.; Fraser, S.; Chambers, P.J.; Rogers, P.; Stanley, G.A. Generation and characterisation of stable ethanol-tolerant mutants of Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2010, 37, 139–149. [Google Scholar] [CrossRef]
- Weber, F.J.; Hage, K.C.; De Bont, J.A.M. Growth of the fungus Cladosporium sphaerospermum with toluene as the sole carbon and energy source. Appl. Environ. Microbiol. 1995, 61, 3562–3566. [Google Scholar] [CrossRef] [Green Version]
- Weber, F.J.; De Bont, J.A.M. Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim. Biophys. Acta 1996, 1286, 225–245. [Google Scholar] [CrossRef]
- Torres, S.; Pandey, A.; Castro, G.R. Organic solvent adaptation of Gram positive bacteria: Applications and biotechnological potentials. Biotechnol. Adv. 2011, 29, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Brandt, B.W.; Kelpin, F.D.L.; Van Leeuwen, I.M.M.; Kooijman, S.A.L.M. Modelling microbial adaptation to changing availability of substrates. Water Res. 2004, 38, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singh, D.K. Biodegradation of persistent organic pollutants in soil, water and pristine sites by cold-adapted microorganisms: Mini review. Int. Biodeterior. Biodegrad. 2015, 100, 98–105. [Google Scholar] [CrossRef]
- Králová, S. Role of fatty acids in cold adaptation of Antarctic psychrophilic Flavobacterium spp. Syst. Appl. Microbiol. 2017, 40, 329–333. [Google Scholar] [CrossRef]
- Brininger, C.; Spradlin, S.; Cobani, L.; Evilia, C. The more adaptive to change, the more likely you are to survive: Protein adaptation in extremophiles. Sem. Cell. Dev. Biol. 2018, 84, 158–169. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Kemmitt, S.J.; Michallon, L.; Guo, S.; Wen, Q.; Brookes, P.C.; Evershed, R.P. The variable response of soil microorganisms to trace concentrations of low molecular weight organic substrates of increasing complexity. Soil Biol. Biochem. 2013, 64, 57–64. [Google Scholar] [CrossRef]
- Wery, J.; De Bont, J.A.M. Solvent-tolerance of pseudomonads: A new degree of freedom in biocatalysis. In Pseudomonas, Volume 3: Biosynthesis of Macromolecules and Molecular Metabolism; Ramos, J.L., Ed.; Kluwer Academic; Plenum Publishers: New York, NY, USA, 2004; pp. 609–634. [Google Scholar]
- Ramos, J.L.; Duque, E.; Gallegos, M.T.; Godoy, P.; Ramos-Gonzalez, M.I.; Rojas, A.; Teran, W.; Segura, A. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 2002, 56, 743–768. [Google Scholar] [CrossRef]
- Heipieper, H.J.; Neumann, G.; Cornelissen, S.; Meinhardt, F. Solvent-tolerant bacteria for biotransformations in two-phase fermentation systems. Appl. Microbiol. Biotechnol. 2007, 74, 961–973. [Google Scholar] [CrossRef]
- Fontanille, P.; Larroche, C. Optimization of isonovalal production from α-pinene oxide using permeabilized cells of Pseudomonas rhodesiae CIP 107491. Appl. Microbiol. Biotechnol. 2003, 60, 534–540. [Google Scholar] [CrossRef]
- Alonso-Calleja, C.; Guerrero-Ramos, E.; Alonso-Hernando, A.; Capita, R. Adaptation and cross-adaptation of Escherichia coli ATCC 12806 to several food-grade biocides. Food Control 2015, 56, 86–94. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C. Surviving the acid test: Responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorenza, S.; Ward, C.H. Microbial adaptation to hydrogen peroxide and biodegradation of aromatic hydrocarbons. J. Ind. Microbiol. Biotechnol. 1997, 18, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Trytek, M.; Fiedurek, J.; Skowronek, M. Biotransformation of (R)-(+)-limonene by the psychrotrophic fungus Mortierella minutissima in H2O2-oxygenated culture. Food Technol. Biotechnol. 2009, 47, 131–136. [Google Scholar]
- Tan, Q.; Day, D.F.; Cadwallader, K.R. Bioconversion of (R)-(+)-limonene by P. digitatum (NRRL 1202). Process Biochem. 1998, 33, 29–31. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R.; Poretti, A.; Da Fonseca, M.M.R. Cell adaptation to solvent, substrate and product: A successful strategy to overcome product inhibition in a bioconversion system. Appl. Microbiol. Biotechnol. 2005, 69, 268–275. [Google Scholar] [CrossRef]
- He, S.; Cui, Y.; Qin, X.; Zhang, F.; Shi, C.; Paoli, G.C.; Shi, X. Influence of ethanol adaptation on Salmonella enteric serovar Enteritidis survival in acidic environments and expression of acid tolerance-related genes. Food Microbiol. 2018, 72, 193–198. [Google Scholar] [CrossRef]
- Fiedurek, J.; Gromada, A.; Słomka, A.; Korniłowicz-Kowalska, T.; Kurek, E.; Melke, J. Catalase activity in arctic microfungi grown at different temperatures. Acta Biol. Hung. 2003, 54, 105–112. [Google Scholar] [CrossRef]
- Fiedurek, J.; Rogalski, J.; Ilczuk, Z.; Leonowicz, A. Screening and mutagenization of molds for the improvement of glucose oxidase production. Enzym. Microb. Technol. 1986, 8, 734–736. [Google Scholar] [CrossRef]
- Bryson, V.; Szybalski, W. Microbial selection. Science 1952, 116, 45–51. [Google Scholar] [CrossRef]
- Bos, C.J.; Debets, A.J.M.; Nachtegaal, H.; Slakhorst, S.M.; Swart, K. Isolation of auxotrophic mutants of Aspergillus niger by filtration enrichment and lytic enzymes. Curr. Genet. 1992, 21, 117–120. [Google Scholar] [CrossRef]
Sample Availability: Samples of the mutant strains are available from the authors. |
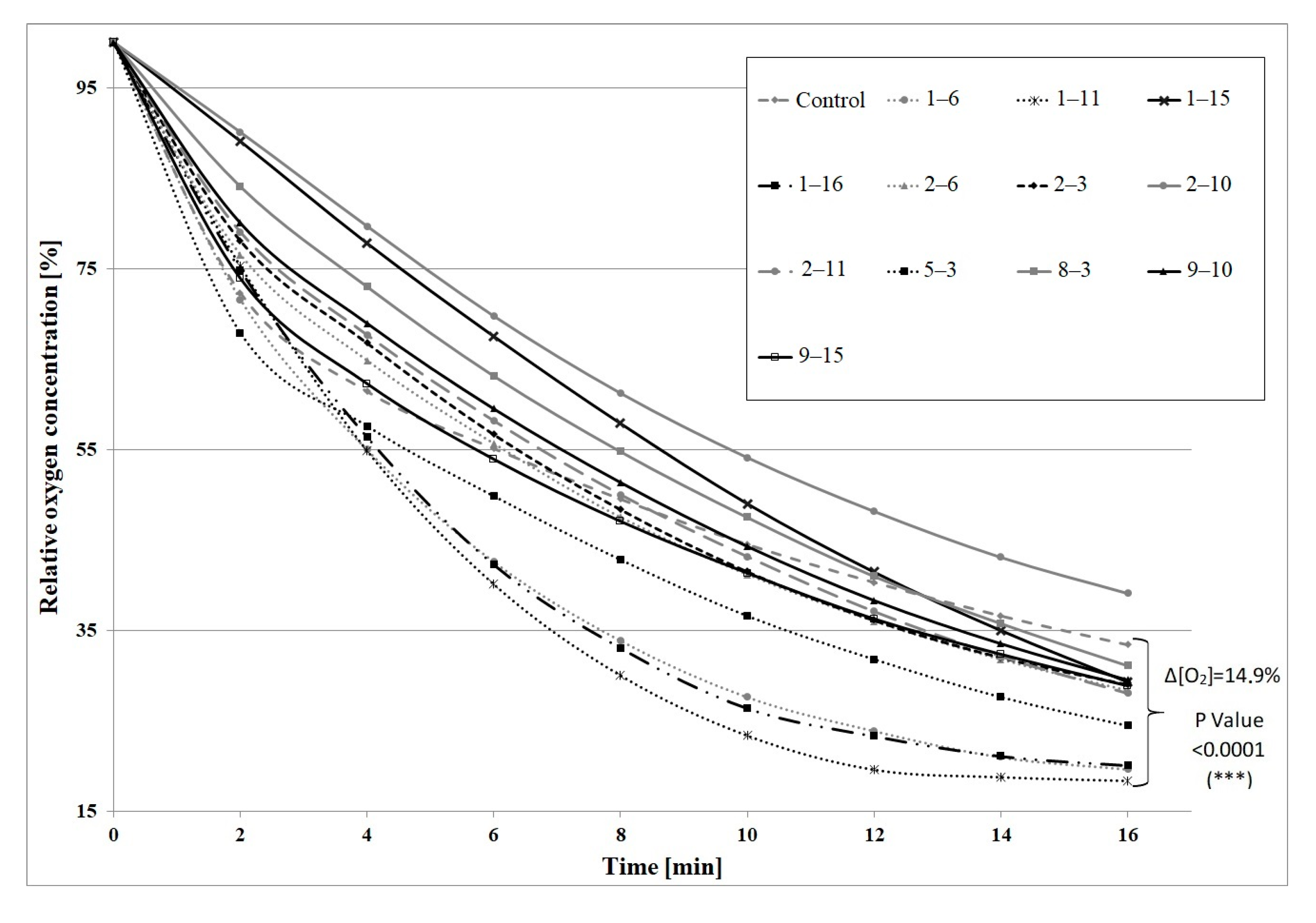
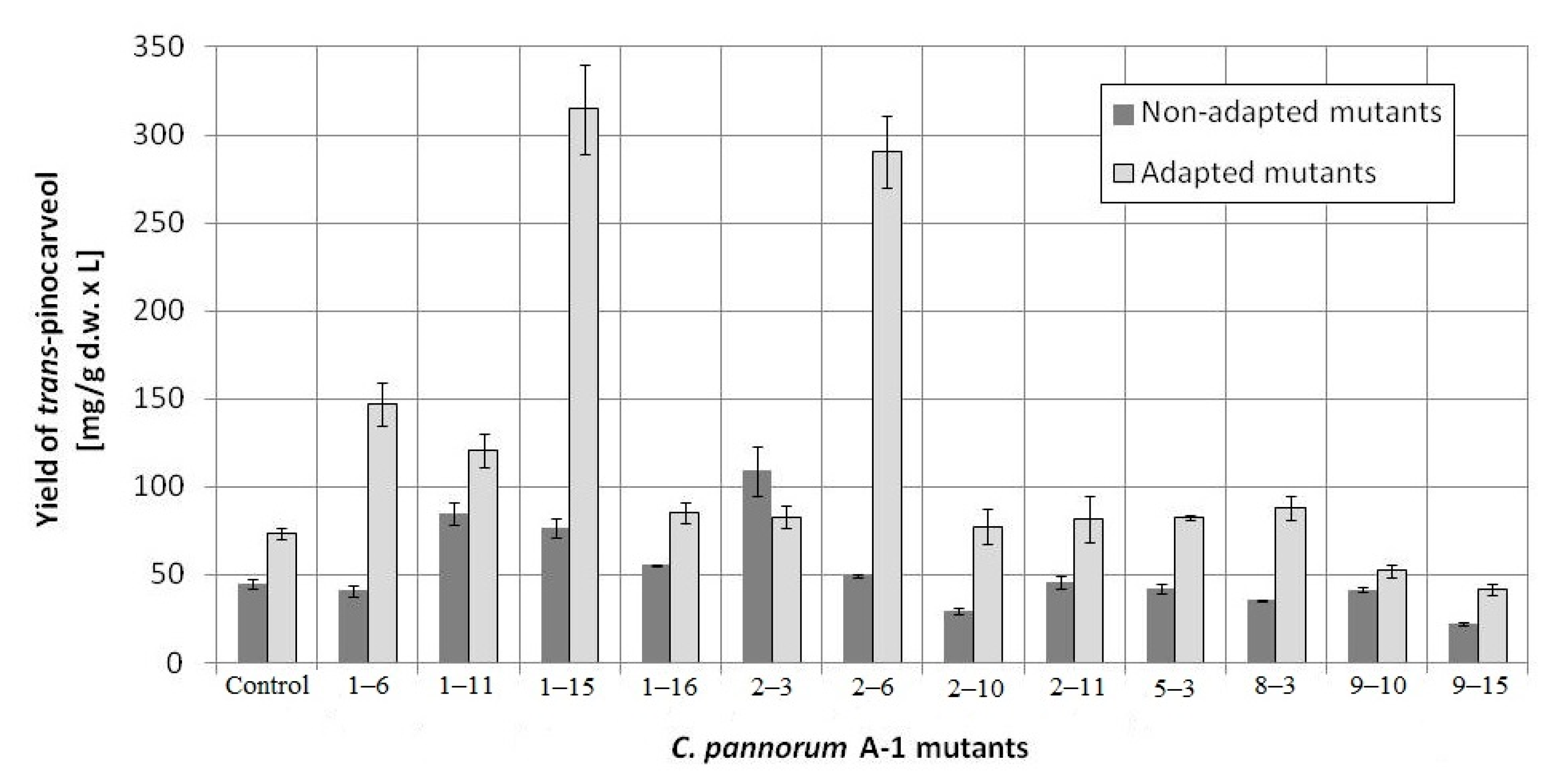



| Variant of Mutagenesis | Survivability [%] | The Most Biotransformation-Active Mutants | Efficiency of Biotransformation [mg/g d.w. × L] |
|---|---|---|---|
| 10 min UV + 5 min NTG | 9.6 | 1–15 1 | 314.7 (±25.3) |
| 1–6 1 | 147.2 (±11.9) | ||
| 1–11 1 | 121.4 (±9.5) | ||
| 8–3 1 | 88.5 (±6.3) | ||
| 1–16 1 | 85.6 (±6.0) | ||
| 10 min UV + 10 min NTG | 1.6 | 2–6 1 | 290.8 (±20.4) |
| 2–3 2 | 109.4 (±14.2) | ||
| 2–11 1 | 82.2 (±13.1) | ||
| 2–10 1 | 77.8 (±10.1) | ||
| 9–10 1 | 52.6 (±3.7) |
| Presence of Hydrogen Peroxide in the Medium After 1-h Incubation | ||
|---|---|---|
| H2O2 Concentration % (v/v) | Wild-Type Strain | Mutant 1–6 |
| Control | − | − |
| 0.1 | − | − |
| 0.5 | + | − |
| 1.0 | + | − |
| 1.5 | + | + |
| 2.5 | + | + |
| 5.0 | + | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutyła, M.; Fiedurek, J.; Gromada, A.; Jędrzejewski, K.; Trytek, M. Mutagenesis and Adaptation of the Psychrotrophic Fungus Chrysosporium pannorum A-1 as a Method for Improving β-pinene Bioconversion. Molecules 2020, 25, 2589. https://doi.org/10.3390/molecules25112589
Kutyła M, Fiedurek J, Gromada A, Jędrzejewski K, Trytek M. Mutagenesis and Adaptation of the Psychrotrophic Fungus Chrysosporium pannorum A-1 as a Method for Improving β-pinene Bioconversion. Molecules. 2020; 25(11):2589. https://doi.org/10.3390/molecules25112589
Chicago/Turabian StyleKutyła, Mateusz, Jan Fiedurek, Anna Gromada, Krzysztof Jędrzejewski, and Mariusz Trytek. 2020. "Mutagenesis and Adaptation of the Psychrotrophic Fungus Chrysosporium pannorum A-1 as a Method for Improving β-pinene Bioconversion" Molecules 25, no. 11: 2589. https://doi.org/10.3390/molecules25112589







