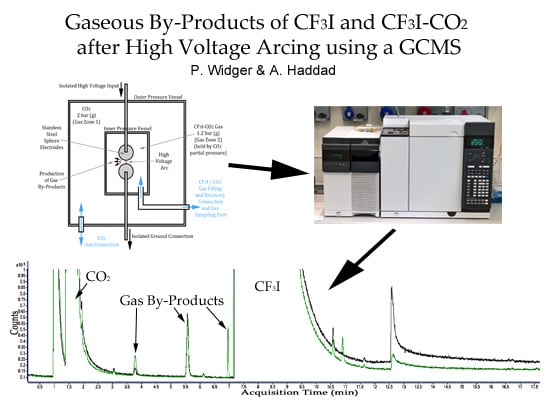
Analysis of Gaseous By-Products of CF3I and CF3I-CO2 after High Voltage Arcing Using a GCMS
Abstract
:1. Introduction
2. Results
2.1. Results of Pure CO2 Gas By-Product Analysis
2.2. Results of Pure CF3I Gas By-Product Analysis
2.3. Results of 30:70% CF3I-CO2 Gas By-Product Analysis
3. Discussion
3.1. Discussion of Pure CF3I and 30:70% CF3I-CO2 Gas By-Product Comparison
3.2. Discussion of 30:70% CF3I-CO2 GCMS Column Comparison
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Gas Detected | CAS Number | Molecular Weight | Labels | Exposure Limits | Exposure Controls | Reference |
|---|---|---|---|---|---|---|
| CO2—Carbon Dioxide | CAS-No. 124-38-9 EC No. 204-696-9 | 44.01 g/mol | H280: Contains gas under pressure; may explode if heated. | TWA 5000 ppm 9150 mg/m3 STEL 15,000 ppm 27,400 mg/m3 TWA 5000 ppm 9000 mg/m3 | P403: Store in a well-ventilated place. | [21] |
| CF3I—Methane, trifluoroiodo- | CAS-No.: 2314-97-8 EC-No.: 219-014-5 Index-No.: 602-086-00-0 | 195.91 g/mol | H341 Suspected of causing genetic defects. | LCLo inhalation 1 pph/4H (10,000 mg/kg) | P280 Wear protective gloves/ protective clothing/ eye protection/ face protection. P410 Protect from sunlight. P502 Refer to manufacturer/ supplier for information on recovery/ recycling | [15] [22,23] |
| CO—Carbon Monoxide | CAS-No. 630-08-0 EC-No. 211-128-3 Index-No. 006-001-00-2 | 28.01 g/mol | H220 Extremely flammable gas. H331 Toxic if inhaled. H360D May damage the unborn child. H372 Causes damage to organs through prolonged or repeated exposure. | STEL 200 ppm TWA 30 ppm LC50 Inhalation-Rat-4 h-1807 ppm | Avoid contact with skin, eyes and clothing. Wash hands before breaks and immediately after handling the product. | [24] |
| CF4—Tetrafluoromethane | CAS number: 75-73-0 | 88.01 g/mol | H280: Contains gas under pressure; may explode if heated. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. | No occupational exposure limit. DNEL not available PNEC not available. | P271: Use only outdoors or in a well-ventilated area. P280: Wear protective gloves/protective clothing/eye protection/face protection. R36/37/38: Irritating to eyes, respiratory system and skin. R44: Risk of explosion if heated under confinement. S36/37/39: Wear suitable protective clothing, gloves and eye/face protection. S45: In case of accident or if you feel unwell, seek medical advice immediately | [25,26] |
| C2F6—Ethane, hexafluoro- | CAS number: 76-16-4 EINECS number: 200-939-8 | 138.01 g/mol | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. | LC inhalation 20 pph/2H (200,000 mg/kg) LD50 4400 mg/kg | P261: Avoid breathing gas. P271: Use only outdoors or in a well-ventilated area. P280: Wear protective gloves/protective clothing/eye protection/face protection. | [27] |
| C2F4—Ethene, tetrafluoro- | CAS No.: 116-14-3 EC No. 204-126-9 UN No. 1081 | 100.02 g/mol | H220 (90.55%): Extremely flammable gas [Danger Flammable gases] H350 (85.07%): May cause cancer [Danger Carcinogenicity] H371 (73.63%): May cause damage to organs [Warning Specific target organ toxicity, single exposure] | LC50 inhalation 40,000 ppm/4H (40,000 mg/kg) LC50 inhalation143 gm/m3/4H (143,000 mg/kg) LC50inhalation 116 gm/m3/4H (116,000 mg/kg) | P201 Obtain special instructions before use, P210 Keep away from heat, hot surface, sparks, open flames and other ignition sources. - No smoking, P260 Do not breathe dust/fume/gas/mist/vapors/spray, P281 Use personal protective equipment as required, P308 + P313, P309 + P311 if exposed or if you feel unwell Call a POISON CENTER or doctor, P377, P381, P403 Store in a well-ventilated place, P405, P410 + P403 Protect from sunlight. | [28] |
| CHF3—Fluoroform | CAS number: 75-46-7 EINECS number: 200-872-4 | 70.01 g/mol | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. | LC50 > 200,000 ppm/2H LC50 > 663,000 ppm/4H | P271: Use only outdoors or in a well-ventilated area. P280: Wear protective gloves/protective clothing/eye protection/face protection. R36/37/38: Irritating to eyes, respiratory system and skin. S36/37/39: Wear suitable protective clothing, gloves and eye/face protection. S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible | [29,30] |
| C2F6O3—Trioxide, bis(trifluoromethyl) | CAS No. 1718-18-9 | 186.01 g/mol | No data available. | No data available. | No data available. | [31] |
| C3F8—Perfluoropropane | CAS number: 76-19-7 EINECS number: 200-941-9 | 188.02 g/mol | H280: Contains gas under pressure; may explode if heated. H319: Causes serious eye irritation. H335: May cause respiratory irritation. | LD 20 mL/kg (20 mg/kg) | P260: Do not breathe gas. P271: Use only outdoors or in a well-ventilated area. P280: Wear protective gloves/protective clothing/eye protection/face protection. | [32] |
| C2HF5—Ethane, pentafluoro | CAS number: 354-33-6 EC No. 206-557-8 | 120.02 g/mol | H280: Contains gas under pressure; may explode if heated. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. | LC50 inhalation 2910 gm/m3/4H (2910,000 mg/kg) LC50inhalation 2735 gm/m3/2H (2,735,000 mg/kg) | P260: Do not breathe gas. P271: Use only outdoors or in a well-ventilated area. P280: Wear protective gloves/protective clothing/eye protection/face protection. | [33,34] |
| C3F6—Hexafluoropropene | CAS-No.: 116-15-4 EC-No.: 204-127-4 Index-No.: 602-061-00-4 | 150.02 g/mol | H332 Harmful if inhaled. H335 May cause respiratory irritation. H371 May cause damage to organs. H373 May cause damage to organs through prolonged or repeated exposure. | LC50 750 ppm/4H LC16 5200 mg/m3/2H LC50 1600 mg/m3/2H LC84 13,400 mg/m3/2H | P260 Do not breathe dust/fume/gas/mist/vapours/spray. P308 + P311 IF exposed or concerned: Call a POISON CENTER/doctor. P410 + P403 Protect from sunlight. Store in a well-ventilated place. | [35,36] |
| C4F8—Cyclobutane, octafluoro- | CAS number: 115-25-3 EINECS number: 204-075-2 | 200.03 g/mol | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. H280: Contains gas under pressure; may explode if heated. | LCLo inhalation 78 pph/2H (780,000 mg/kg) | P271: Use only outdoors or in a well-ventilated area. P261: Avoid breathing gas. P280: Wear protective gloves/protective clothing/eye protection/face protection. | [37] |
| C2H3F3—Ethane, 1,1,1-trifluoro- | CAS number: 420-46-2 | 84.04 g/mol | H220: Extremely flammable gas. H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. | LC50 inhalation 54 pph/4H (540,000 mg/kg) | P210: Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P280: Wear protective gloves/protective clothing/eye protection/face protection. | [38,39] |
| C2F5I—Pentfluoroethyliodide | CAS-No.: 354-64-3 EC-No.: 206-566-7 | 245.92 g/mol | H315 Causes skin irritation. H319 Causes serious eye irritation. H335 May cause respiratory irritation. | LC inhalation 40,000 ppm/4H (40,000 mg/kg) | P261 Avoid breathing dust/fume/gas/mist/vapours/spray. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P410 + P403 Protect from sunlight. Store in a well-ventilated place. EUH044 Risk of explosion if heated under confinement. 2 | [40,41] |
| C2F9I—Tetrafluoro(pentafluoroethyl)iodine | CAS-No.: 20636-76-4 | 321.91 g/mol | No data available | No data available | No data available | [42] |
| I2—Iodine (CF3I test only) | CAS-No.: 7553-56-2 EC-No.: 231-442-4 Index-No.: 053-001-00-3 | 253.81 g/mol | H312 + H332 Harmful in contact with skin or if inhaled. H315 Causes skin irritation. H319 Causes serious eye irritation. H335 May cause respiratory irritation. H372 Causes damage to organs (Thyroid) through prolonged or repeated exposure if swallowed. H400 Very toxic to aquatic life. | STEL 0.1 ppm LD50 Oral-Rat-14,000 mg/kg LC50 Inhalation-Rat-4 h- > 4.588 mg/L LC50 Dermal-Rat-male-1425 mg/kg | P261 Avoid breathing dust. P273 Avoid release to the environment. P280 Wear protective gloves/protective clothing. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P314 Get medical advice/attention if you feel unwell. | [43] |
| C3F7I—Perfluoropropyl iodide | CAS-No.: 754-34-7 EC-No.: 212-045-5 | 295.93 g/mol | H315 (100%): Causes skin irritation H319 (100%): Causes serious eye irritation H335 (75%): May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] | LC50 Inhalation-2 h-404,000 mg/m3 | P261 Avoid breathing dust/fume/gas/mist/vapors/spray, P271 Use only outdoors or in a well-ventilated area, P280 Wear protective protection, P302 + P352 If on skin wash with plenty of water, P304 + P340 If inhaled remove victim to fresh air, P305 + P351 If in eyes rinse cautiously with water for several minutes, P312 Call a poison centre or doctor if you feel unwell, P321, P362 Take off contaminated clothing, P403 + P233 Store in a well-ventilated place, P405 Store locked up. | [44,45] |
| C3F7IO—Tetrafluoro-1 trifluoromethoxy 1-iodoethane 1,2,2,2-Tetrafluoro-1-iodoethyl trifluoromethyl ether | CAS-No.: 139604-89-0 | 311.92 g/mol | H302 Harmful if swallowed [Warning Acute toxicity, oral] H312 Harmful in contact with skin. H315 Causes skin irritation H319 Causes serious eye irritation H332 Harmful if inhaled H335 May cause respiratory irritation [Warning Specific target organ toxicity, single exposure; Respiratory tract irritation] | No data available | P261 Avoid breathing dust/fume/gas/mist/vapors/spray, P264 Wash thoroughly after handling, P271 Use only outdoors or in a well-ventilated area, P280 Wear protective protection, P301 + P312 f swallowed call a poison centre if you feel unwell, P302 + P352 If on skin wash with plenty of water, P304 + P312 If inhaled call a poison centre or doctor if you feel unwell, P304 + P340 If inhaled remove victim to fresh air, P305 + P351 + P338 If in eyes rinse cautiously with water for several minutes, P312 Call a POISON CENTER or doctor if you feel unwell, P321, P322, P330 Rinse mouth, P332 + P313 If skin irritation occurs get medical advice/attention, P337 + P313 If eye irritation persists get medical advice/attention, P362 Take off contaminated clothing, P363 Wash contaminated clothing before reuse, P403 + P233 Store in a well-ventilated place & Keep container tightly closed, P405 Store locked up. | [46] |
References
- Widger, P.; Haddad, A.M. Evaluation of SF6 Leakage from Gas Insulated Equipment on Electricity Networks in Great Britain. Energies 2018, 11, 2037. [Google Scholar] [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.M.; Collins, W.; Fuglestvedt, J.; Jianping, H.; Koch, D.; Lamarque, J.F.; David, L.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Working Group I Contribution to Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- National Grid. National Grid Electricity Transmission Network Innovation Allowance Annual Summary. 2016/2017. Available online: https://www.nationalgrid.com/sites/default/files/documents/National%20Grid%20Electricity%20Transmission%20NIA%20Annual%20Summary%202016-17.pdf (accessed on 12 September 2018).
- ABB. AirPlus TM: An Alternative to SF6 as an Insulation and Switching Medium in Electrical Switchgear. Available online: https://library.e.abb.com/public/3405a31190934a8c98997eca8fc811be/ABB%20Review%202-2016_AirPlus_An%20Alternative%20to%20SF6.pdf (accessed on 12 September 2018).
- Beroual, A.; Haddad, A. Recent Advances in the Quest for a New Insulation Gas with a Low Impact on the Environment to Replace Sulphur Hexafluoride (SF6) Gas in High-Voltage Power Network Applications. Energies 2017, 10, 1216. [Google Scholar] [CrossRef]
- Widger, P.; Haddad, A.; Griffiths, H. Breakdown performance of vacuum circuit breakers using alternative CF3I-CO2 insulation gas mixture. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 14–21. [Google Scholar] [CrossRef]
- Chen, L.; Widger, P.; Kamarudin, M.S.; Griffiths, H.; Haddad, A. CF3I Gas Mixtures: Breakdown Characteristics and Potential for Electrical Insulation. IEEE Trans. Power Deliv. 2017, 32, 1089–1097. [Google Scholar] [CrossRef]
- Widger, P.; Griffiths, H.; Haddad, A. Insulation strength of CF3I-CO2 gas mixtures as an alternative to SF6 in MV switch disconnectors. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 330–338. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zhang, J.; Xiao, S.; Xie, B.; Chen, D.; Gao, Y.; Tang, J. Assessment on the toxicity and application risk of C4F7N: A new SF6 alternative gas. J. Hazard. Mater. 2019, 368, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Rabie, M.; Franck, C.M. Assessment of Eco-friendly Gases for Electrical Insulation to Replace the Most Potent Industrial Greenhouse Gas SF6. Environ. Sci. Technol. 2018, 52, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Xiao, S.; Tang, J.; Tian, S.; Deng, Z. Decomposition Mechanism of C5F10O: An Environmentally Friendly Insulation Medium. Environ. Sci. Technol. 2017, 51, 10127–10136. [Google Scholar] [CrossRef] [PubMed]
- Kamarol, M.; Nakayama, Y.; Hara, T.; Ohtsuka, S.; Hikita, M. Gas Decomposition Analysis of CF3I under AC Partial Discharge of Non-uniform Electric Field Configuration. In Proceedings of the 10th Japan-Korea Joint Symposium on Electrical Discharge and High Voltage Engineering, Shibaura Institute of Technology, Tokyo, Japan, 15–17 November 2007; Paper 16P-15. pp. 161–164. [Google Scholar]
- Aalco. Stainless Steel: 1.4301 Sheet and Plate. 24 May 2018. Available online: http://www.aalco.co.uk/online-tools/product-finder/product.aspx?productid=2083 (accessed on 10 September 2018).
- Tosoh Corporation, (Tosoh Corporation, Tokyo, Japan). CF3I (Trifluoroiodomethane) Safety data sheet according to Regulation (EC) No. 1907/2006, Cas No. 2314-97-8. Personal communication, Available upon request. 2011. [Google Scholar]
- BOC, Carbon dioxide Factsheet. 2017. Available online: https://www.boconline.co.uk/en/legacy/attachment?files=tcm:t410-54560,tcm:410-54560,tcm:10-54560 (accessed on 9 September 2018).
- Kasuya, H.; Katagiri, H.; Kawamura, Y.; Saruhashi, D.; Nakamura, Y.; Mizoguchi, H.; Yanabu, S. Measurement of Decomposed Gas Density of CF3I-CO2 Mixture. In Proceedings of the 16th International Symposium on High Voltage Engineering (ISH 2009), South African Inst. of Electrical Engineers, Cape Town, South Africa, 24–28 August 2009; Paper C-41. pp. 1–4. [Google Scholar]
- BOC, Helium Purity level. 2018. Available online: https://www.boconline.co.uk/shop/en/uk/helium--cp-grade--300bar-cylinder-171698#product1 (accessed on 12 September 2018).
- Vacuum pump final vacuum level, Dilo B078R06 portable vacuum pump unit specifications, Dilo GmbH, Babenhausen, 2012 Product Catalogue. Available online: https://www.dilo-gmbh.com/fileadmin/user_upload/8._Downloads/2._englisch/2._SF6-Gashandling/2._SF6-Servicegeraete/Mini_Series_-_modular.pdf (accessed on 22 April 2019).
- Widger, P.; Haddad, A. Solid by-products of a CF3I-CO2 insulating gas mixtures on electrodes after lightning impulse breakdown. J. Phys. Commun. 2017, 1, 025010. [Google Scholar] [CrossRef]
- BOC, Carbon Dioxide Materials Safety Data Sheet. 2017. Available online: https://www.boconline.co.uk/en/legacy/attachment?files=tcm:4410-39607,tcm:410-39607,tcm:10-39607 (accessed on 19 March 2019).
- Sigma Aldrich, CF3I Material Safety Data Sheet. Version 5.4 Revision Date: 18.04.2018. Available online: https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=171441&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2FALDRICH%2F171441%3Flang%3Den (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, CF3I. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/16843#section=Top (accessed on 19 March 2019).
- Sigma Aldrich, CO Material Safety Data Sheet. Version 5.4 Revision Date: 24.05.2016. Available online: https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=295116&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F295116%3Flang%3Den (accessed on 19 March 2019).
- Apollo Scientific, CF4 Material Safety Data Sheet. Revision No. 2: 20/03/2013. Available online: http://www.apolloscientific.co.uk/downloads/msds/PC1650_msds.pdf (accessed on 19 March 2019).
- BOC Online, CF4 Material Safety Data Sheet. Version 1.2, Revision date: 04.11.2011. Available online: https://www.boconline.co.uk/en/images/sg_116_tetrafluoromethane_r14_tcm410-64642.pdf (accessed on 19 March 2019).
- Apollo Scientific, C2F6 Material Safety Data Sheet. Version 3, Revision date: 28/01/2016. Available online: http://www.apolloscientific.co.uk/downloads/msds/PC7201_msds.pdf (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, C2F4. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8301#section=Top (accessed on 19 March 2019).
- Apollo Scientific, CHF3 Material Safety Data Sheet. Version 3, Revision date: 12/09/2011. Available online: http://www.apolloscientific.co.uk/downloads/msds/PC7400_msds.pdf (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, CHF3. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6373#section=Top (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, C2F6O3. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/549862#section=Top (accessed on 19 March 2019).
- Apollo Scientific, C3F8 Material Safety Data Sheet. Version 4, Revision date: 19/04/2018. Available online: http://www.apolloscientific.co.uk/downloads/msds/PC6210_msds.pdf (accessed on 19 March 2019).
- Apollo Scientific, C2HF5 Material Safety Data Sheet. Version 3, Revision date: 19/04/2018. Available online: http://www.apolloscientific.co.uk/downloads/msds/PC5590_msds.pdf (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, C2HF5. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9633#section=Top (accessed on 19 March 2019).
- Sigma Aldrich, C3F6 Material Safety Data Sheet. Version 5.2 Revision Date: 24.02.2016. Available online: https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=295388&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F295388%3Flang%3Den (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, C3F6. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8302#section=Top (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, C4F8. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/8263#section=Top (accessed on 19 March 2019).
- Apollo Scientific, C2H3F3 Material Safety Data Sheet. Version 3, Revision date: 22/06/2016. Available online: http://www.apolloscientific.co.uk/downloads/msds/PC7298_msds.pdf# (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, C2H3F3. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9868#section=Top (accessed on 19 March 2019).
- Sigma Aldrich, C2F5I Material Safety Data Sheet. Version 5.1 Revision Date: 25.02.2016. Available online: https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=331015&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2FALDRICH%2F331015%3Flang%3Den (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, C2F5I. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9636#section=Top (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, C2F9I. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/550350#section=Top (accessed on 19 March 2019).
- Sigma Aldrich, I2 Material Safety Data Sheet. Version 5.5 Revision Date 11.07.2018. Available online: https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=229695&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2F229695%3Flang%3Den (accessed on 19 March 2019).
- Sigma Aldrich, C3F7I Material Safety Data Sheet. Version 5.0 Revision Date 22.02.2013. Available online: https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=GB&language=en&productNumber=P10402&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fproduct%2Faldrich%2Fp10402%3Flang%3Den (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, C3F7I. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/33977#section=Top (accessed on 19 March 2019).
- U.S. National Library of Medicine National Center for Biotechnology Information, Pubchem, C3F7IO. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/550245#section=Top (accessed on 19 March 2019).
Sample Availability: Not Available. |








| By-product Labelled Number in Figure 4 | CF3I and CF3I-CO2 Gas Sample–Detected before Breakdown | CF3I and CF3I-CO2 Gas Sample–By-products Detected after Breakdown | CF3I Gas Sample only–By-products Detected after Breakdown | CF3I-CO2 Gas Sample only–By-products Detected after Breakdown |
|---|---|---|---|---|
| 1 | CO—Carbon Monoxide | |||
| 2 | CF4—Tetrafluoromethane | CF4 or C2F6O3—Trioxide, bis(trifluoromethyl) | ||
| 3 | CO2—Carbon Dioxide | |||
| 4 | C2F6—Ethane, hexafluoro- | |||
| 5 | C2F4—Ethene, tetrafluoro- | |||
| 6 | CHF3—Fluoroform | |||
| 7 | C2F6O3—Trioxide, bis(trifluoromethyl) | |||
| 8 | C3F8—Perfluoropropane | |||
| 9 | C2HF5—Ethane, pentafluoro | |||
| 10 | C3F6 or C4F8—Cyclobutane, octafluoro- | |||
| 11 | C2F6O3 or C2H3F3—Ethane, 1,1,1-trifluoro- | |||
| 12 | CF3I—Methane, trifluoroiodo- | |||
| 13 | C2F5I—Pentfluoroethyliodide | C2F5I or C2F9I—Tetrafluoro(pentafluoroethyl)iodine | ||
| 14 | H2O—Water | |||
| 15 | I2—Iodine (CF3I test only) | |||
| 16 | C3F7I—Perfluoropropyl iodide | |||
| 17 | C3F7IO—Tetrafluoro-1 trifluoromethoxy 1-iodoethane |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widger, P.; Haddad, A. Analysis of Gaseous By-Products of CF3I and CF3I-CO2 after High Voltage Arcing Using a GCMS. Molecules 2019, 24, 1599. https://doi.org/10.3390/molecules24081599
Widger P, Haddad A. Analysis of Gaseous By-Products of CF3I and CF3I-CO2 after High Voltage Arcing Using a GCMS. Molecules. 2019; 24(8):1599. https://doi.org/10.3390/molecules24081599
Chicago/Turabian StyleWidger, Phillip, and Abderrahmane (Manu) Haddad. 2019. "Analysis of Gaseous By-Products of CF3I and CF3I-CO2 after High Voltage Arcing Using a GCMS" Molecules 24, no. 8: 1599. https://doi.org/10.3390/molecules24081599