Anthocyanin Encapsulated by Ferulic Acid-Grafted-Maltodextrin (FA-g-MD) Microcapsules Potentially Improved its Free Radical Scavenging Capabilities Against H2O2-Induced Oxidative Stress
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Microcapsules
2.2. In Vitro Release Profile of ANC
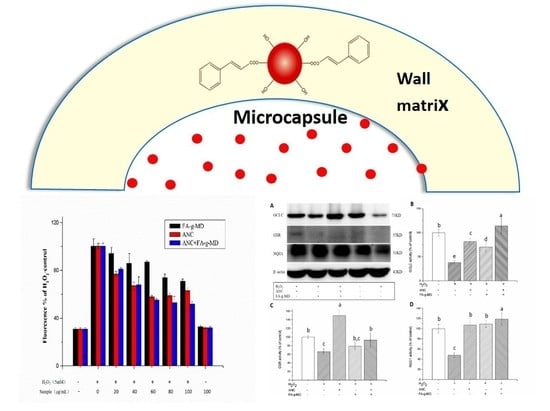
2.3. Antioxidant Capacity of ANC, FA-g-MD and Their Combination
2.4. UV-vis Absorption Spectrum of ANC and FA-g-MD
2.5. Modulation of Cellular ROS Level
2.6. Expression of Antioxidant Enzymes
3. Materials and Methods
3.1. Chemicals, Cells and Media
3.2. Preparation of Microcapsules
3.3. Scanning Electron Microscope Analysis (SEM)
3.4. Encapsulation Yield (EY) and encapsulation efficiency (EE)
3.5. Volumetric Density
3.6. Moisture Content and Water Activity
3.7. In vitro ANC Release
3.8. DPPH Radical Scavenging Ability Assay
3.9. Assay of Lipid Peroxidation Inhibition
3.10. UV-vis Analysis
3.11. Cell Culture and Viability Assays
3.12. ROS Assay
3.13. Protein Expression (Western Blot Analysis)
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ANC | Anthocyanin |
| FA-g-MD | Ferulic acid-grafted-maltodextrin |
| ROS | Reactive oxygen species |
| DPPH | 2,2′-diphenyl-1-picrylhydrazyl radical |
| DCF-DA | 2′,7′-dichlorofluorescin diacetate |
| NQO1 | quinone oxidoreductase 1 |
| GSR | glutathione reductase |
| γ-GCLC | γ-glutamate cysteine ligase catalytic subunit |
References
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Current Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [Green Version]
- Myung, S.-K.; Ju, W.; Cho, B.; Oh, S.-W.; Park, S.M.; Koo, B.-K.; Park, B.-J. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. BMJ Brit. Med. J. 2013, 346, f10. [Google Scholar] [CrossRef]
- Aruoma, O.I. Antioxidant actions of plant foods: Use of oxidative DNA damage as a tool for studying antioxidant efficacy. Free Radical Res. 2009, 30, 419–427. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and Antioxidant Properties of Anthocyanin Rich Extracts from Blueberry and Blackcurrant Juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [Green Version]
- Jing, P.; Qian, B.; Zhao, S.; Qi, X.; Ye, L.; Giusti, M.M.; Wang, X. Effect of glycosylation patterns of Chinese eggplant anthocyanins and other derivatives on antioxidant effectiveness in human colon cell lines. Food Chem. 2015, 172, 183–189. [Google Scholar] [CrossRef]
- Shi, M.; Bai, J.; Zhao, L.; Yu, X.; Liang, J.; Liu, Y.; Nord, W.; Li, Y. Co-loading and intestine-specific delivery of multiple antioxidants in pH-responsive microspheres based on TEMPO-oxidized polysaccharides. Carbohyd. Polym. 2017, 157, 858–8657. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins encapsulated b PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Aβ1–42-induced oxidative stress. J. Nanobiotech. 2017, 15, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.H.; Chan, L.W.; Heng, P.W.S. Alginate/starch composites as wall material to achieve microencapsulation with high oil loading. J. Microencapsul. 2008, 26, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-Drying Microencapsulation of Anthocyanins by Natural Biopolymers: A Review. Dry. Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Arozarena, I.; Marín-Arroyo, M.R. Optimization of a Wall Material Formulation to Microencapsulate a Grape Seed Extract Using a Mixture Design of Experiments. Food Bioprocess Tech. 2012, 6, 941–951. [Google Scholar] [CrossRef]
- Bai, H.; Liu, R.; Chen, H.L.; Zhang, W.; Wang, X.; Zhang, X.D.; Li, W.L.; Hai, C.X. Enhanced antioxidant effect of caffeic acid phenethyl ester and Trolox in combination against radiation induced-oxidative stress. Chem. Biol. Interact. 2014, 207, 7–15. [Google Scholar] [CrossRef]
- Chu, C.; Lu, F.J.; Yeh, R.H.; Li, Z.L.; Chen, C.H. Synergistic antioxidant activity of resveratrol with genistein in high-glucose treated Madin-Darby canine kidney epithelial cells. Biomed. Rep. 2016, 4, 349–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantan, R.; Tocharus, J.; Suksamrarn, A.; Tocharus, C. Synergistic effect of atorvastatin and Cyanidin-3-glucoside on angiotensin II-induced inflammation in vascular smooth muscle cells. Exp. Cell Res. 2016, 342, 104–112. [Google Scholar] [CrossRef]
- Sharif, H.R.; Goff, H.D.; Majeed, H.; Shamoon, M.; Liu, F.; Nsor-Atindana, J.; Haider, J.; Liang, R.; Zhong, F. Physicochemical properties of β-carotene and eugenol co-encapsulated flax seed oil powders using OSA starches as wall material. Food Hydrocolloids 2017, 73 (Supp. C), 274–283. [Google Scholar] [CrossRef]
- Aewsiri, T.; Benjakul, S.; Visessanguan, W.; Eun, J.-B.; Wierenga, P.A.; Gruppen, H. Antioxidative activity and emulsifying properties of cuttlefish skin gelatin modified by oxidised phenolic compounds. Food Chem. 2009, 117, 160–168. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Characterisation of ferulic acid incorporated starch–chitosan blend films. Food Hydrocolloid. 2008, 22, 826–835. [Google Scholar] [CrossRef]
- Wrigstedt, P.; Kylli, P.; Pitkanen, L.; Nousiainen, P.; Tenkanen, M.; Sipila, J. Synthesis and antioxidant activity of hydroxycinnamic acid xylan esters. J. Agric. Food Chem. 2010, 58, 6937–6943. [Google Scholar] [CrossRef] [PubMed]
- Woranuch, S.; Yoksan, R.; Akashi, M. Ferulic acid-coupled chitosan: Thermal stability and utilization as an antioxidant for biodegradable active packaging film. Carbohyd. Polym. 2015, 115, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Hou, C.-J.; Fa, H.-B.; Huo, D.-Q.; Yang, M. Synthesis and antioxidant property of hydroxycinnamoyl maltodextrin derivatives. Int. J. Food Sci. Technol. 2016, 51, 2450–2459. [Google Scholar] [CrossRef]
- García-Tejeda, Y.V.; Salinas-Moreno, Y.; Martínez-Bustos, F. Acetylation of normal and waxy maize starches as encapsulating agents for maize anthocyanins microencapsulation. Food Bioprod. Process. 2015, 94, 717–726. [Google Scholar] [CrossRef]
- Ah med, M.; Akter, M.S.; Lee, J.C.; Eun, J.B. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT–Food Sci. Technol. 2010, 43, 1309–1322. [Google Scholar]
- Ma, Y.; Hou, C.-J.; Wu, H.-X.; Fa, H.-B.; Li, J.-J.; Shen, C.-H.; Li, D.; Huo, D.-Q. Synthesis of maltodextrin-grafted-cinnamic acid and evaluation on its ability to stabilize anthocyanins via microencapsulation. J. Microencapsul. 2016, 33, 554–562. [Google Scholar] [CrossRef]
- Oidtmann, J.; Schantz, M.; Mader, K.; Baum, M.; Berg, S.; Betz, M.; Kulozik, U.; Leick, S.; Rehage, H.; Schwarz, K.; et al. Preparation and comparative release characteristics of three anthocyanin encapsulation systems. J. Agric. Food Chem. 2012, 60, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.; Parola, A.J.; Lima, J.C.; Pina, F. Solvent effects on the thermal and photochemical reactions of 4’-iodo-8-methoxyflavylium and their consequences on the coloring phenomena caused by anthocyanins in plants. Chemistry-Eur. J. 2006, 12, 7906–7912. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Szeląg, M.; Urbaniak, A.; Bluyssen, H.A.R. A theoretical antioxidant pharmacophore for natural hydroxycinnamic acids. Open Chem. 2015, 13. [Google Scholar] [CrossRef]
- Kylli, P.; Nousiainen, P.; Biely, P.; SIPILÄ, J.; Tenkanen, M.; Heinonen, M. Antioxidant Potential of Hydroxycinnamic Acid. J. Agric. Food Chem. 2008, 56, 4797–4805. [Google Scholar] [CrossRef]
- Rustioni, L.; Di Meo, F.; Guillaume, M.; Failla, O.; Trouillas, P. Tuning color variation in grape anthocyanins at the molecular scale. Food Chem. 2013, 141, 4349–4357. [Google Scholar] [CrossRef] [Green Version]
- Sousa, A.; Araujo, P.; Cruz, L.; Bras, N.F.; Mateus, N.; De Freitas, V. Evidence for copigmentation interactions between deoxyanthocyanidin derivatives (oaklins) and common copigments in wine model solutions. J. Agric. Food Chem. 2014, 62, 6995–7001. [Google Scholar] [CrossRef]
- Stephanie, G.; Nathalie, M.; Abert-Vian, M.; Njara, R.; Dangles, O. Chemical Synthesis of Hydroxycinnamic Acid Glucosides and Evaluation of Their Ability To Stabilize Natural Colors via Anthocyanin Copigmentation. J. Agric. Food Chem. 2007, 55, 7573–7579. [Google Scholar]
- Wolfe, K.L.; Liu, R.H. Structure-activity relationships of flavonoids in the cellular antioxidant activity assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants Foods and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Stiebitz, H.; Bytof, G.; Lantz, I.; Baum, M.; Eisenbrand, G.; Janzowski, C. Antioxidant effectiveness of coffee extracts and selected constituents in cell-free systems and human colon cell lines. Mol. Nutr. Food Res. 2010, 54, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mou, D.; Du, Y. Procyanidins: Extraction and micro- encapsulation. J. Sci. Food. Agr. 2007, 87, 2192–2197. [Google Scholar] [CrossRef]
- Santiago-Adame, R.; Medina-Torres, L.; Gallegos-Infante, J.A.; Calderas, F.; Gonzalez-Laredo, R.F.; Rocha-Guzman, N.E.; Ochoa-Martínez, L.A.; Bernad-Bernad, M.J. Spray drying-microencapsulation of cinnamon infusions(Cinnamomum zeylanicum) with maltodextrin. LWT–Food Sci. Technol. 2015, 64, 571–577. [Google Scholar] [CrossRef]
- Tulini, F.L.; Souza, V.B.; Thomazini, M.; Silva, M.P.; Massarioli, A.P.; Alencar, S.M.; Pallone, E.M.J.A.; Genovese, M.I.; Favaro-Trindade, C.S. Evaluation of the release profile, stability andantioxidant activity proanthocyanidin-rich cinnamon (Cinnamomum zeylanicum) extract co-encapsulated with α-tocopherol by spray chilling. Food Res. Int. 2017, 95, 117–124. [Google Scholar] [CrossRef]
- Kahkonen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef]
- Soenen, S.J.; Himmelreich, U.; Nuytten, N.; De Cuyper, M. Cytotoxic effects of iron oxide nanoparticles and implications for safety in cell labelling. Biomaterials 2011, 32, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader11Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee by the United States Department of Agriculture and does not imply its approval to the exclusion of other products that may be suitable. Free Radical Bio. Med. 1999, 27, 612–616. [Google Scholar]
- Cilla, A.; Laparra, J.M.; Alegria, A.; Barbera, R.; Farre, R. Antioxidant effect derived from bioaccessible fractions of fruit beverages against H2O2-induced oxidative stress in Caco-2 cells. Food Chem. 2008, 106, 1180–1187. [Google Scholar] [CrossRef]
- Bellion, P.; Olk, M.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Formation of hydrogen peroxide in cell culture media by apple polyphenols and its effect on antioxidant biomarkers in the colon cell line HT-29. Mol. Nutr. Food Res. 2009, 53, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kingsley, P.J.; Marnett, L.J.; Eling, T.E. The Role of NAG-1/GDF15 in the Inhibition of Intestinal Polyps in APC/Min Mice by Sulindac. Cancer Prev. Res. 2011, 4, 150. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Feng, Y.; Zeng, W.; Luo, H. Anthocyanin Encapsulated by Ferulic Acid-Grafted-Maltodextrin (FA-g-MD) Microcapsules Potentially Improved its Free Radical Scavenging Capabilities Against H2O2-Induced Oxidative Stress. Molecules 2019, 24, 1596. https://doi.org/10.3390/molecules24081596
Ma Y, Feng Y, Zeng W, Luo H. Anthocyanin Encapsulated by Ferulic Acid-Grafted-Maltodextrin (FA-g-MD) Microcapsules Potentially Improved its Free Radical Scavenging Capabilities Against H2O2-Induced Oxidative Stress. Molecules. 2019; 24(8):1596. https://doi.org/10.3390/molecules24081596
Chicago/Turabian StyleMa, Yi, Yunhui Feng, Wanling Zeng, and Huibo Luo. 2019. "Anthocyanin Encapsulated by Ferulic Acid-Grafted-Maltodextrin (FA-g-MD) Microcapsules Potentially Improved its Free Radical Scavenging Capabilities Against H2O2-Induced Oxidative Stress" Molecules 24, no. 8: 1596. https://doi.org/10.3390/molecules24081596