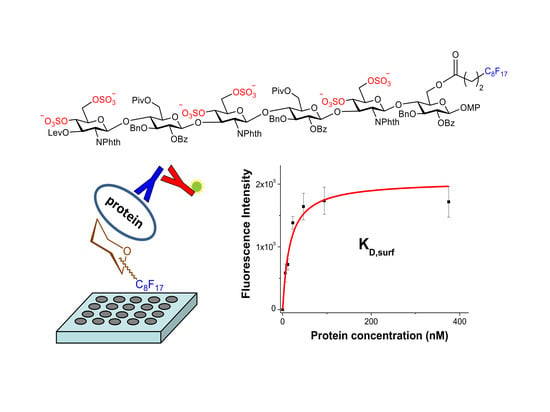
Synthesis of a Fluorous-Tagged Hexasaccharide and Interaction with Growth Factors Using Sugar-Coated Microplates
Abstract
:1. Introduction
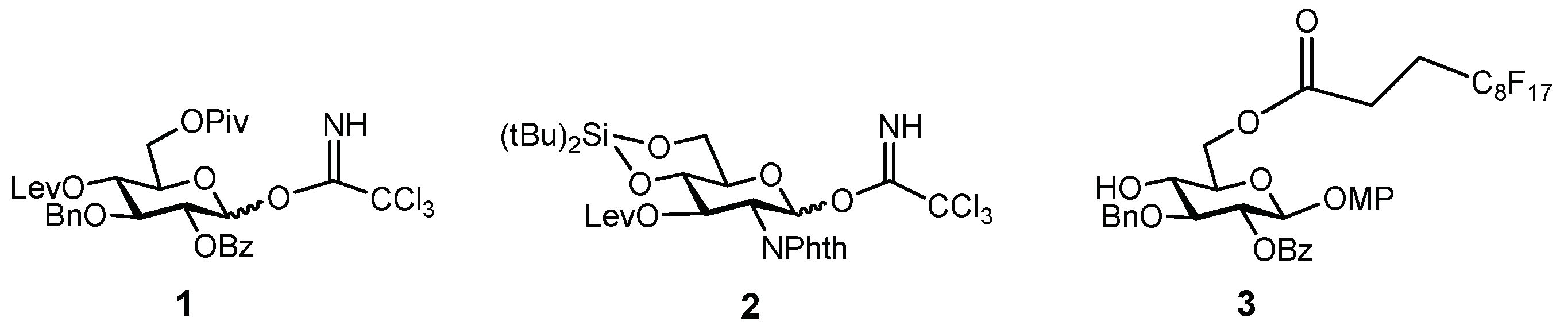
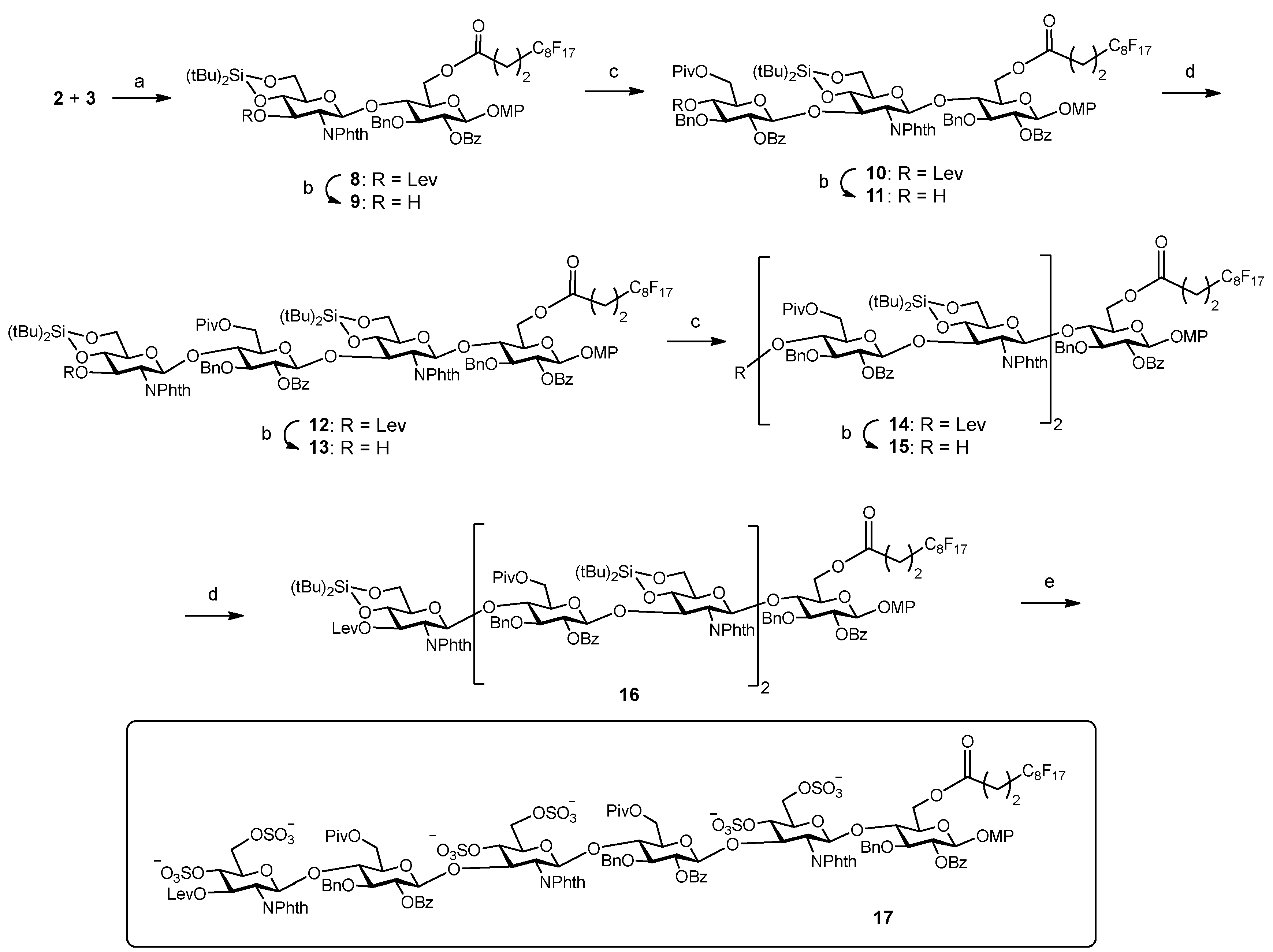
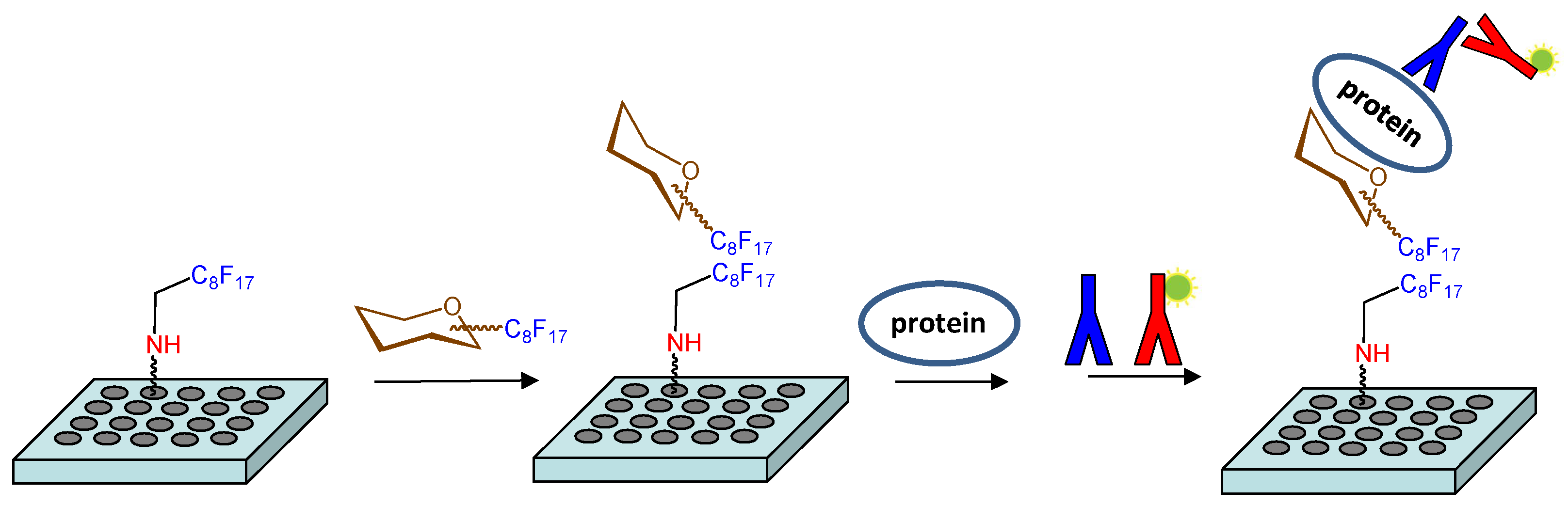
2. Results and Discussion
3. Materials and Methods
3.1. General Synthetic Procedures
3.2. General Procedure for F-SPE
3.3. Immobilization of Fluorous-Tagged Sugar on Microtiter Plates and Determination of Surface Dissociation Constants (KD, surf)
3.4. Procedure for Synthesis of Hexasaccharide 17
3.4.1. Synthesis of 4-Methoxyphenyl 2-deoxy-3-O-levulinoyl-2-phthalimido-β-d-glucopyranoside (5)
3.4.2. Synthesis of 4-Methoxyphenyl 4,6-O-di-tert-butylsilylidene-2-deoxy-3-O-levulinoyl-2-phthalimido-β-d-glucopyranoside (6)
3.4.3. Synthesis of 4,6-O-di-tert-Butylsilylidene-2-deoxy-3-O-levulinoyl-2-phthalimido-α,β-d-glucopyranose (7)
3.4.4. Synthesis of O-(4,6-O-di-tert-Butylsilylidene-2-deoxy-3-O-levulinoyl-2-phthalimido-α,β-d-glucopyranosyl) trichloroacetimidate (2)
3.4.5. Synthesis of 4-Methoxyphenyl O-(4,6-O-di-tert-butylsilylidene-2-deoxy-3-O-levulinoyl-2-phthalimido-β-d-glucopyranosyl)-(1→4)-O-(2-O-benzoyl-3-O-benzyl-6-O-pivaloyl-β-d-glucopyranosyl)-(1→3)-O-(4,6-O-di-tert-butylsilylidene-2-deoxy-2-phthalimido-β-d-glucopyranosyl)-(1→4)-O-(2-O-benzoyl-3-O-benzyl-6-O-pivaloyl-β-d-glucopyranosyl)-(1→3)-O-(4,6-O-di-tert-butylsilylidene-2-deoxy-2-phthalimido-β-d-glucopyranosyl)-(1→4)-2-O-benzoyl-3-O-benzyl-6-O-4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoroundecanoyl-β-d-glucopyranoside (16)
3.4.6. Synthesis of 4-Methoxyphenyl O-(2-deoxy-3-O-levulinoyl-2-phthalimido-4,6-di-O-sulfo-β-d-glucopyranosyl)-(1→4)-O-(2-O-benzoyl-3-O-benzyl-6-O-pivaloyl-β-d-glucopyranosyl)-(1→3)-O-(2-deoxy-2-phthalimido-4,6-di-O-sulfo-β-d-glucopyranosyl)-(1→4)-O-(2-O-benzoyl-3-O-benzyl-6-O-pivaloyl-β-d-glucopyranosyl)-(1→3)-O-(2-deoxy-2-phthalimido-4,6-di-O-sulfo-β-d-glucopyranosyl)-(1→4)-2-O-benzoyl-3-O-benzyl-6-O-4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoroundecanoyl-β-d-glucopyranoside (17)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Muramatsu, T. Midkine: A Promising Molecule for Drug Development to Treat Diseases of the Central Nervous System. Curr. Pharm. Des. 2011, 17, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Kadomatsu, K.; Kishida, S.; Tsubota, S. The heparin-binding growth factor midkine: The biological activities and candidate receptors. J. Biochem. 2013, 153, 511–521. [Google Scholar] [CrossRef]
- Deepa, S.S.; Umehara, Y.; Higashiyama, S.; Itoh, N.; Sugahara, K. Specific molecular interactions of oversulfated chondroitin sulfate E with various heparin-binding growth factors—Implications as a physiological binding partner in the brain and other tissues. J. Biol. Chem. 2002, 277, 43707–43716. [Google Scholar] [CrossRef]
- Solera, C.; Macchione, G.; Maza, S.; Kayser, M.M.; Corzana, F.; de Paz, J.L.; Nieto, P.M. Chondroitin Sulfate Tetrasaccharides: Synthesis, Three-Dimensional Structure and Interaction with Midkine. Chem. Eur. J. 2016, 22, 2356–2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gama, C.I.; Tully, S.E.; Sotogaku, N.; Clark, P.M.; Rawat, M.; Vaidehi, N.; Goddard, W.A.; Nishi, A.; Hsieh-Wilson, L.C. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat. Chem. Biol. 2006, 2, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Rogers, C.J.; Clark, P.M.; Tully, S.E.; Abrol, R.; Garcia, K.C.; Goddard, W.A., III; Hsieh-Wilson, L.C. Elucidating glycosaminoglycan-protein-protein interactions using carbohydrate microarray and computational approaches. Proc. Natl. Acad. Sci. USA 2011, 108, 9747–9752. [Google Scholar] [CrossRef]
- Liu, P.; Chen, L.; Toh, J.K.C.; Ang, Y.L.; Jee, J.-E.; Lim, J.; Lee, S.S.; Lee, S.-G. Tailored chondroitin sulfate glycomimetics via a tunable multivalent scaffold for potentiating NG/TrkA-induced neurogenesis. Chem. Sci. 2015, 6, 450–456. [Google Scholar] [CrossRef]
- Desai, U.R. The promise of sulfated synthetic small molecules as modulators of glycosaminoglycan function. Future Med. Chem. 2013, 5, 1363–1366. [Google Scholar] [CrossRef]
- Weiss, R.J.; Esko, J.D.; Tor, Y. Targeting heparin and heparan sulfate protein interactions. Org. Biomol. Chem. 2017, 15, 5656–5668. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Mancera, R.L. Heparin/heparan sulphate-based drugs. Drug Discovery Today 2010, 15, 1058–1069. [Google Scholar] [CrossRef]
- Dominguez-Rodriguez, P.; Reina, J.J.; Gil-Caballero, S.; Nieto, P.M.; de Paz, J.L.; Rojo, J. Glycodendrimers as Chondroitin Sulfate Mimetics: Synthesis and Binding to Growth Factor Midkine. Chem. Eur. J. 2017, 23, 11338–11345. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, W.; Xu, X.J.; Sun, H.F.; Zhao, J.H.; Meng, X.B.; Wu, M.Y.; Li, Z.J. Synthesis of Fucosylated Chondroitin Sulfate Glycoclusters: A Robust Route to New Anticoagulant Agents. Chem. Eur. J. 2018, 24, 1694–1700. [Google Scholar] [CrossRef]
- Loka, R.S.; Yu, F.; Sletten, E.T.; Nguyen, H.M. Design, synthesis, and evaluation of heparan sulfate mimicking glycopolymers for inhibiting heparanase activity. Chem. Commun. 2017, 53, 9163–9166. [Google Scholar] [CrossRef]
- Tyler, P.C.; Guimond, S.E.; Turnbull, J.E.; Zubkova, O.V. Single-Entity Heparan Sulfate Glycomimetic Clusters for Therapeutic Applications. Angew. Chem. Int. Ed. 2015, 54, 2718–2723. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.I.; Sheng, G.J.; Chang, S.-K.; Hsieh-Wilson, L.C. Tailored Glycopolymers as Anticoagulant Heparin Mimetics. Angew. Chem. Int. Ed. 2013, 52, 11796–11799. [Google Scholar] [CrossRef] [Green Version]
- Sheng, G.J.; Oh, Y.I.; Chang, S.-K.; Hsieh-Wilson, L.C. Tunable Heparan Sulfate Mimetics for Modulating Chemokine Activity. J. Am. Chem. Soc. 2013, 135, 10898–10901. [Google Scholar] [CrossRef] [PubMed]
- Kuhnast, B.; El Hadri, A.; Boisgard, R.; Hinnen, F.; Richard, S.; Caravano, A.; Nancy-Portebois, V.; Petitou, M.; Tavitian, B.; Dolle, F. Synthesis, radiolabeling with fluorine-18 and preliminary in vivo evaluation of a heparan sulphate mimetic as potent angiogenesis and heparanase inhibitor for cancer applications. Org. Biomol. Chem. 2016, 14, 1915–1920. [Google Scholar] [CrossRef]
- Lee, S.S.; Fyrner, T.; Chen, F.; Alvarez, Z.; Sleep, E.; Chun, D.S.; Weiner, J.A.; Cook, R.W.; Freshman, R.D.; Schallmo, M.S.; et al. Sulfated glycopeptide nanostructures for multipotent protein activation. Nat. Nanotechnol. 2017, 12, 821–829. [Google Scholar] [CrossRef]
- Johnstone, K.D.; Karoli, T.; Liu, L.; Dredge, K.; Copeman, E.; Li, C.P.; Davis, K.; Hammond, E.; Bytheway, I.; Kostewicz, E.; et al. Synthesis and Biological Evaluation of Polysulfated Oligosaccharide Glycosides as Inhibitors of Angiogenesis and Tumor Growth. J. Med. Chem. 2010, 53, 1686–1699. [Google Scholar] [CrossRef] [Green Version]
- Ferro, V.; Liu, L.; Johnstone, K.D.; Wimmer, N.; Karoli, T.; Handley, P.; Rowley, J.; Dredge, K.; Li, C.P.; Hammond, E.; et al. Discovery of PG545: A Highly Potent and Simultaneous Inhibitor of Angiogenesis, Tumor Growth, and Metastasis. J. Med. Chem. 2012, 55, 3804–3813. [Google Scholar] [CrossRef]
- Afosah, D.K.; Al-Horani, R.A.; Sankaranarayanan, N.V.; Desai, U.R. Potent, Selective, Allosteric Inhibition of Human Plasmin by Sulfated Non-Saccharide Glycosaminoglycan Mimetics. J. Med. Chem. 2017, 60, 641–657. [Google Scholar] [CrossRef] [PubMed]
- De Paz, J.L.; Nieto, P.M. Improvement on binding of chondroitin sulfate derivatives to midkine by increasing hydrophobicity. Org. Biomol. Chem. 2016, 14, 3506–3509. [Google Scholar] [CrossRef] [Green Version]
- Maza, S.; Gandia-Aguado, N.; de Paz, J.L.; Nieto, P.M. Fluorous-tag assisted synthesis of a glycosaminoglycan mimetic tetrasaccharide as a high-affinity FGF-2 and midkine ligand. Bioorg. Med. Chem. 2018, 26, 1076–1085. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Pedersen, C.M. Catalytic Glycosylations in Oligosaccharide Synthesis. Chem. Rev. 2018, 118, 8285–8358. [Google Scholar] [CrossRef]
- Zhu, X.M.; Schmidt, R.R. New Principles for Glycoside-Bond Formation. Angew. Chem. Int. Ed. 2009, 48, 1900–1934. [Google Scholar] [CrossRef] [PubMed]
- Jaipuri, F.A.; Pohl, N.L. Toward solution-phase automated iterative synthesis: Fluorous-tag assisted solution-phase synthesis of linear and branched mannose oligomers. Org. Biomol. Chem. 2008, 6, 2686–2691. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Curran, D.P. Synthetic applications of fluorous solid-phase extraction (F-SPE). Tetrahedron 2006, 62, 11837–11865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macchione, G.; de Paz, J.L.; Nieto, P.M. Synthesis of hyaluronic acid oligosaccharides and exploration of a fluorous-assisted approach. Carbohydr. Res. 2014, 394, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Bhaduri, S.; Pohl, N.L.B. Fluorous-Tag Assisted Syntheses of Sulfated Keratan Sulfate Oligosaccharide Fragments. Org. Lett. 2016, 18, 1414–1417. [Google Scholar] [CrossRef]
- Karst, N.; Jacquinet, J.C. Chemical synthesis of β-d-GlcpA(2SO4)-(1→3)-d-GalpNAc(6SO4), the disaccharide repeating unit of shark cartilage chondroitin sulfate D, and of its methyl β-d-glycoside derivative. J. Chem. Soc., Perkin Trans. 2000, 1, 2709–2717. [Google Scholar] [CrossRef]
- Dinkelaar, J.; Gold, H.; Overkleeft, H.S.; Codee, J.D.C.; van der Marel, G.A. Synthesis of Hyaluronic Acid Oligomers using Chemoselective and One-Pot Strategies. J. Org. Chem. 2009, 74, 4208–4216. [Google Scholar] [CrossRef]
- Mydock, L.K.; Demchenko, A.V. Mechanism of chemical O-glycosylation: From early studies to recent discoveries. Org. Biomol. Chem. 2010, 8, 497–510. [Google Scholar] [CrossRef]
- Yu, Y.; Kononov, A.; Delbianco, M.; Seeberger, P.H. A Capping Step During Automated Glycan Assembly Enables Access to Complex Glycans in High Yield. Chem. Eur. J. 2018, 24, 6075–6078. [Google Scholar] [CrossRef] [PubMed]
- Maza, S.; de Paz, J.L.; Nieto, P.M. Microwave-assisted sulfonation of heparin oligosaccharides. Tetrahedron Lett. 2011, 52, 441–443. [Google Scholar] [CrossRef]
- Maza, S.; Mar Kayser, M.; Macchione, G.; Lopez-Prados, J.; Angulo, J.; de Paz, J.L.; Nieto, P.M. Synthesis of chondroitin/dermatan sulfate-like oligosaccharides and evaluation of their protein affinity by fluorescence polarization. Org. Biomol. Chem. 2013, 11, 3510–3525. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.S.; Jaipuri, F.A.; Pohl, N.L. Fluorous-based carbohydrate microarrays. J. Am. Chem. Soc. 2005, 127, 13162–13163. [Google Scholar] [CrossRef] [PubMed]
- Mamidyala, S.K.; Ko, K.-S.; Jaipuri, F.A.; Park, G.; Pohl, N.L. Noncovalent fluorous interactions for the synthesis of carbohydrate microarrays. J. Fluorine Chem. 2006, 127, 571–579. [Google Scholar] [CrossRef]
- Chen, G.-S.; Pohl, N.L. Synthesis of fluorous tags for incorporation of reducing sugars into a quantitative microarray platform. Org. Lett. 2008, 10, 785–788. [Google Scholar] [CrossRef]
- Tang, S.-L.; Linz, L.B.; Bonning, B.C.; Pohl, N.L.B. Automated Solution-Phase Synthesis of Insect Glycans to Probe the Binding Affinity of Pea Enation Mosaic Virus. J. Org. Chem. 2015, 80, 10482–10489. [Google Scholar] [CrossRef]
- Maza, S.; Macchione, G.; Ojeda, R.; Lopez-Prados, J.; Angulo, J.; de Paz, J.L.; Nieto, P.M. Synthesis of amine-functionalized heparin oligosaccharides for the investigation of carbohydrate-protein interactions in microtiter plates. Org. Biomol. Chem. 2012, 10, 2146–2163. [Google Scholar] [CrossRef]
- Liang, P.H.; Wang, S.K.; Wong, C.H. Quantitative analysis of carbohydrate-protein interactions using glycan microarrays: Determination of surface and solution dissociation constants. J. Am. Chem. Soc. 2007, 129, 11177–11184. [Google Scholar] [CrossRef] [PubMed]
- Medve, L.; Achilli, S.; Serna, S.; Zuccotto, F.; Varga, N.; Thepaut, M.; Civera, M.; Vives, C.; Fieschi, F.; Reichardt, N.; Bernardi, A. On-Chip Screening of a Glycomimetic Library with C-Type Lectins Reveals Structural Features Responsible for Preferential Binding of Dectin-2 over DC-SIGN/R and Langerin. Chem. Eur. J. 2018, 24, 14448–14460. [Google Scholar] [CrossRef] [PubMed]
- Mena-Barragan, T.; de Paz, J.L.; Nieto, P.M. Unexpected loss of stereoselectivity in glycosylation reactions during the synthesis of chondroitin sulfate oligosaccharides. Beilstein J. Org. Chem. 2019, 15, 137–144. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound 17 is available from the authors. |

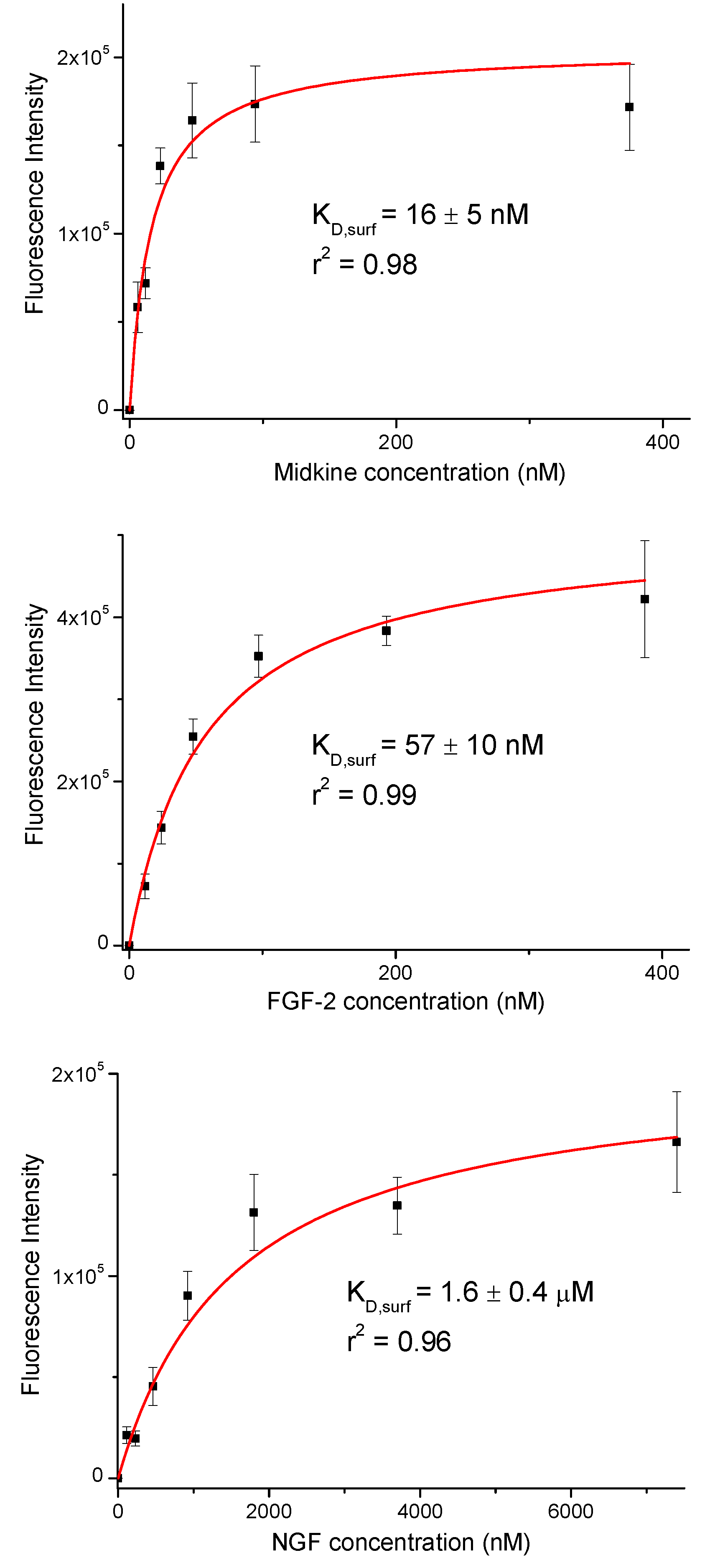
| Midkine | FGF-2 | NGF | |
|---|---|---|---|
| IC50 (nM) | 450 ± 30 | 6300 ± 1600 | not determined |
| KD,surf (nM) | 16 ± 5 | 57 ± 10 | 1600 ± 400 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maza, S.; de Paz, J.L.; Nieto, P.M. Synthesis of a Fluorous-Tagged Hexasaccharide and Interaction with Growth Factors Using Sugar-Coated Microplates. Molecules 2019, 24, 1591. https://doi.org/10.3390/molecules24081591
Maza S, de Paz JL, Nieto PM. Synthesis of a Fluorous-Tagged Hexasaccharide and Interaction with Growth Factors Using Sugar-Coated Microplates. Molecules. 2019; 24(8):1591. https://doi.org/10.3390/molecules24081591
Chicago/Turabian StyleMaza, Susana, José L. de Paz, and Pedro M. Nieto. 2019. "Synthesis of a Fluorous-Tagged Hexasaccharide and Interaction with Growth Factors Using Sugar-Coated Microplates" Molecules 24, no. 8: 1591. https://doi.org/10.3390/molecules24081591