Biocompatible Organic Coatings Based on Bisphosphonic Acid RGD-Derivatives for PEO-Modified Titanium Implants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Bioactive Bifunctional Molecules
2.2. PEO Coating Characterization
2.3. XPS Analysis of the Composite PEO Coating Functionalized with RGD Derivatives
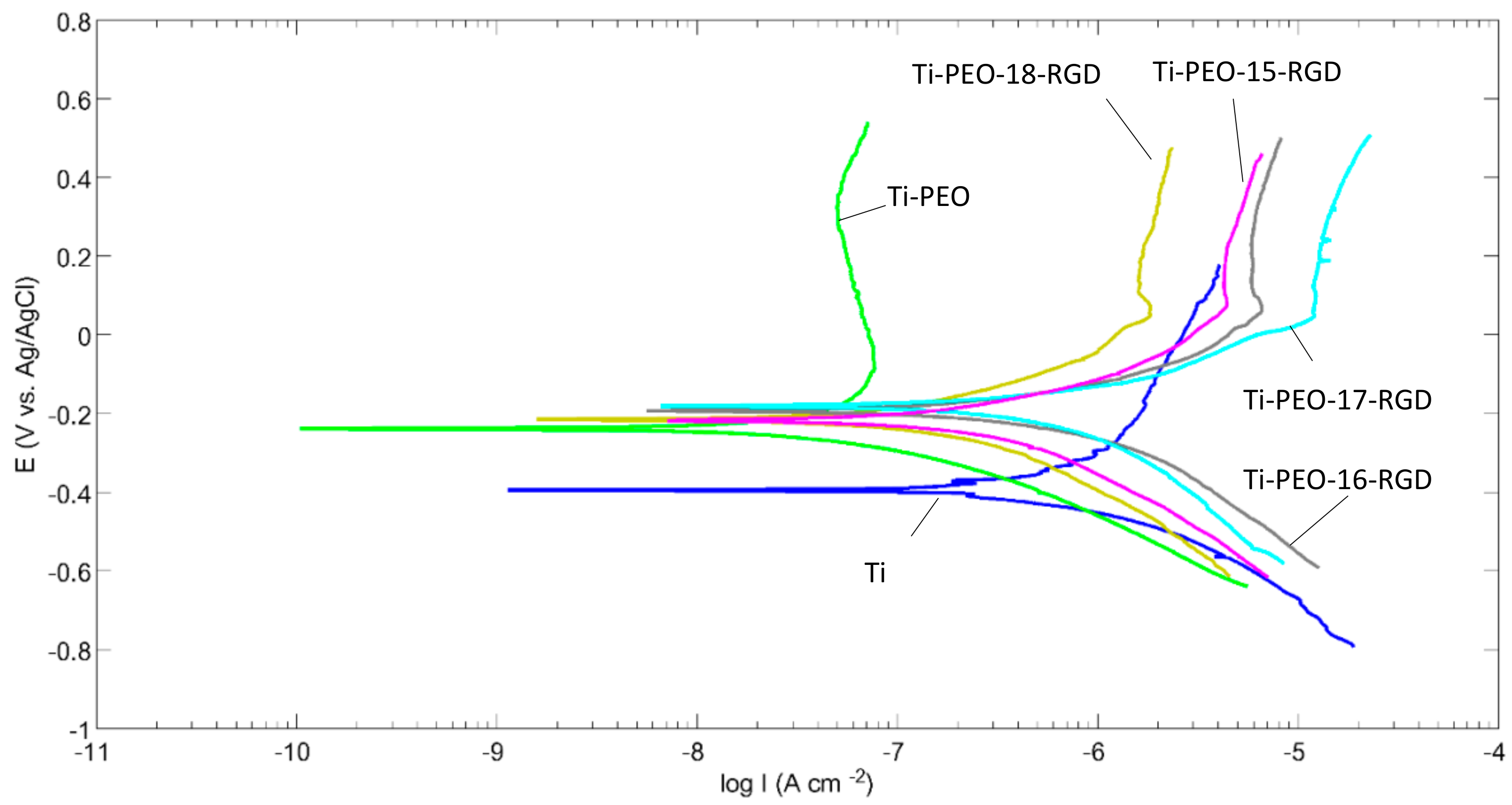
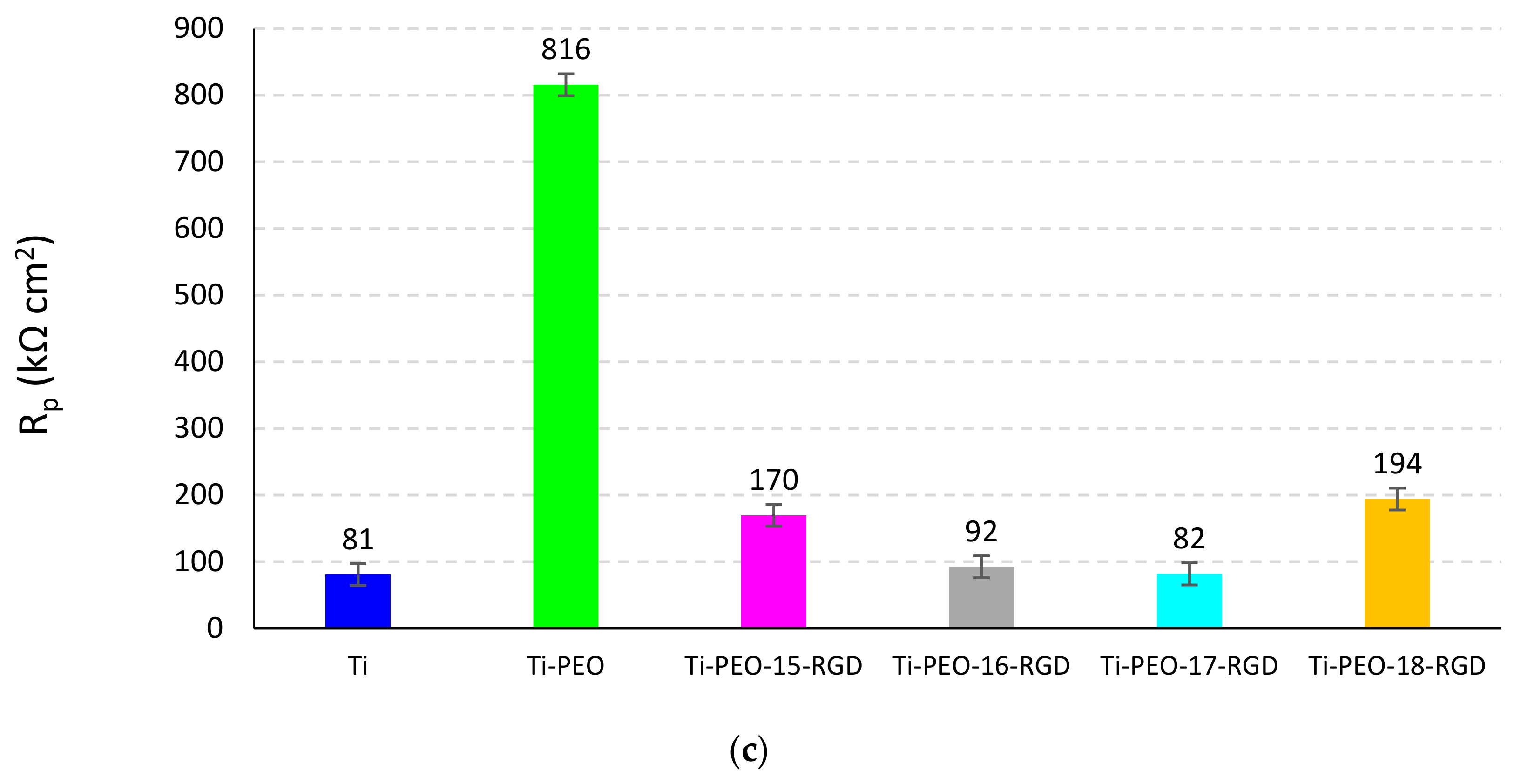
2.4. Electrochemical Behavior of the Composite PEO Coating Functionalized with RGD Derivatives
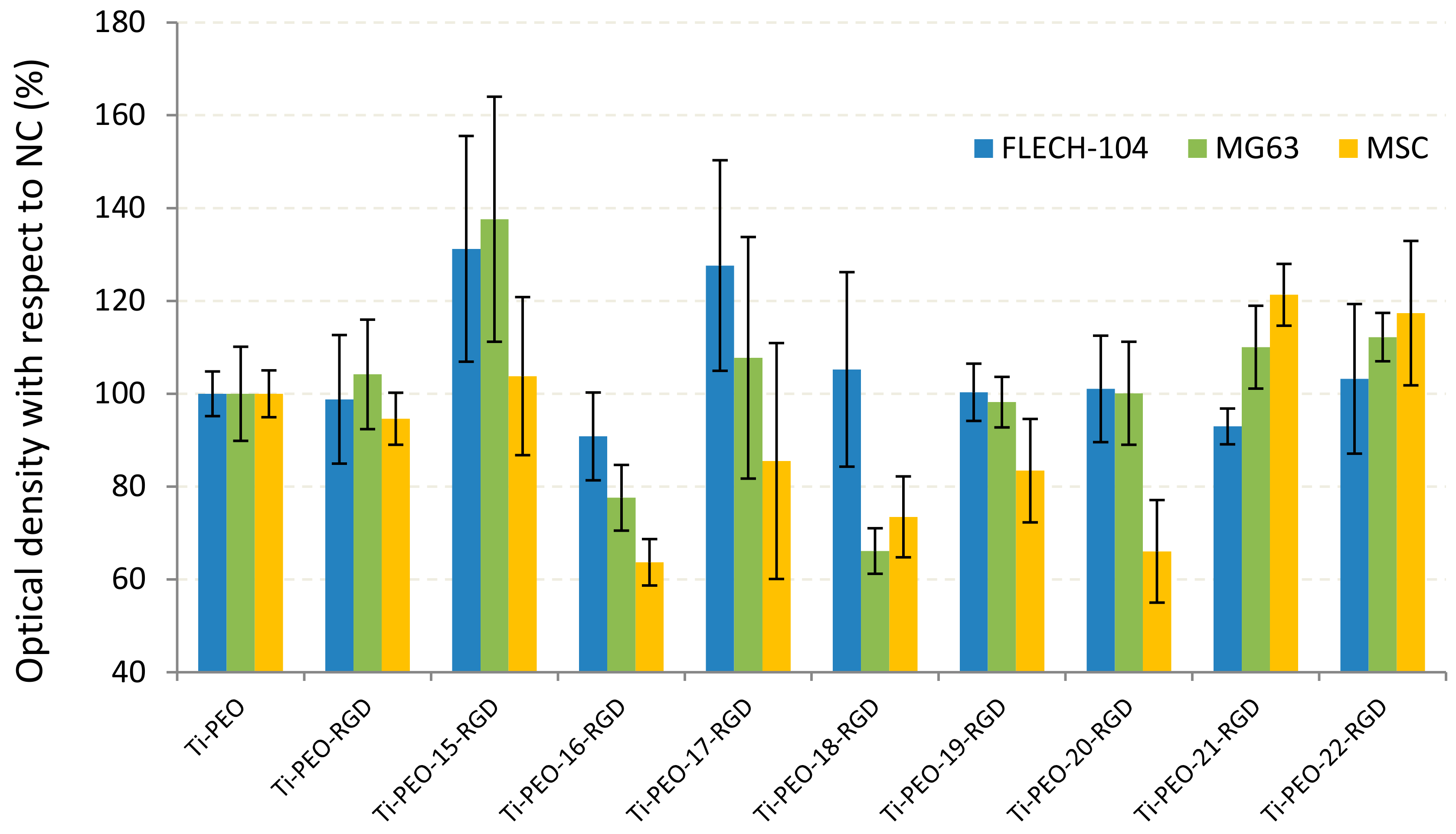
2.5. In Vitro Test Results Supporting The Efficiency of Proposed PEO Coating Functionalized with RGD Derivatives
3. Materials and Methods
3.1. General Information
3.2. Synthesis of RGD-Functionalized Derivatives
3.2.1. Synthesis of Amino-1-hydroxyalkan-1,1-diyl-bisphosphonic acids 1–3
3.2.2. Synthesis of (N-Maleimidoalkyl)succinimide Esters (4–6)
3.2.3. Synthesis of Conjugates of Aminobisphosphonic Acids with Maleimidosuccinimide Linkers 7–14
3.2.4. Synthesis of RGDC Derivatives 15–22
3.3. Metal Sample Preparation and PEO Coating
3.4. Surface Characterization
3.5. Electrochemical Tests
3.6. In Vitro Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Mohedano, M.; Guzman, R.; Arrabal, R.; Lõpez Lacomba, J.L.; Matykina, E. Bioactive plasma electrolytic oxidation coatings—The role of the composition, microstructure, and electrochemical stability. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1524–1537. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, J.M.; Nagay, B.E.; Ribeiro, A.L.R.; da Cruz, N.C.; Rangel, E.C.; Fais, L.M.G.; Vaz, L.G.; Barão, V.A.R. Functionalization of an experimental Ti-Nb-Zr-Ta alloy with a biomimetic coating produced by plasma electrolytic oxidation. J. Alloy. Compd. 2019, 770, 1038–1048. [Google Scholar] [CrossRef]
- Sharifi, H.; Aliofkhazraei, M.; Darband, G.B.; Shrestha, S. A review on adhesion strength of peo coatings by scratch test method. Surf. Rev. Lett. 2018, 25. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.; Munir, K.; Wen, C. Calcium phosphate-based composite coating by micro-arc oxidation (MAO) for biomedical application: A review. Crit. Rev. Solid State Mater. Sci. 2018, 43, 392–416. [Google Scholar] [CrossRef]
- Anderson, J.M. Biological responses to materials. Ann. Rev. Mater. Sci. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Shekaran, A.; García, A.J. Extracellular matrix-mimetic adhesive biomaterials for bone repair. J. Biomed. Mater. Res. Part A 2011, 96A, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Meyers, S.R.; Grinstaff, M.W. Biocompatible and bioactive surface modifications for prolonged in vivo efficacy. Chem. Rev. 2012, 112, 1615–1632. [Google Scholar] [CrossRef] [Green Version]
- Tejero, R.; Anitua, E.; Orive, G. Toward the biomimetic implant surface: Biopolymers on titanium-based implants for bone regeneration. Prog. Polym. Sci. 2014, 39, 1406–1447. [Google Scholar] [CrossRef]
- Mas-Moruno, C. 3-Surface functionalization of biomaterials for bone tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Park, J.M.; Koak, J.Y.; Jang, J.H.; Han, C.H.; Kim, S.K.; Heo, S.J. Osseointegration of anodized titanium implants coated with fibroblast growth factor-fibronectin (FGF-FN) fusion protein. Int. J. Oral Maxillofac. Implant. 2006, 21, 859–866. [Google Scholar]
- Kazek-Kęsik, A.; Jaworska, J.; Krok-Borkowicz, M.; Gołda-Cępa, M.; Pastusiak, M.; Brzychczy-Włoch, M.; Pamuła, E.; Kotarba, A.; Simka, W. Hybrid oxide-polymer layer formed on Ti-15Mo alloy surface enhancing antibacterial and osseointegration functions. Surf. Coat. Technol. 2016, 302, 158–165. [Google Scholar] [CrossRef]
- Zaporozhets, T.S.; Puz’, A.V.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Smolina, T.P.; Besednova, N.N. Biocompatibility of modified osteoinductive calcium-phosphate coatings of metal implants. Bull. Exp. Biol. Med. 2017, 162, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Parfenov, E.V.; Parfenova, L.V.; Dyakonov, G.S.; Danilko, K.V.; Mukaeva, V.R.; Farrakhov, R.G.; Lukina, E.S.; Valiev, R.Z. Surface functionalization via PEO coating and RGD peptide for nanostructured titanium implants and their in vitro assessment. Surf. Coat. Technol. 2019, 357, 669–683. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.J.; Textor, M.; Spencer, N.D.; Wieland, M.; Keller, B.; Sigrist, H. Immobilization of the cell-adhesive peptide Arg–Gly–Asp–Cys (RGDC) on titanium surfaces by covalent chemical attachment. J. Mater. Sci. Mater. Med. 1997, 8, 867–872. [Google Scholar] [CrossRef]
- Rezania, A.; Johnson, R.; Lefkow, A.R.; Healy, K.E. Bioactivation of metal oxide surfaces. 1. surface characterization and cell response. Langmuir 1999, 15, 6931–6939. [Google Scholar] [CrossRef]
- Zreiqat, H.; Akin, F.; Howlett, C.; Markovic, B.; Haynes, D.; Lateef, S.; Hanley, L. Differentiation of human bone-derived cells grown on GRGDSP-peptide bound titanium surfaces. J. Biomed. Mater. Res. 2003, 64, 105–113. [Google Scholar] [CrossRef]
- Barber, T.A.; Gamble, L.J.; Castner, D.G.; Healy, K.E. In vitro characterisation of peptide-modified p(AAm-co-EG/AAc) IPN-coated titanium implants. J. Orthop. Res. 2006, 24, 1366–1376. [Google Scholar] [CrossRef]
- Barber, T.A.; Golledge, S.L.; Castner, D.G.; Healy, K.E. Peptide-modified p(AAm-co-EG/AAc) IPNs grafted to bulk titanium modulate osteoblast behavior in vitro. J. Biomed. Mater. Res. Part A 2003, 64A, 38–47. [Google Scholar] [CrossRef]
- Gawalt, E.S.; Avaltroni, M.J.; Danahy, M.P.; Silverman, B.M.; Hanson, E.L.; Midwood, K.S.; Schwarzbauer, J.E.; Schwartz, J. Bonding organics to Ti alloys: Facilitating human osteoblast attachment and spreading on surgical implant materials. Langmuir 2003, 19, 200–204. [Google Scholar] [CrossRef]
- Pallu, S.; Bourget, C.; Bareille, R.; Labrugère, C.; Dard, M.; Sewing, A.; Jonczyk, A.; Vernizeau, M.; Christine Durrieu, M.; Amédée-Vilamitjana, J. The effect of cyclo-DfKRG peptide immobilization on titanium on the adhesion and differentiation of human osteoprogenitor cells. Biomaterials 2005, 26, 6932–6940. [Google Scholar] [CrossRef] [PubMed]
- Bagno, A.; Piovan, A.; Dettin, M.; Chiarion, A.; Brun, P.; Gambaretto, R.; Fontana, G.; Di Bello, C.; Palu, G.; Castagliuolo, I. Human osteoblast-like cell adhesion on titanium substrates covalently functionalized with synthetic peptides. Bone 2007, 40, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Secchi, A.G.; Grigoriou, V.; Shapiro, I.M.; Cavalcanti-Adam, E.A.; Composto, R.J.; Ducheyne, P.; Adams, C.S. RGDS peptides immobilized on titanium alloy stimulate bone cell attachment, differentiation and confer resistance to apoptosis. J. Biomed. Mater. Res. Part A 2007, 83, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Kaemmerer, P.; Heller, M.; Brieger, J.; Klein, M.; Al-Nawas, B.; Gabriel, M. Immobilisation of linear and cyclic RGD-peptides on titanium surfaces and their impact on endothelial cell adhesion and proliferation. Eur. Cell Mater. 2011, 21, 364–372. [Google Scholar] [CrossRef]
- Ryu, J.-J.; Park, K.; Kim, H.-S.; Jeong, C.-M.; Huh, J.-B. Effects of anodized titanium with Arg-Gly-Asp (RGD) peptide immobilized via chemical grafting or physical adsorption on bone cell adhesion and differentiation. Int. J. Oral Maxillofac Implant. 2013, 28, 963–972. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Junjian, C.; Chengzhi, C.; Lin, S.; Sa, L.; Li, R.; Yingjun, W. Multi-biofunctionalization of a titanium surface with a mixture of peptides to achieve excellent antimicrobial activity and biocompatibility. J. Mater. Chem. B 2015, 3, 30–33. [Google Scholar] [CrossRef]
- Heller, M.; Kumar, V.; Pabst, A.; Brieger, J.; Al-Nawas, B.; Kaemmerer, P. Osseous response on linear and cyclic RGD-peptides immobilized on titanium surfaces in vitro and in vivo: RGD-peptides influence osseous healing. J. Biomed. Mater. Res. Part A 2018, 106, 419–427. [Google Scholar] [CrossRef]
- Ortiz-Hernandez, M.; Rappe, S.K.; Molmeneu, M.; Mas-Moruno, C.; Guillem-Marti, J.; Punset, M.; Caparros, C.; Calero, J.; Franch, J.; Fernandez-Fairen, M.; et al. two different strategies to enhance osseointegration in porous titanium: Inorganic thermo-chemical treatment versus organic coating by peptide adsorption. Int. J. Mol. Sci. 2018, 19, 2574. [Google Scholar] [CrossRef] [Green Version]
- Tosatti, S.; Schwartz, Z.; Campbell, C.T.; Cochran, D.L.; VandeVondele, S.; Hubbell, J.A.; Denzer, A.; Simpson, J.; Wieland, M.; Lohmann, C.; et al. RGD-containing peptide GCRGYGRGDSPG reduces enhancement of osteoblast differentiation by poly(l-lysine)-graft-poly(ethylene glycol)-coated titanium surfaces. J. Biomed. Mater. Res. 2004, 68A, 458–472. [Google Scholar] [CrossRef]
- Maddikeri, R.R.; Tosatti, S.; Schuler, M.; Chessari, S.; Textor, M.; Richards, R.G.; Harris, L.G. Reduced medical infection related bacterial strains adhesion on bioactive RGD modified titanium surfaces: A first step toward cell selective surfaces. J. Biomed. Mater. Res. Part A 2008, 84A, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Petrie, T.; Raynor, J.E.; Reyes, C.D.; Burns, K.L.; Collard, D.M.; García, A. The effect of integrin-specific bioactive coatings on tissue healing and implant osseointegration. Biomaterials 2008, 29, 2849–2857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, B.F.; Schuler, M.; Tosatti, S.; Textor, M.; Schwartz, Z.; Boyan, B. Osteoblast response to titanium surfaces functionalized with extracellular matrix peptide biomimetics. Clin. Oral Implant. Res. 2011, 22, 865–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schliephake, H.; Scharnweber, D.; Dard, M.; Roessler, S.; Sewing, A.; Meyer, J.; Hoogestraat, D. Effect of RGD peptide coating of titanium implants on periimplant bone formation in the alveolar crest. Clin. Oral Implant. Res. 2002, 13, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, S.; Illert, T.; Bierbaum, S.; Scharnweber, D.; Zwipp, H.; Schneiders, W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials 2006, 27, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- Chua, P.-H.; Neoh, K.-G.; Kang, E.-T.; Wang, W. Surface functionalization of titanium with hyaluronic acid/chitosan polyelectrolyte multilayers and RGD for promoting osteoblast functions and inhibiting bacterial adhesion. Biomaterials 2008, 29, 1412–1421. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, Q.; Li, X.; Zhang, F.; Zhao, S. Fabrication and in vitro evaluation of the collagen/hyaluronic acid PEM coating crosslinked with functionalized RGD peptide on titanium. Acta Biomater. 2012, 8, 866–877. [Google Scholar] [CrossRef]
- Jin, C.; Ren, L.-F.; Ding, H.-Z.; Shi, G.-S.; Lin, H.-S.; Zhang, F. Enhanced attachment, proliferation, and differentiation of human gingival fibroblasts on titanium surface modified with biomolecules. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 2167–2177. [Google Scholar] [CrossRef]
- Zhao, B.; Tian, W.; Feng, H.; Lee, I.-S.; Cui, F. Effects of RGD peptide grafting to titanium dental implants on the adhesion of human gingival fibroblasts and epithelial cells. Curr. Appl. Phys. 2005, 5, 407–410. [Google Scholar] [CrossRef]
- Karaman, O.; Kelebek, S.; Demirci, E.A.; iBİŞ, F.; Ulu, M.; Ercan, U. Synergistic Effect of Cold Plasma Treatment and RGD Peptide Coating on Cell Proliferation over Titanium Surfaces. Tissue Eng. Regener. Med. 2017, 15, 13–24. [Google Scholar] [CrossRef]
- Yamamichi, N.; Pugdee, K.; Chang, W.-J.; Lee, S.-A.; Yoshinari, M.; Hayakawa, T.; Abiko, Y. Gene expression monitoring in osteoblasts on fibronectin derived peptide coated titanium. Dent. Mater. J. 2008, 27, 744–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oya, K.; Tanaka, Y.; Saito, H.; Kurashima, K.; Nogi, K.; Tsutsumi, H.; Tsutsumi, Y.; Doi, H.; Nomura, N.; Hanawa, T. Calcification by MC3T3-E1 cells on RGD peptide immobilized on titanium through electrodeposited PEG. Biomaterials 2009, 30, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-W.; Kurashima, K.; Tustusmi, Y.; An, C.-H.; Suh, J.-Y.; Doi, H.; Nomura, N.; Noda, K.; Hanawa, T. Bone healing of commercial oral implants with RGD immobilization through electrodeposited poly(ethylene glycol) in rabbit cancellous bone. Acta Biomater. 2011, 7, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Nogués, M.; Buxadera-Palomero, J.; Ginebra, M.-P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. All-in-one trifunctional strategy: A cell adhesive, bacteriostatic and bactericidal coating for titanium implants. Colloids Surf. B Biointerfaces 2018, 169, 30–40. [Google Scholar] [CrossRef]
- Okamoto, K.; Matsuura, T.; Hosokawa, R.; Akagawa, Y. RGD peptides regulate the specific adhesion scheme of osteoblasts to hydroxyapatite but not to titanium. J. Dent. Res. 1998, 77, 481–487. [Google Scholar] [CrossRef]
- Ferris, D.M.; Moodie, G.D.; Dimond, P.M.; Giorani, C.W.D.; Ehrlich, M.G.; Valentini, R.F. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials 1999, 20, 2323–2331. [Google Scholar] [CrossRef]
- Elmengaard, B.; Bechtold, J.E.; Soballe, K. In vivo study of the effect of RGD treatment on bone ongrowth on press-fit titanium alloy implants. Biomaterials 2005, 26, 3521–3526. [Google Scholar] [CrossRef]
- Reyes, C.D.; Petrie, T.A.; Burns, K.L.; Schwartz, Z.; Garcia, A.J. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2007, 28, 3228–3235. [Google Scholar] [CrossRef] [Green Version]
- Pallu, S.; Fricain, J.C.; Bareille, R.; Bourget, C.; Dard, M.; Sewing, A.; Amédée, J. Cyclo-DfKRG Peptide Modulates In Vitro and In Vivo Behavior of Human Osteoprogenitor Cells on Titanium Alloys. Acta Biomater. 2009, 5, 3581–3592. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Dorfner, P.; Manzenrieder, F.; Neubauer, S.; Reuning, U.; Burgkart, R.; Kessler, H. Behavior of primary human osteoblasts on trimmed and sandblasted Ti6Al4V surfaces functionalized with integrin αvβ3-selective cyclic RGD peptides. J. Biomed. Mater. Res. Part A 2013, 101A, 87–97. [Google Scholar] [CrossRef]
- Yuran, S.; Dolid, A.; Reches, M. Resisting bacteria and attracting cells: Spontaneous formation of a bifunctional peptide-based coating by on-surface assembly approach. ACS Biomater. Sci. Eng. 2018, 4, 4051–4061. [Google Scholar] [CrossRef]
- Auernheimer, J.; Zukowski, D.; Dahmen, C.; Kantlehner, M.; Enderle, A.; Goodman, S.L.; Kessler, H. Titanium implant materials with improved biocompatibility through coating with phosphonate-anchored cyclic RGD peptides. ChemBioChem 2005, 6, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Zorn, G.; Gotman, I.; Gutmanas, E.Y.; Adadi, R.; Salitra, G.; Sukenik, C.N. Surface Modification of Ti45Nb Alloy with an Alkylphosphonic Acid Self-Assembled Monolayer. Chem. Mater. 2005, 17, 4218–4226. [Google Scholar] [CrossRef]
- Bly, R.A.; Cao, Y.; Moore, W.A.; Soboyejo, W. Investigation of the effects of alkane phosphonic acid/RGD coatings on cell spreading and the interfacial strength between human osteosarcoma cells and Ti–6Al–4V. Mater. Sci. Eng. C 2007, 27, 83–89. [Google Scholar] [CrossRef]
- Heijink, A.; Schwartz, J.; Zobitz, M.E.; Nicole Crowder, K.; Lutz, G.E.; Sibonga, J.D. Self-assembled monolayer films of phosphonates for bonding RGD to titanium. Clin. Orthop. Relat. Res. 2008, 466, 977–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillou-Buffello, D.; Bareille, R.; Gindre, M.; Sewing, A.; Laugier, P.; Amédée, J. Additive Effect of RGD Coating to Functionalized Titanium Surfaces on Human Osteoprogenitor Cell Adhesion and Spreading. Tissue Eng. Part A 2008, 14, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Beuvelot, J.; Portet, D.; Lecollinet, G.; Moreau, M.F.; Baslé, M.F.; Chappard, D.; Libouban, H. In vitro kinetic study of growth and mineralization of osteoblast-like cells (Saos-2) on titanium surface coated with a RGD functionalized bisphosphonate. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Queffélec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface modification using phosphonic acids and esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef]
- Goura, J.; Chandrasekhar, V. Molecular metal phosphonates. Chem. Rev. 2015, 115, 6854–6965. [Google Scholar] [CrossRef]
- Bujoli, B.; Queffelec, C. Fine-Tuning the Functionality of Inorganic Surfaces Using Phosphonate Chemistry. In Tailored Organic-Inorganic Materials; John Wiley & Sons: New York, NY, USA, 2015; pp. 299–318. [Google Scholar]
- Gawalt, E.S.; Avaltroni, M.J.; Koch, N.; Schwartz, J. Self-assembly and bonding of alkanephosphonic acids on the native oxide surface of titanium. Langmuir 2001, 17, 5736–5738. [Google Scholar] [CrossRef]
- Silverman, B.M.; Wieghaus, K.A.; Schwartz, J. Comparative properties of siloxane vs. phosphonate monolayers on a key titanium alloy. Langmuir 2005, 21, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Portet, D.; Denizot, B.; Rump, E.; Lejeune, J.-J.; Jallet, P. nonpolymeric coatings of iron oxide colloids for biological use as magnetic resonance imaging contrast agents. J. Colloid Interface Sci. 2001, 238, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lecollinet, G.; Delorme, N.; Edely, M.; Gibaud, A.; Bardeau, J.F.; Hindré, F.; Boury, F.; Portet, D. Self-assembled monolayers of bisphosphonates: Influence of side chain steric hindrance. Langmuir 2009, 25, 7828–7835. [Google Scholar] [CrossRef] [PubMed]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Han, Y.; Hong, S.H.; Xu, K. Structure and in vitro bioactivity of titania-based films by micro-arc oxidation. Surf. Coat. Technol. 2003, 168, 249–258. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low, C.T.J.; Wood, R.J.K.; Stevens, K.T.; Archer, J.; Poeton, A.R.; Ryder, A. Plasma electrolytic oxidation (PEO) for production of anodised coatings on lightweight metal (Al, Mg, Ti) alloys. Trans. Inst. Met. Finish. 2009, 87, 122–135. [Google Scholar] [CrossRef]
- Jung, O.; Smeets, R.; Kopp, A.; Porchetta, D.; Hiester, P.; Heiland, M.; Friedrich, R.E.; Precht, C.; Hanken, H.; Gröbe, A.; et al. PEO-generated surfaces support attachment and growth of cells in vitro with no additional benefit for micro-roughness in Sa (0.2–4 μm). In Vivo 2016, 30, 27–33. [Google Scholar]
- Parfenov, E.V.; Yerokhin, A.; Nevyantseva, R.R.; Gorbatkov, M.V.; Liang, C.J.; Matthews, A. Towards smart electrolytic plasma technologies: An overview of methodological approaches to process modelling. Surf. Coat. Technol. 2015, 269, 2–22. [Google Scholar] [CrossRef] [Green Version]
- Yerokhin, A.; Parfenov, E.V.; Matthews, A. In situ impedance spectroscopy of the plasma electrolytic oxidation process for deposition of Ca- and P-containing coatings on Ti. Surf. Coat. Technol. 2016, 301, 54–62. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sharkeev, Y.P.; Sinebryukhov, S.L.; Khrisanfova, O.A.; Legostaeva, E.V.; Zavidnaya, A.G.; Puz’, A.V.; Khlusov, I.A.; Opra, D.P. Functional coatings formed on the titanium and magnesium alloys as implant materials by plasma electrolytic oxidation technology: Fundamental principles and synthesis conditions. Corros Rev. 2016, 34. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Gonzalez Tenorio, R.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Matykina, E. Tailoring of antibacterial and osteogenic properties of Ti6Al4V by plasma electrolytic oxidation. Appl. Surf. Sci. 2018, 454, 157–172. [Google Scholar] [CrossRef]
- Uezono, M.; Takakuda, K.; Kikuchi, M.; Suzuki, S.; Moriyama, K. Hydroxyapatite/collagen nanocomposite-coated titanium rod for achieving rapid osseointegration onto bone surface. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Noordin, S.; Masri, B.A.; Garbuz, D.S.; Duncan, C.P.; Hancock, R.E.W.; Wang, R. Drug release and bone growth studies of antimicrobial peptide-loaded calcium phosphate coating on titanium. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100 B, 1344–1352. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Zavidnaya, A.G.; Egorkin, V.S.; Puz’, A.V.; Mashtalyar, D.V.; Sergienko, V.I.; Yerokhin, A.L.; Matthews, A. Composite hydroxyapatite–PTFE coatings on Mg–Mn–Ce alloy for resorbable implant applications via a plasma electrolytic oxidation-based route. J. Taiwan Inst. Chem. Eng. 2014, 45, 3104–3109. [Google Scholar] [CrossRef]
- Nielsen, O.; Buchardt, O. Facile Synthesis of Reagents Containing a Terminal Maleimido Ligand Linked to an Active Ester. Synthesis 1991, 1991, 819–821. [Google Scholar] [CrossRef]
- Ede, N.J.; Tregear, G.W.; Haralambidis, J. Routine preparation of thiol oligonucleotides: Application to the synthesis of oligonucleotide-peptide hybrids. Bioconjugate Chem. 1994, 5, 373–378. [Google Scholar] [CrossRef]
- Tyler, P.C.; Young, R.N.; Rodan, G.A.; Ruel, R. Prostaglandin Analog for Treating Osteoporosis. US Patent No. 5,409,911, 1995. [Google Scholar]
- Moon, S.; Arrabal, R.; Matykina, E. 3-Dimensional structures of open-pores in PEO films on AZ31 Mg alloy. Mater. Lett. 2015, 161, 439–441. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Pedeferri, M. Anodic oxidation of titanium: From technical aspects to biomedical applications. J. Appl. Biomater. Biomech. 2011, 9, 55–69. [Google Scholar] [CrossRef]
- Zorn, G.; Gotman, I.; Gutmanas, E.Y.; Adadi, R.; Sukenik, C.N. Surface modification of Ti45Nb alloy by immobilization of RGD peptide via self assembled monolayer. J. Mater. Sci. Mater. Med. 2007, 18, 1309–1315. [Google Scholar] [CrossRef]
- Zoulalian, V.; Zürcher, S.; Tosatti, S.; Textor, M.; Monge, S.; Robin, J.J. Self-assembly of poly (ethylene glycol)-poly (alkyl phosphonate) terpolymers on titanium oxide surfaces: Synthesis, interface characterization, investigation of nonfouling properties, and long-term stability. Langmuir 2010, 26, 74–82. [Google Scholar] [CrossRef]
- Kovács, R.; Grün, A.; Németh, O.; Garadnay, S.; Greiner, I.; Keglevich, G. The synthesis of pamidronic derivatives in different solvents: An optimization and a mechanistic study. Heteroat. Chem. 2014, 25, 186–193. [Google Scholar] [CrossRef]
- Paterson, M.J.; Eggleston, I.M. Convenient preparation of N-maleoyl amino acid succinimido esters using N-trifluoroacetoxysuccinimide. Synth. Commun. 2008, 38, 303–308. [Google Scholar] [CrossRef]
- Song, H.Y.; Ngai, M.H.; Song, Z.Y.; MacAry, P.A.; Hobley, J.; Lear, M.J. Practical synthesis of maleimides and coumarin-linked probes for protein and antibody labelling via reduction of native disulfides. Org. Biomol. Chem. 2009, 7, 3400–3406. [Google Scholar] [CrossRef] [PubMed]
- Leonard, N.M.; Brunckova, J. One-pot synthesis of succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC). Org. Prep. Proced. Int. 2012, 44, 180–183. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with imageJ. Biophoton. Int. 2004, 11, 36–41. [Google Scholar]
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. I. Matrix-flushing method for quantitative multicomponent analysis. J. Appl. Crystallogr. 1974, 7, 519–525. [Google Scholar] [CrossRef]
- Scully, J.R. Polarization resistance method for determination of instantaneous corrosion rates. Corrosion 2000, 56, 199–218. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



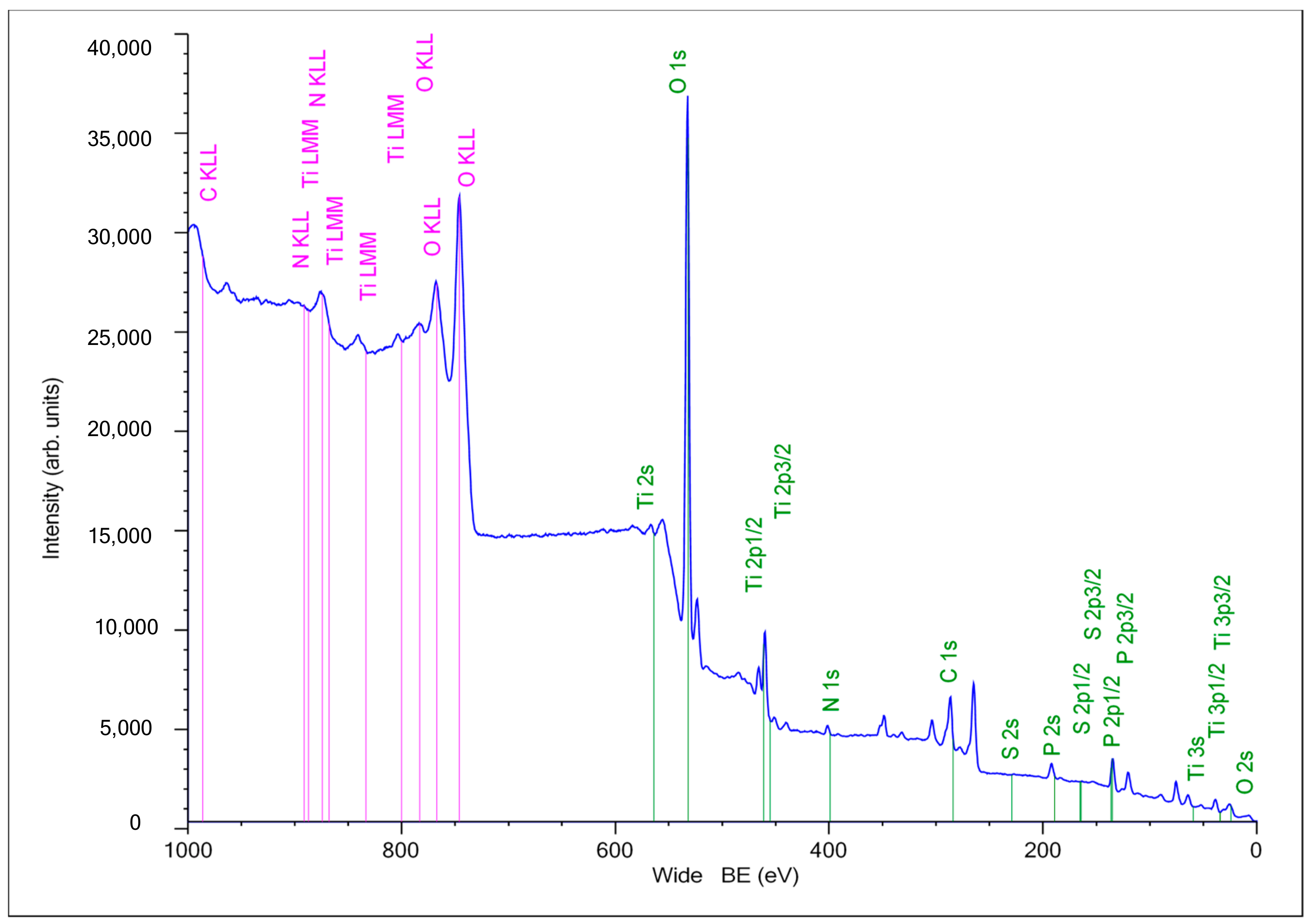

| Sample | XPS Atomic Composition (at %) | Atomic Ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| N1s | P2p | C1s | O1s | Ti2p | S2p | Ti2p/C1s | Ti2p/P2p | |
| Ti-PEO | 1.48 | 3.57 | 7.00 | 70.49 | 17.36 | 0.00 | 2.48 | 4.86 |
| Ti-PEO-15-RGD | 5.43 | 6.96 | 13.34 | 67.25 | 6.72 | 0.30 | 0.50 | 0.97 |
| Ti-PEO-16-RGD | 4.87 | 4.60 | 13.54 | 60.59 | 16.39 | 0.00 | 1.21 | 3.56 |
| Ti-PEO-17-RGD | 2.37 | 6.06 | 15.48 | 65.20 | 10.79 | 0.00 | 0.70 | 1.78 |
| Ti-PEO-18-RGD | 5.86 | 3.01 | 25.92 | 57.20 | 7.50 | 0.50 | 0.29 | 2.49 |
| Ti-PEO-19-RGD | 2.88 | 3.52 | 14.32 | 67.96 | 11.10 | 0.21 | 0.78 | 3.15 |
| Ti-PEO-20-RGD | 2.35 | 6.00 | 18.03 | 67.36 | 6.27 | 0.00 | 0.35 | 1.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parfenova, L.V.; Lukina, E.S.; Galimshina, Z.R.; Gil’fanova, G.U.; Mukaeva, V.R.; Farrakhov, R.G.; Danilko, K.V.; Dyakonov, G.S.; Parfenov, E.V. Biocompatible Organic Coatings Based on Bisphosphonic Acid RGD-Derivatives for PEO-Modified Titanium Implants. Molecules 2020, 25, 229. https://doi.org/10.3390/molecules25010229
Parfenova LV, Lukina ES, Galimshina ZR, Gil’fanova GU, Mukaeva VR, Farrakhov RG, Danilko KV, Dyakonov GS, Parfenov EV. Biocompatible Organic Coatings Based on Bisphosphonic Acid RGD-Derivatives for PEO-Modified Titanium Implants. Molecules. 2020; 25(1):229. https://doi.org/10.3390/molecules25010229
Chicago/Turabian StyleParfenova, Lyudmila V., Elena S. Lukina, Zulfia R. Galimshina, Guzel U. Gil’fanova, Veta R. Mukaeva, Ruzil G. Farrakhov, Ksenia V. Danilko, Grigory S. Dyakonov, and Evgeny V. Parfenov. 2020. "Biocompatible Organic Coatings Based on Bisphosphonic Acid RGD-Derivatives for PEO-Modified Titanium Implants" Molecules 25, no. 1: 229. https://doi.org/10.3390/molecules25010229








