1. Introduction
Pectin methylesterase (PE, EC 3.1.1.11) is a ubiquitous enzyme in plants, bacteria, and fungi [
1,
2]. PE catalyses the removal of methylester from homogalacturonan domains in pectin to release methanol and protons in the apoplast, which affects the properties of the pectin matrix by influencing calcium–pectate interactions [
3]. Via demethoxylation process, pectin aggregates into calcium-linked gel structures, which increases wall porosity and reduces apoplastic pH, thought to activate local hydrolases including polygalacturonases and pectin lyases, thereby softening the cell wall and inducing the ripening of fruits [
4].
In recent years, much research has been conducted to explore the substances with PE inhibitory activity (S
PEI) which are substances capable of reducing the rate of de-esterification of PE. In the food industry, S
PEI were proposed to have several potential applications because of the strong interaction between inhibitory substances and PE. S
PEI may be used in fruit maceration and juice clarification processes by their controlled degradation of pectin [
5]. Additionally, in wine production, S
PEI may reduce the methanol content by attenuating the activity of endogenous PE in fruit tissue [
6]. Several inhibitory substances have been identified from plants: side-branched uronic acid in potato tubers; proteins (~16.3 kDa) in kiwifruit [
7],
Arabidopsis [
8] and pepper leaves [
9]; and catechins in green tea [
10].
Jelly fig (
Ficus awkeotsang Makino) is a native woody vine growing in Taiwan [
11]. The seeds are used to make a jelly dessert, called “Ai-Yu-Tung,” in Asian countries [
12]. For preparing the jelly dessert, the achenes from jelly fig (JFA) fruit are rubbed gently with the addition of hard water. The aqueous extract, which is rich in pectin, will then spontaneously form a pudding-like gel by demethoxylation of pectin catalyzed by endogenous PE. However, when achenes are crushed along with the process, the gelation ability as well as PE activity is eliminated. With this phenomenon, some S
PEI are assumed to exist in seeds to be released from the achenes. For elucidating the mechanism of PE activity removal, the identification of S
PEI in jelly fig achenes (JFA) is needed.
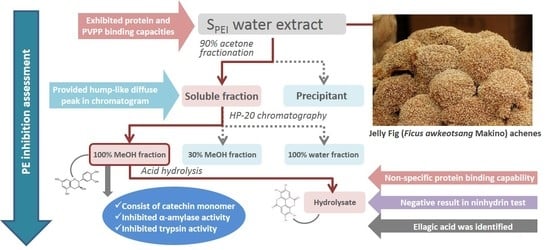
The aims of this study are to (i) isolate and characterize the JFA-SPEI by performing a series of PE inhibition-guided purification and identification experiments including membrane separation, acetone precipitation, adsorbent precipitation, macroporous resin chromatography and acid hydrolysis, (ii) further reveal the composition of SPEI by high-performance liquid chromatography-ultraviolet (HPLC-UV) and mass spectrometry (MS), and (iii) interpret the extensive enzyme inhibitory effect of JFA-SPEI by conducting trypsin and α-amylase inhibition experiments. In addition, since the JFA-SPEI were identified to consist predominantly of complex tannins in our study, total tannins content were determined within each isolated fraction as well.
3. Discussion
Recent evidence demonstrated that S
PEI has an important physiological role in PE regulation in plants because of an ability to inhibit plant PE activity [
17,
18,
19]. Therefore, S
PEI has several potential applications in a food-technological context [
20]. These endogenous substances found in several plant species such as kiwi fruit,
Arabidopsis, pepper and broccoli, were identified as proteinaceous compounds [
7,
8,
9,
17]. Active S
PEI were found in JFA and characterized as thermotolerable polypeptides of 3.5 to 4.5 kDa by Jiang et al. [
11,
21]. However, in our study, several findings indicated that JFA-S
PEI mostly consisted of endogenous ellagitannins and proanthocyanidins. Jiang et al. revealed that JFA-S
PEI extracts had remarkable inhibitory effect on PE activity after a 30-min boiling treatment and were considered thermotolerable substances [
11], and they identified JFA-S
PEI extracts by membrane separation followed by Sepharose G-50 chromatography, the S
PEI showed competitive inhibition with substrates [
21]. However, by following these procedures, we found several polymeric phenolic characteristics such as color transition after acid hydrolysis and a hump diffuse peak profile in chromatograms of isolated fractions with PE inhibitory activity. Therefore, we aimed to characterize the composition of JFA-S
PEI by using a series of PE inhibition-guided purification experiments.
S
PEI in JFA showed high thermostability because of remarkable inhibition of PE activity after the heating process described previously. Jiang, Li, Chang, and Chang, (2002) reported that the 3~10-kDa MWCO fraction of JFA-S
PEI crude extracts conferred greater inhibition of PE activity than other fractions [
21], but we found inhibitory substances in the >10-kDa MWCO fraction. Furthermore, with acetone precipitation, PE inhibition was performed with both the >10-kDa and <10-kDa MWCO fraction of the acetone-soluble part. This finding is probably due to the synthesis of the non-covalent interaction between S
PEI and protein that formed a complex structure and was retained in the high MW fraction, then the addition of acetone broke the binding and led to substance release to the low MW fraction. Hence, acetone precipitation could be considered a purification process of JFA.
A negative result from the ninhydrin test clearly revealed that in the presence of JFA-SPEI, very few or no free amino groups remained after hydrolysis. Moreover, the results of PVPP and protein interaction assessment were direct evidence that JFA-SPEI had protein-binding capacity and was implicated with phenolic compounds. Crude extract solution showed clear-turbid transition and significantly reduced PE inhibition after PVPP/proteins were introduced. This is an indication of complex formation and was probably due to SPEI mostly consisting of phenolic compounds.
In addition, Jiang, Li, Chang, and Chang, (2002) reported that incubation of JFA-S
PEI with trypsin reduced PE inhibition to ~3%, which suggests that its cleavage ability toward polypeptide chain contributed to the inhibition of PE activity [
21]. However, we found evidences that reduced PE activity may involve tannic compounds but not protein. Pre-treatment of trypsin with S
PEI fractions reduced the hydrolysis activity of trypsin toward model protein, which provide strong support for our hypothesis that the reduced PE inhibitory activity of JFA-S
PEI involved a precipitated complex formed between S
PEI and trypsin but not trypsin-catalyzed hydrolysis.
Several phenolic compounds have been found to possess inhibitory effect of PE activity. Lewis et al. (2008) found that green tea catechins can be used to inhibit PE activity across plant taxa and that the inhibitory interaction occurred at the substrate binding site of PE [
10]. Chen et al. (2009) showed that the addition of gallic acid and coumaric acid in the wine-making process may reduce methanol content by a mixed inhibition pattern of PE activity [
22]. Previous studies indicated that phenolic compounds might have potential inhibitory activity toward PE.
We found a hump diffuse peak profile in HPLC chromatogram of both JFA-SPEI fractions that disappeared after acid hydrolysis, followed by downregulation of PE inhibition. This finding may be due to tannin content responsible for the reduced PE activity. Moreover, ellagic acid was observed after hydrolysis, but no gallic acid was released during this process. However, MS spectra showed molecular ion species consistent with procyanidin oligomers containing singly linked units. Therefore, we concluded that the purified JFA-SPEI was a mixture of ellagitannins and condensed tannin, and these tannic compounds may play a more important role than the proteinaceous inhibitor found in JFA.
The term “tannin” was suggested to be reserved for phenolic compounds with a sufficient degree of hydroxylation and molecular size to form complexes with proteins and other polymers under suitable conditions of pH and concentration [
23]. Low MW phenolics including phenolic acids and simple flavonoids may bind protein but cannot crosslink the complexes, as is required for precipitation [
24]. Our results are consistent with these findings. The precipitated complexes we observed after the addition of soy protein, lysozyme and BSA may be due to the formation of ellagitannin/condensed tannin–protein complexes.
Tannins are widely considered to reduce activities of enzymes via non-specific protein binding. The inhibition activities of trypsin, α-amylase and lipase were found positively and linearly related to the degree of polymerization of tannins [
25]. As well, condensed tannins inhibited activities of lactate dehydrogenase, alcohol dehydrogenase and α-glucosidase [
26]. In our study, JFA-S
PEI with tannic substances inhibited digestive enzyme including α-amylase and trypsin, and may further be regarded as non-specific inhibitory substance of PE. On the other hand, hydrolysis of dietary polysaccharide such as starch is the dominant source of blood glucose that causes hyperglycemia [
26]. This reaction is carried out by a group of hydrolysis enzymes that includes pancreatic α-amylase. It is believed that inhibition of these enzymes can be a crucial strategy for management of type 2 diabetes [
27]. Therefore, JFA-S
PEI may lead to potential diabetes prevention effects.
Total tannin content of each fraction was determined along with the isolation process. We found relatively high tannin contents in fractions that conferred high PE inhibitory activities. This result provides firm evidence for the fact that JFA-S
PEI consists predominantly of tannic substances. In phytophysiology, endogenous tannins of plants were reported to affect the physiological condition of plants. For example, gallo- and ellagitannins were found to powerfully inactivate the early stages of pectolysis of low-methoxy pectin catalyzed by pectinase [
28]. Furthermore, to our knowledge, our study is the first to demonstrate that tannins inhibit the activity of plant source PE. The results suggest that these endogenous polyphenol compounds via inhibiting PE may regulate the physiologic development of some tannin-rich plant organs.
4. Materials and Methods
4.1. Materials and Chemicals
Jelly fig (Ficus awkeotsang Makino) and, pea (Pisum sativum L.) for PE extraction, were purchased from a local market in Taipei, Taiwan. Soy protein was from Gemfont (Taipei). Methylene blue and sodium chloride were from Riedel-de Haën (Seelze, Germany). Acetone, methanol, hydrochloride solution and fluorescein isothiocyanate (FITC)-casein were from Merck (Darmstadt, Germany). Trypsin solution was from ThermoFisher Scientific (Waltham, MA, USA). Pancreatic α-amylase was from Megazyme (Bray, Ireland). Methyl red was from Ferak (Berlin, Germany). Polyvinylpolypyrrolidone (PVPP), ninhydrin, pectin, lipase, tannase, tannic acid, Folin–Ciocalteu reagent, sodium carbonate, dinitrosalicylic acid, bovine serum albumin (BSA) were from Sigma Aldrich (St. Louis, MO, USA). The DIAION® HP-20 macroporous resin was from Mitsubishi Chemical (Tokyo, Japan). All chemicals were of reagent grade.
4.2. Preparation of JFA-SPEI Crude Extract and Pectin Methylesterase (PE) Solution
Preparation of S
PEI crude extract from JFA was as described previously [
21]. In brief, JFA was first washed with water flowing continually in a cheesecloth bag to remove pectin and to obtain JFA residue. An amount of 50 g dried JFA residue was introduced into 500 mL distilled water and gently stirred overnight at room temperature to eliminate the remaining pectin. JFA residue suspension was homogenized with a polytron homogenizer (Kinematica AG Littau, Switzerland) at 2000 rpm for 20 min to extract S
PEI, the extraction solution was centrifuged at 3000×
g for 10 min and supernatant was collected. The residual precipitate was then extracted twice by the same procedure; the supernatants were combined and heated at 95 °C for 15 min to deactivate the PE, then the solution was diluted with 3% NaCl solution to a volume of 1 L and boiled to eliminate residual PE. Resulted solution was centrifuged at 6500×
g for 20 min to obtain the supernatant as a S
PEI crude extract.
Extracting PE from pea-pod shells was as described previously [
11]. Briefly, 500 g pea-pod shells were homogenized for 2 min in 2 L cold (4 °C) distilled water. The mixture was filtered through cheesecloth to obtain the residues. Pea-pod residues were then extracted with a two-fold (
v/
w) volume of 0.3 M NaCl solution; the extract was filtered and the filtrate as PE solution was stored at 4 °C for further use.
4.3. Fractionation of SPEI Crude Extract
Preliminary fractionation involved acetone precipitation. Ice-cold acetone at 90% (v/v) was gently added with constant stirring. The mixture was incubated overnight at −20 °C or below. The solution was then filtered with filter paper and the soluble part was concentrated by rotary evaporation. The ASfr was obtained by reconstitution to the original volume with distilled water. The solid content was dried at room temperature and considered the acetone-precipitated fraction of SPEI crude extract (APfr).
For further fractionation, an amount of 60 mL ASfr underwent Diaion HP-20 chromatography with a water–methanol gradient system to obtain a water-eluted fraction and 30% and 100% methanol-eluted fraction (60 mL for each fraction).
Membrane separation (MW cutoff [MWCO] 10 kDa) involved using an Amicon ultraflitration cell equipped with an ultrafiltration membrane (Millipore) to obtain <10 and >10 kDa fractions from SPEI crude extract.
4.4. Evaluation of PE Inhibitory Activity
To evaluate the inhibitory activity of S
PEI, 200 μL PE solution was mixed with 800 μL S
PEI solution at various concentrations and incubated at 30 °C for 10 min, then 5 mL of 0.5% pectin solution (dissolved in 0.1 M NaCl) was added into the reaction mixture and incubated at 40 °C for 1 h. At the end of incubation, the mixture was heated at 95 °C for 15 min to terminate the enzyme catalysis reaction. PE inhibitory activity was evaluated by using methyl red-methylene blue indicator. Through this indicator, the cleavage of methylester groups and release of hydrogen ions could be detected by monitoring the pH-induced chromogenic transition with visual observation (
Figure 5). An amount of 20 mg methyl red powder was dissolved in 60 mL ethanol and diluted with distilled water to a volume of 100 mL; methylene blue was dissolved in 100 mL distilled water to a concentration of 20 ppm. The indicator solution was obtained by mixing these two solutions. Eventually, 100 μL of methyl red-methylene blue indicator was introduced into the solution and PE activity was estimated by measuring absorbance at 527 nm, the values of which showed linear changes along with increasing concentration of hydrogen ions. PE inhibition was calculated as percentage of control. The blanked absorbance of pectin solution with the addition of PE and without adding S
PEI was considered as 100%.
4.5. Chemical Characterization of SPEI
To characterize JFA-S
PEI, we used several assessments and treatments, including PVPP/protein treatment, ninhydrin test, acid hydrolysis and trypsin inhibition test. To explore the effect of interaction between S
PEI and PVPP on PE inhibitory activity, 200 mg PVPP was introduced into 10 mL S
PEI crude extract and stirred at room temperature for 1 h, then centrifuged at 3000×
g for 10 min [
29]. The supernatant was diluted to the original volume with distilled water, and PE inhibitory activity of S
PEI was performed. Proteins including soybean protein, lysozyme and BSA were added to S
PEI crude extract to determine the effect on PE inhibitory activity of S
PEI. Protein solutions at 1% (
w/
v) were mixed with the same volume of S
PEI crude extract solution and incubated at 4 °C overnight, then underwent the PE inhibition analysis. PE inhibition was also calculated.
To further identify the composition of JFA-SPEI, we used acid hydrolysis by incubation with 1 N HCl at 121 °C for 30 min. The pH value was adjusted to 6 with approximately 200 μL of 40% NaOH. The hydrolyzed ASfr fraction was then analysed by high-performance liquid chromatography (HPLC) to detect hydrolysis products.
The ninhydrin test was performed as described [
30]. The 100% MeOHfr from HP-20 chromatography was dried by rotating evaporation, and 0.1 g of the dried substances underwent acid hydrolysis as described previously. After cooling to room temperature, the pH was adjusted to 5 with 1 M NaOH. The sample was then dehydrated, and dried materials were reconstituted with 50% ethanol and introduced into 0.2% ninhydrin solution. The reaction was allowed to proceed for 15 min in a boiling water bath. After cooling to room temperature, the sample was diluted with 50% ethanol and the absorbance at 570 was measured. Hydrolyzed gelatin was used as a polypeptide control.
4.6. HPLC and MS Analysis
HPLC involved use of an analytical-model 584 solvent delivery gradient HPLC module (ESA Biosciences, Chelmsford, MA, USA), a Rheodyne injector model 7725i with a 20 μL sample loop (Rheodyne, Cotati, CA, USA), a 250 × 4.6 mm column, 5-μm Water Polarity TMdC18 protected by a guard cartridge (Hichrom 5C18, Berkshire, UK) and a UV detector model UV6000LP (Thermo Separation Products, San Jose, CA, USA). Chromatographic separation was performed at 25 °C by use of a linear mobile phase gradient including 0.1% acetic acid in water (solvent A) and 0.1% acetic acid in methanol (solvent B) at flow rate 0.65 mL/min. Elution involved a gradient starting with 1% B in A to reach 50% B at 60 min and then returning to the initial conditions for 10 min (1% B in A). Samples were recorded at 267 nm. For analysing the composition of 100% MeOHfr, elution involved a gradient starting with 1% B in A to reach 60% B at 30 min, 80% B at 40 min, and 90% at 50 min and then returning to the initial conditions for 10 min at a flow rate of 0.75 mL/min. Samples were recorded at 255 nm and 267 nm. Gallic acid and ellagic acid were identified according to their retention times by chromatographic comparisons with authentic standards. MS detection of the 100% MeOHfr was conducted with an electrospray source for the Finnigan LCQ advantage electrospray ion-trap mass spectrometer (Thermal; San Jose, CA, USA) at a constant flow rate of 10 μL/min by using a model 22 medical syringe infusion pump (Harvard Apparatus, South Natick, MA, USA) with a 100-μL syringe. The MS spectrum was performed with a sheath gas flow rate of 50 arb, an AuxSweep gas flow rate of 10 arb, a spray voltage of −3.5 kV and a capillary temperature of 225 °C.
4.7. Digestive Enzyme Inhibition Assay
A trypsin inhibition test was used to explore whether SPEI could affect the activity of trypsin on a model protein. ASfr and the 100% MeOHfr were diluted 100 times with distilled water and mixed with 0.5 μg/mL trypsin at 1:19 volume. The mixture was incubated at 28 °C for 10 min, then a 100 μL aliquot was mixed with 100 μL of 0.5 μg/mL FITC-casein solution. The reaction was allowed to proceed at 28 °C for 20 min. Fluorescence emission was obtained with excitation 485 nm and emission 538 nm. A calibration curve was produced with FITC-casein from 10 to 500 ng/mL.
For the α-amylase inhibition, a mixture of 80 μL ASfr or the 100% MeOHfr and 20 μL 0.9% sodium chloride solution containing α-amylase (13 U/mL) were incubated at 37 °C for 10 min. After preincubation, 50 μL 1% soluble starch in 0.9% sodium chloride solution were added to each tube at timed intervals. The reaction mixtures were then incubated at 37 °C for 10 min followed by the addition of 100 μL dinitrosalicylic acid color reagent. The test tubes were then placed in a boiling water bath for 5 min to stop the reaction and cooled to room temperature. The reaction mixture was then diluted with 1 mL distilled water and absorbance was read at 540 nm. The digestive enzymes inhibition was calculated as percentage of control. The blanked absorbance of reaction solution with the addition of digestive enzymes and without adding SPEI was considered as 100%.
4.8. Determination of Tannins Level
Total tannins contents were determined as described [
31] with modification. Firstly, total phenol content of sample was assessed. For the assay, 400 μL of diluted sample were added to 2 mL of 1:10 diluted Folin–Ciocalteu reagent. After 4 min, 1.6 mL of saturated sodium carbonate solution (75 g/L) was added. After 2 h of incubation at room temperature, the absorbance at 760 nm was measured in triplicate. Tannic acid (0–3 μg/mL) was used for calibration of standard curve. The results were expressed as milligram tannic acid equivalent (mg TAE)/g dry weight of JFA.
For determining total tannins content, subsequently, 1 g of casein was added into 18 mL of the diluted sample. The mixture was kept under mechanical agitation for 3 h at room temperature, and then centrifuged at 3000 rpm for 20 min. The supernatant was collected and assessed for total phenolic content by method mentioned above. The tannins level is calculated as the difference between the total phenol level and the non-complex residual phenol level, once the tannins are removed from the medium through complexation with casein.
4.9. Statistical Analysis
One-way analysis of variance (ANOVA) with Duncan’s multiple comparison was used to determine statistical significance. Differences were considered statistically significant at P < 0.05.