Effects of Temperature and Host Concentration on the Supramolecular Enantiodifferentiating [4 + 4] Photodimerization of 2-Anthracenecarboxylate through Triplet-Triplet Annihilation Catalyzed by Pt-Modified Cyclodextrins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enantiodifferentiating Photocyclodimerization of 2-Anthracenecarboxylate (AC) Mediated by Pt-2
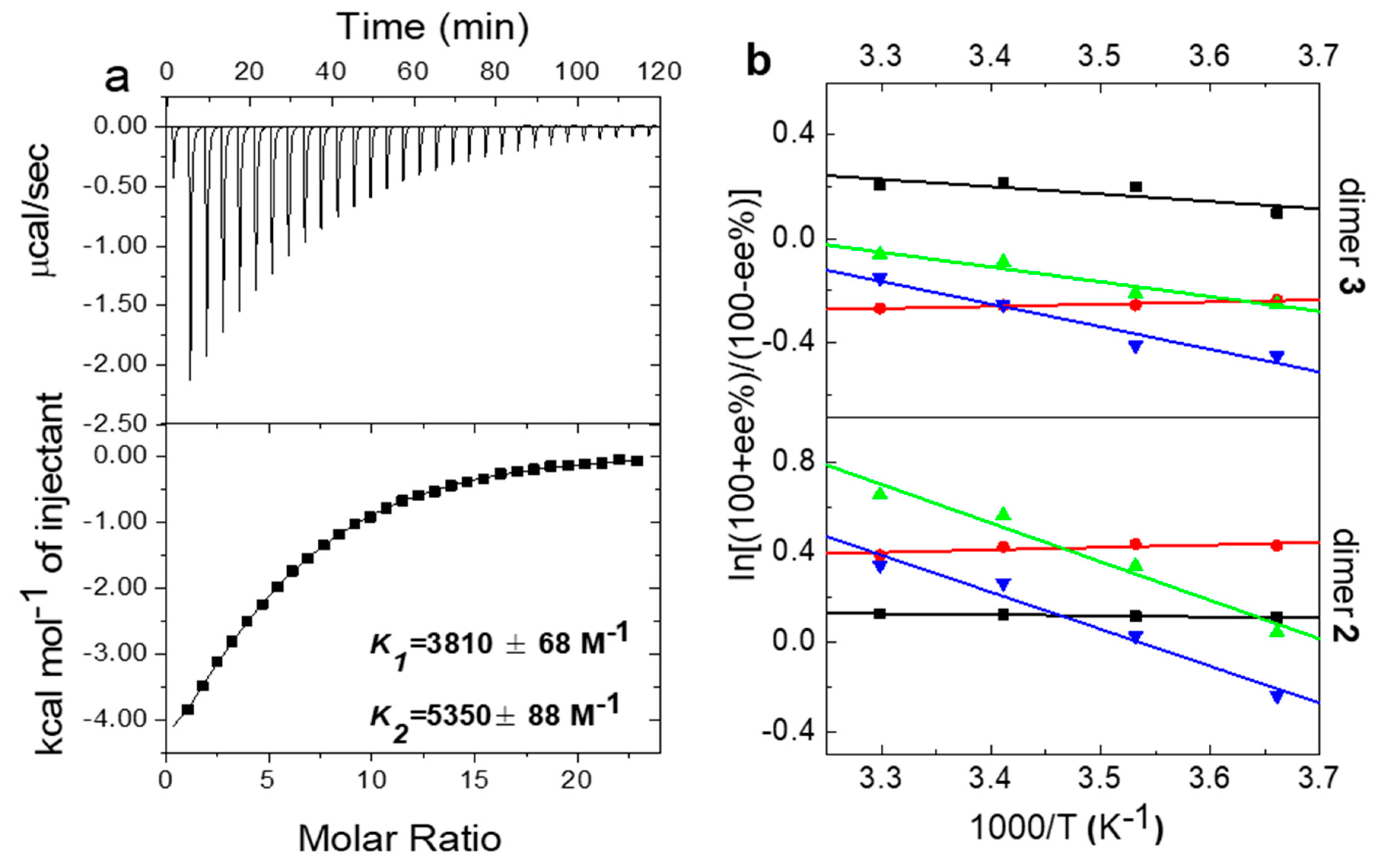
2.1.1. Characterization of Pt-2
2.1.2. Effects of Pt-2/AC Ratio
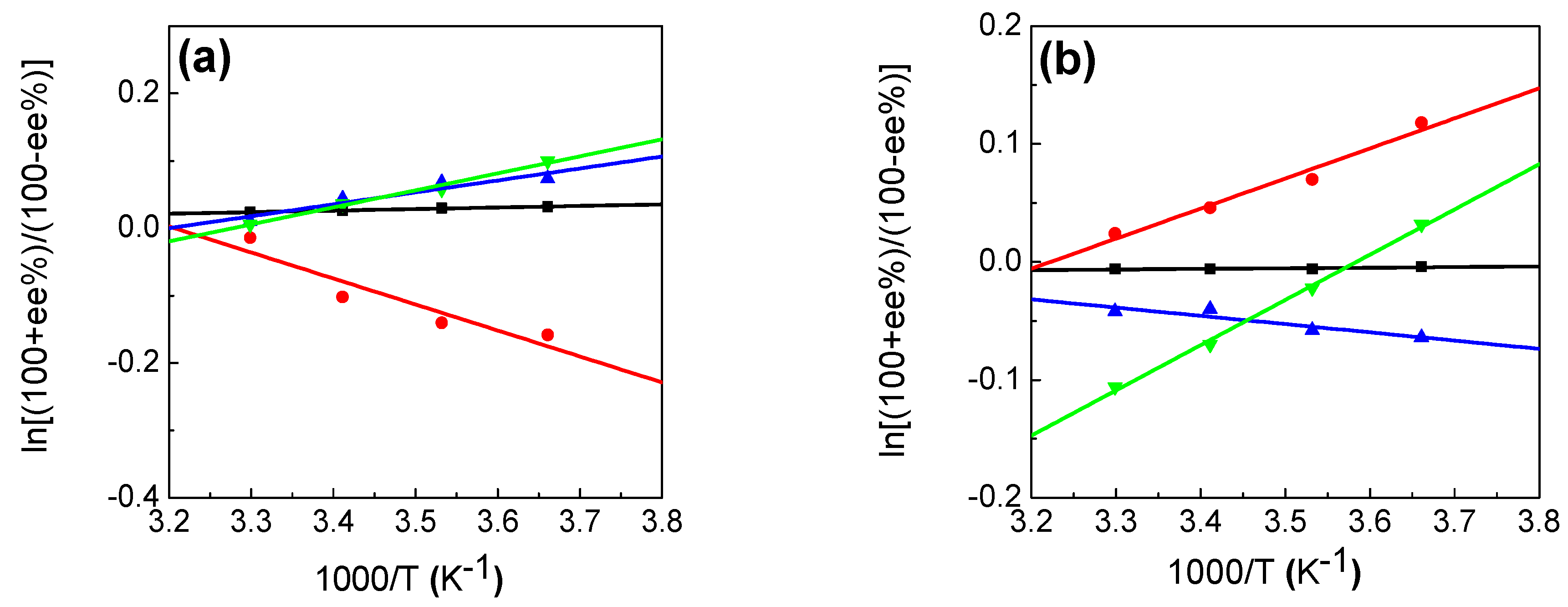
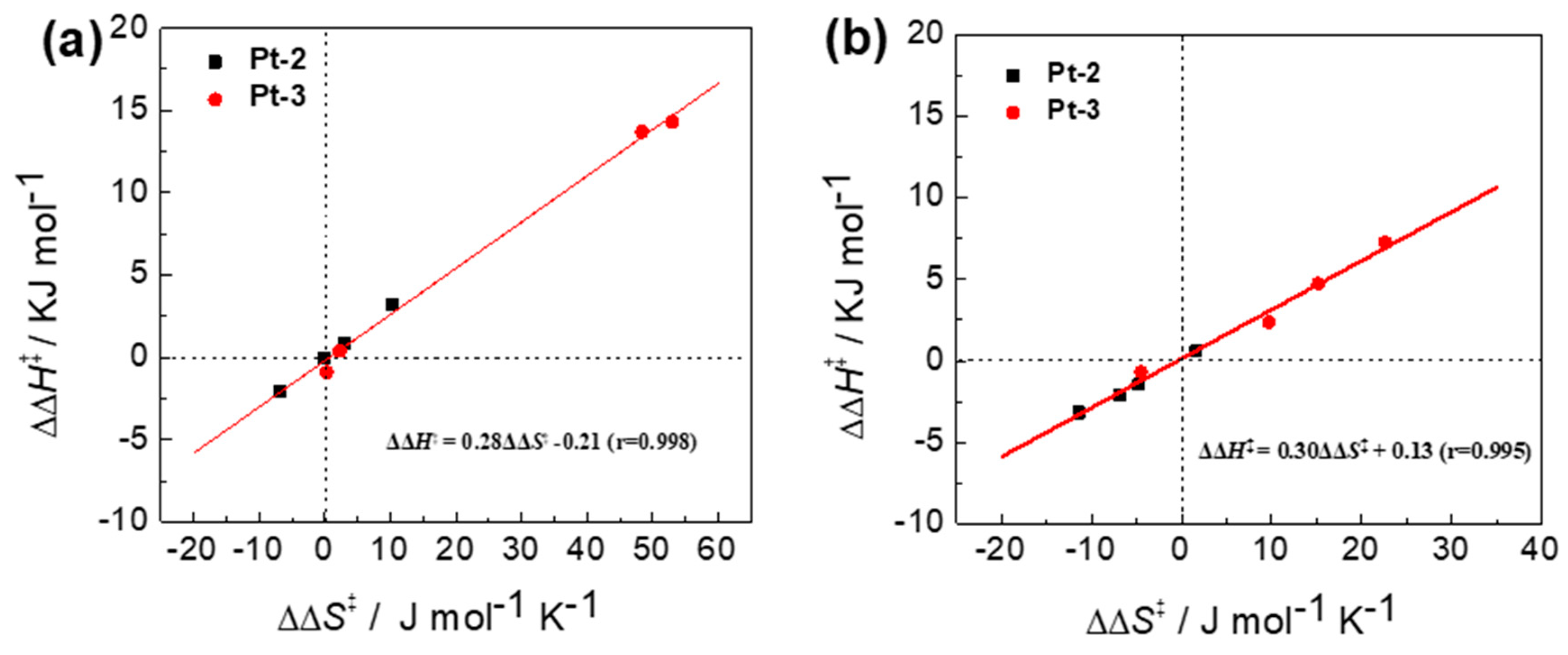
2.1.3. Effects of Temperature
2.2. Temperature Dependence of the Photocyclodimerization of AC Mediated by Pt-3
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Photoreaction and Product Analysis
3.3. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huo, H.; Shen, X.; Wang, C.; Zhang, L.; Rose, P.; Chen, L.A.; Harms, K.; Marsch, M.; Hilt, G.; Meggers, E. Asymmetric photoredox transition-metal catalysis activated by visible light. Nature 2014, 515, 100–103. [Google Scholar] [CrossRef]
- Nakamura, A.; Inoue, Y. Electrostatic manipulation of enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate within γ-cyclodextrin cavity through chemical modification. inverted product distribution and enhanced enantioselectivity. J. Am. Chem. Soc. 2005, 127, 5338–5339. [Google Scholar] [CrossRef]
- Yang, C.; Nakamura, A.; Fukuhara, G.; Origane, Y.; Mori, T.; Wada, T.; Inoue, Y. Pressure and temperature-controlled enantiodifferentiating [4 + 4] photocyclodimerization of 2-anthracenecarboxylate mediated by secondary face- and skeleton-modified γ-cyclodextrins. J. Org. Chem. 2006, 71, 3126–3136. [Google Scholar] [CrossRef]
- Yang, C.; Nakamura, A.; Wada, T.; Inoue, Y. Enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid mediated by γ-cyclodextrins with a flexible or rigid cap. Org. Lett. 2006, 8, 3005–3008. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mori, T.; Inoue, Y. Supramolecular enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate mediated by capped γ-cyclodextrins: Critical control of enantioselectivity by cap rigidity. J. Org. Chem. 2008, 73, 5786–5794. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Mori, T.; Origane, Y.; Ho Ko, Y.; Selvapalam, N.; Kim, K.; Inoue, Y. Highly stereoselective photocyclodimerization of γ-cyclodextrin-appended anthracene mediated by γ-cyclodextrin and cucurbit[8]uril: A dramatic steric effect operating outside the binding site. J. Am. Chem. Soc. 2008, 130, 8574–8575. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, C.; Fukuhara, G.; Mori, T.; Liu, Y.; Inoue, Y. Supramolecular FRET photocyclodimerization of anthracenecarboxylate with naphthalene-capped γ-cyclodextrin. Beilstein J. Org. Chem. 2011, 7, 290–297. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yang, C.; Ke, C.; Fukuhara, G.; Mori, T.; Liu, Y.; Inoue, Y. Wavelength-controlled supramolecular photocyclodimerization of anthracenecarboxylate mediated by γ-cyclodextrins. Chem. Commun. 2011, 47, 6849–6851. [Google Scholar] [CrossRef]
- Yang, C.; Ke, C.; Liang, W.; Fukuhara, G.; Mori, T.; Liu, Y.; Inoue, Y. Dual supramolecular photochirogenesis: Ultimate stereocontrol of photocyclodimerization by a chiral scaffold and confining host. J. Am. Chem. Soc. 2011, 133, 13786–13789. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yan, Z.; Ji, J.; Wu, W.; Yang, C.; Nishijima, M.; Fukuhara, G.; Mori, T.; Inoue, Y. Ammonia-driven chirality inversion and enhancement in enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate mediated by diguanidino-γ-cyclodextrin. J. Am. Chem. Soc. 2014, 136, 6916–6919. [Google Scholar] [CrossRef]
- Yi, J.; Liang, W.; Wei, X.; Yao, J.; Yan, Z.; Su, D.; Zhong, Z.; Gao, G.; Wu, W.; Yang, C. Switched enantioselectivity by solvent components and temperature in photocyclodimerization of 2-anthracenecarboxylate with 6 A,6 X-diguanidio-γ-cyclodextrins. Chin. Chem. Lett. 2018, 29, 87–90. [Google Scholar] [CrossRef]
- Yang, C.; Ke, C.; Kahee, F.; Yuan, D.; Mori, T.; Inoue, Y. pH-Controlled supramolecular enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate with capped γ-cyclodextrins. Aust. J. Chem. 2008, 61, 565–568. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Q.; Yamauchi, M.; Yao, J.; Zhou, D.; Nishijima, M.; Fukuhara, G.; Mori, T.; Liu, Y.; Inoue, Y. Manipulating γ-cyclodextrin-mediated photocyclodimerization of anthracenecarboxylate by wavelength, temperature, solvent and host. Photochem. Photobiol. Sci. 2014, 13, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jiang, L.; Liang, W.; Gui, J.; Xu, D.; Wu, W.; Nakai, Y.; Nishijima, M.; Fukuhara, G.; Mori, T.; et al. Inherently chiral azonia[6]helicene-modified β-cyclodextrin: Synthesis, characterization, and chirality sensing of underivatized amino acids in water. J. Org. Chem. 2016, 81, 3430–3434. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, Y.; Liang, W.; Wu, W.; Huang, Q.; Yu, X.; Xu, W.; Zhou, D.; Yang, C. Temperature-driven braking of γ-cyclodextrin-curcubit[6]uril-cowheeled[4]rotaxanes. Chin. Chem. Lett. 2018. [Google Scholar] [CrossRef]
- Kawanami, Y.; Umehara, H.; Mizoguchi, J.; Nishijima, M.; Fukuhara, G.; Yang, C.; Mori, T.; Inoue, Y. Cross- versus homo-photocyclodimerization of anthracene and 2-anthracenecarboxylic acid mediated by a chiral hydrogen-bonding template. Factors controlling the cross-/homo-selectivity and enantioselectivity. J. Org. Chem. 2013, 78, 3073–3085. [Google Scholar] [CrossRef]
- Kawanami, Y.; Pace, T.C.; Mizoguchi, J.; Yanagi, T.; Nishijima, M.; Mori, T.; Wada, T.; Bohne, C.; Inoue, Y. Supramolecular complexation and enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid with 4-aminoprolinol derivatives as chiral hydrogen-bonding templates. J. Org. Chem. 2009, 74, 7908–7921. [Google Scholar] [CrossRef]
- Kawanami, Y.; Katsumata, S.Y.; Nishijima, M.; Fukuhara, G.; Asano, K.; Suzuki, T.; Yang, C.; Nakamura, A.; Mori, T.; Inoue, Y. Supramolecular photochirogenesis with a higher-order complex: Highly accelerated exclusively head-to-head photocyclodimerization of 2-anthracenecarboxylic acid via 2:2 complexation with prolinol. J. Am. Chem. Soc. 2016, 138, 12187–12201. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.; Westkamper, F.; Grimme, S.; Bach, T. Catalytic enantioselective reactions driven by photoinduced electron transfer. Nature 2005, 436, 1139–1140. [Google Scholar] [CrossRef]
- Gui, J.; Yan, Z.; Peng, Y.; Yi, J.; Zhou, D.; Su, D.; Zhong, Z.; Gao, G.; Wu, W.; Yang, C. Enhanced head-to-head photodimers in the photocyclodimerization of anthracenecarboxylic acid with a cationic pillar[6]arene. Chin. Chem. Lett. 2016, 27, 1017–1021. [Google Scholar] [CrossRef]
- Alagesan, M.; Kanagaraj, K.; Wan, S.; Sun, H.; Su, D.; Zhong, Z.; Zhou, D.; Wu, W.; Gao, G.; Zhang, H.; et al. Enantiodifferentiating [4 + 4] photocyclodimerization of 2-anthracenecarboxylate mediated by a self-assembled iron tetrahedral coordination cage. J. Photochem. Photobiol. A: Chem. 2016, 331, 95–101. [Google Scholar] [CrossRef]
- Wada, T.; Nishijima, M.; Fujisawa, T.; Sugahara, N.; Mori, T.; Nakamura, A.; Inoue, Y. Bovine serum albumin-mediated enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate. J. Am. Chem. Soc. 2003, 125, 7492–7493. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, M.; Pace, T.C.; Nakamura, A.; Mori, T.; Wada, T.; Bohne, C.; Inoue, Y. Supramolecular photochirogenesis with biomolecules. Mechanistic studies on the enantiodifferentiation for the photocyclodimerization of 2-anthracenecarboxylate mediated by bovine serum albumin. J. Am. Chem. Soc. 2007, 129, 3478–3479. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Inoue, Y. Supramolecular photochirogenesis. Chem. Soc. Rev. 2014, 43, 4123–4143. [Google Scholar] [CrossRef] [PubMed]
- Richard, B.; Dominik, L.; Maturi, M.M.; Thorsten, B. Enantioselective catalysis of photochemical reactions. Angew. Chem. Int. Ed. 2015, 54, 3872–3890. [Google Scholar]
- Yan, Z.; Wu, W.; Yang, C.; Inoue, Y. Catalytic supramolecular photochirogenesis. Supramol. Catal. 2015, 2, 9–24. [Google Scholar] [CrossRef]
- Ke, C.; Yang, C.; Mori, T.; Wada, T.; Liu, Y.; Inoue, Y. Catalytic enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylic acid mediated by a non-sensitizing chiral metallosupramolecular host. Angew. Chem. Int. Ed. 2009, 48, 6675–6677. [Google Scholar] [CrossRef] [PubMed]
- Brimioulle, R.; Bach, T. Enantioselective Lewis acid catalysis of intramolecular enone [2+2] photocycloaddition reactions. Science 2013, 342, 840–843. [Google Scholar] [CrossRef]
- Rao, M.; Kanagaraj, K.; Fan, C.; Ji, J.; Xiao, C.; Wei, X.; Wu, W.; Yang, C. Photocatalytic supramolecular enantiodifferentiating dimerization of 2-anthracenecarboxylic acid through triplet-triplet annihilation. Org. Lett. 2018, 20, 1680–1683. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Guo, H. Triplet–triplet annihilation based upconversion: From triplet sensitizers and triplet acceptors to upconversion quantum yields. RSC Adv. 2011, 1, 937–950. [Google Scholar] [CrossRef]
- Singhrachford, N.T.; Castellano, F.N. Photon upconversion based on sensitized triplet–triplet annihilation. Coordin. Chem. Rev. 3. Photochem. Photobid. A: Chem. 2010, 254, 2560–2573. [Google Scholar] [CrossRef]
- Islangulov, R.R.; Lott, R.; Weder, R.; Castellano, F.N. Noncoherent low-power upconversion in solid polymer films. J. Am. Chem. Soc. 2007, 129, 12652–12653. [Google Scholar] [CrossRef] [PubMed]
- Trupke, T.; Shalav, A.; Richards, B.S.; Würfel, P.; Green, M.A. Efficiency enhancement of solar cells by luminescent up-conversion of sunlight. Sol. Ene. Mater. Solar Cells 2006, 90, 3327–3338. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H. Encapsulated triplet-triplet annihilation-based upconversion in the aqueous phase for sub-band-gap semiconductor photocatalysis. J. Am. Chem. Soc. 2012, 134, 17478–17481. [Google Scholar] [CrossRef] [PubMed]
- Mattiello, S.; Monguzzi, A.; Pedrini, J.; Sassi, M.; Villa, C.; Torrente, Y.; Marotta, R.; Meinardi, F.; Beverina, L. Self-assembled dual dye-doped nanosized micelles for high-contrast up-conversion bioimaging. Adv. Func. Mater. 2016, 26, 8447–8454. [Google Scholar] [CrossRef]
- Monguzzi, A.; Tubino, R.; Meinardi, F. Upconversion-induced delayed fluorescence in multicomponent organic systems: Role of Dexter energy transfer. Physical Review B 2008, 77, 155122. [Google Scholar] [CrossRef]
- Fan, C.; Wu, W.; Chruma, J.J.; Zhao, J.; Yang, C. Enhanced triplet-triplet energy transfer and upconversion fluorescence through host-guest complexation. J. Am. Chem. Soc. 2016, 138, 15405–15412. [Google Scholar] [CrossRef] [PubMed]
- Griesbeck, A.; Meierhenrich, U.J. Asymmetric photochemistry and photochirogenesis. Angew. Chem. Int. Ed. 2002, 17, 3147–3154. [Google Scholar] [CrossRef]
- Inoue, Y.; Ramamurthy, V. Chiral Photochemistry; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Ramamurthy, V.; Inoue, Y. Supramolecular Photochemistry; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Vallavoju, N.; Sivaguru, J. Supramolecular photocatalysis: Combining confinement and non-covalent interactions to control light initiated reactions. Chem. Soc. Rev. 2014, 43, 4084–4101. [Google Scholar] [CrossRef]
- Wakai, A.; Fukasawa, H.; Yang, C.; Mori, T.; Inoue, Y. Theoretical and experimental investigations of circular dichroism and absolute configuration determination of chiral anthracene photodimers. J. Am. Chem. Soc. 2012, 134, 4990–4997. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Inoue, Y. Supramolecular catalysis of the enantiodifferentiating [4 + 4] photocyclodimerization of 2-anthracenecarboxylate by γ-cyclodextrin. J. Am. Chem. Soc. 2003, 125, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yu, X.; Zhang, Y.; Wenting, L.; Ji, J.; Yao, J.; Rao, M.; Wu, W.; Yang, C. Enhanced irregular photodimers and switched enantioselectivity by solvent and temperature in the photocyclodimerization of 2-anthracenecarboxylate with modified β-cyclodextrins. J. Photochem. Photobio. A: Chem. 2019, 371, 374–381. [Google Scholar] [CrossRef]
- Wei, X.; Wu, W.; Matsushita, R.; Yan, Z.; Zhou, D.; Chruma, J.J.; Nishijima, M.; Fukuhara, G.; Mori, T.; Inoue, Y.; et al. Supramolecular photochirogenesis driven by higher-order complexation: Enantiodifferentiating photocyclodimerization of 2-anthracenecarboxylate to slipped cyclodimers via a 2:2 complex with β-cyclodextrin. J. Am. Chem. Soc. 2018, 140, 3959–3974. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Chen, J.W.; Liu, Y.; Xia, W.; Wang, L. The use of calixarenes in asymmetric catalysis. Curr. Org. Chem. 2011, 15, 39–61. [Google Scholar] [CrossRef]
- Xu, W.; Liang, W.; Wu, W.; Fan, C.; Rao, M.; Su, D.; Zhong, Z.; Yang, C. Supramolecular Assembly-Improved Triplet-Triplet Annihilation Upconversion in Aqueous Solution. Chem. Eur. J. 2018, 24, 16677–16685. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, T.; Kokubu, T. Acceleration of the photodimerization of water-soluble anthracenes included by β- and γ-cyclodextrins. J. Incl. Phenom. 1984, 2, 815–822. [Google Scholar] [CrossRef]
- Wu, W.; Sun, J.; Ji, S.; Wu, W.; Zhao, J.; Guo, H. Tuning the emissive triplet excited states of platinum(II) Schiff base complexes with pyrene, and application for luminescent oxygen sensing and triplet-triplet-annihilation based upconversions. Dalton Trans 2011, 40, 11550–11561. [Google Scholar] [CrossRef] [PubMed]
- Kajtár, M.; Horváth-Toró, C.; Kuthi, E.; Szejtli, J.A. A simple rule for predicting circular-dichroism induced in aromatic guests by cyclodextrin hosts in inclusion complexes. Chim.Acad. Sci. Hung 1982, 110, 327–355. [Google Scholar]
Sample Availability: Samples of the compounds Pt-1, Pt-2, Pt-3 are available from the authors. |


| Sen. | λ/nm | [Sen.]/μM | Conv% b | Relative Yield (%) b | ee% b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 2 | 3 | 5 | 6 | ||||
| β-CD | 365 | 100 | 37 | 42 | 31 | 15 | 9 | 2 | 1 | −1 | 0 | 46 | 31 [49] |
| γ-CD | 365 | 10 | 97 | 40 | 37 | 14 | 9 | - c | - c | 8 | 4 | - c | - c |
| Pt-1 | 532 | 50 | 53 | 37 | 37 | 16 | 10 | - c | - c | - c | - c | - c | - c |
| Pt-2 | 365 | 1 | 90 | 39 | 36 | 15 | 9 | - c | - c | 0.0 | 0.3 | - c | - c |
| 10 | 93 | 42 | 36 | 14 | 8 | - c | - c | 1.3 | 0.3 | - c | - c | ||
| 50 | 98 | 39 | 36 | 15 | 10 | - c | - c | −0.1 | 0.7 | - c | - c | ||
| 100 | 97 | 38 | 36 | 16 | 10 | - c | - c | −5.1 | 2.3 | - c | - c | ||
| 532 | 1 | 1 | 40 | 38 | 14 | 9 | - c | - c | 1.6 | −0.2 | - c | - c | |
| 10 | 75 | 40 | 38 | 14 | 8 | - c | - c | 2.2 | −2.0 | - c | - c | ||
| 50 | 80 | 40 | 39 | 13 | 8 | - c | - c | 2.0 | −2.8 | - c | - c | ||
| 100 | 97 | 41 | 40 | 12 | 7 | - c | - c | 1.8 | −3.5 | - c | - c | ||
| λ/nm | T/°C | Conv% b | [Sen.] | Relative Yield (%) b | ee% b | HH/HT | Anti/syn | Anti/syn | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| /mM | 1 | 2 | 3 | 4 | 2 | 3 | 1/2 | 3/4 | ||||
| 365 | 0 | 98 | 0.01 | 39 | 38 | 14 | 9 | 1.6 | 3.4 | 0.3 | 1.0 | 1.5 |
| 10 | 96 | 0.01 | 41 | 40 | 12 | 7 | 1.5 | 3.2 | 0.2 | 1.0 | 1.6 | |
| 20 | 93 | 0.01 | 42 | 37 | 13 | 8 | 1.3 | 2.9 | 0.3 | 1.1 | 1.7 | |
| 30 | 71 | 0.01 | 44 | 36 | 12 | 8 | 1.2 | 2.7 | 0.3 | 1.2 | 1.5 | |
| 0 | 80 | 0.1 | 37 | 40 | 13 | 10 | −7.9 | 5.9 | 0.3 | 0.9 | 1.4 | |
| 10 | 96 | 0.1 | 37 | 37 | 15 | 10 | −7.0 | 3.5 | 0.3 | 1.0 | 1.5 | |
| 20 | 97 | 0.1 | 38 | 36 | 16 | 10 | −5.1 | 2.3 | 0.4 | 1.0 | 1.5 | |
| 30 | 96 | 0.1 | 37 | 34 | 18 | 11 | −0.7 | 1.2 | 0.4 | 1.1 | 1.6 | |
| 532 | 0 | 53 | 0.01 | 43 | 38 | 12 | 7 | 3.7 | −3.2 | 0.2 | 1.1 | 1.8 |
| 10 | 54 | 0.01 | 42 | 39 | 12 | 7 | 3.4 | −2.9 | 0.2 | 1.1 | 1.7 | |
| 20 | 75 | 0.01 | 40 | 38 | 14 | 8 | 2.2 | −2.0 | 0.3 | 1.1 | 1.8 | |
| 30 | 76 | 0.01 | 42 | 37 | 14 | 8 | 0.5 | −2.1 | 0.3 | 1.1 | 1.9 | |
| 0 | 75 | 0.1 | 42 | 37 | 12 | 9 | 5.0 | 1.6 | 0.3 | 1.1 | 1.4 | |
| 10 | 89 | 0.1 | 41 | 37 | 12 | 9 | 2.9 | −1.1 | 0.3 | 1.1 | 1.4 | |
| 20 | 97 | 0.1 | 41 | 40 | 12 | 7 | 1.8 | −3.5 | 0.2 | 1.0 | 1.6 | |
| 30 | 95 | 0.1 | 45 | 40 | 9 | 5 | 0.3 | −5.3 | 0.2 | 1.1 | 1.8 | |
| [Sen.]/mM | λ/nm | Dimer 2 | Dimer 3 | ||
|---|---|---|---|---|---|
| ΔΔH‡ (KJ mol−1) | ΔΔS‡ (J mol−1 K−1) | ΔΔH‡ (KJ mol−1) | ΔΔS‡ (J mol−1 K−1) | ||
| 0.01 | 365 | −0.2 | −0.4 | −0.3 | −0.6 |
| 532 | −1.5 | −4.7 | 0.6 | 1.6 | |
| 0.1 | 365 | 3.2 | 10.3 | −2.1 | −6.8 |
| 532 | −2.1 | −6.9 | −3.2 | −11.4 | |
| λ/nm | T/°C | Conv% b | [sen.] /mM | Relative Yield (%) b | ee% b | HH/HT | Anti/syn 1/2 | Anti/syn 3/4 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 2 | 3 | |||||||
| 365 | 0 | 74 | 0.01 | 37 | 39 | 13 | 11 | 5.5 | 4.9 | 0.3 | 0.9 | 1.2 |
| 10 | 90 | 0.01 | 36 | 38 | 14 | 11 | 5.7 | 9.9 | 0.3 | 0.9 | 1.2 | |
| 20 | 92 | 0.01 | 37 | 38 | 14 | 11 | 6.0 | 10.7 | 0.3 | 1.0 | 1.3 | |
| 30 | 95 | 0.01 | 37 | 37 | 15 | 11 | 6.3 | 10.2 | 0.3 | 1.0 | 1.4 | |
| 0 | 85 | 1 | 25 | 24 | 26 | 26 | 21.1 | −11.8 | 1.1 | 1.1 | 1.0 | |
| 10 | 88 | 1 | 28 | 28 | 23 | 22 | 21.4 | −12.7 | 0.8 | 1.0 | 1.1 | |
| 20 | 81 | 1 | 28 | 29 | 23 | 21 | 20.8 | −12.8 | 0.8 | 1.0 | 1.1 | |
| 30 | 87 | 1 | 30 | 30 | 21 | 18 | 19.1 | −13.4 | 0.7 | 1.0 | 1.2 | |
| 532 | 0 | 63 | 0.01 | 38 | 54 | 5 | 3 | 2.1 | −12.4 | 0.1 | 0.7 | 1.9 |
| 10 | 81 | 0.01 | 37 | 56 | 5 | 2 | 16.7 | −10.5 | 0.1 | 0.8 | 2.3 | |
| 20 | 90 | 0.01 | 34 | 56 | 7 | 4 | 27.4 | −4.5 | 0.1 | 0.6 | 1.9 | |
| 30 | 95 | 0.01 | 35 | 59 | 4 | 2 | 31.6 | −3.0 | 0.1 | 0.6 | 1.8 | |
| 0 | 93 | 1 | 39 | 44 | 11 | 6 | −11.9 | −22.3 | 0.2 | 0.9 | 1.7 | |
| 10 | 93 | 1 | 36 | 47 | 11 | 6 | 1.3 | −20.3 | 0.2 | 0.8 | 2.0 | |
| 20 | 96 | 1 | 33 | 48 | 13 | 6 | 13.0 | −12.7 | 0.2 | 0.7 | 2.0 | |
| 30 | 99 | 1 | 32 | 47 | 14 | 7 | 16.9 | −7.6 | 0.3 | 0.7 | 2.0 | |
| Host | [Sen.]/mM | λ/nm | Dimer 2 | Dimer 3 | ||
|---|---|---|---|---|---|---|
| ΔΔH‡ (KJ mol−1) | ΔΔS‡ (J mol−1 K−1) | ΔΔH‡ (KJ mol−1) | ΔΔS‡ (J mol−1 K−1) | |||
| Pt-3 | 0.01 | 365 | 0.4 | 2.3 | 2.4 | 9.7 |
| 532 | 14.3 | 53.0 | 4.7 | 15.2 | ||
| 1 | 365 | −0.9 | 0.3 | −0.7 | −4.5 | |
| 532 | 13.7 | 48.4 | 7.3 | 22.6 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, M.; Wu, W.; Yang, C. Effects of Temperature and Host Concentration on the Supramolecular Enantiodifferentiating [4 + 4] Photodimerization of 2-Anthracenecarboxylate through Triplet-Triplet Annihilation Catalyzed by Pt-Modified Cyclodextrins. Molecules 2019, 24, 1502. https://doi.org/10.3390/molecules24081502
Rao M, Wu W, Yang C. Effects of Temperature and Host Concentration on the Supramolecular Enantiodifferentiating [4 + 4] Photodimerization of 2-Anthracenecarboxylate through Triplet-Triplet Annihilation Catalyzed by Pt-Modified Cyclodextrins. Molecules. 2019; 24(8):1502. https://doi.org/10.3390/molecules24081502
Chicago/Turabian StyleRao, Ming, Wanhua Wu, and Cheng Yang. 2019. "Effects of Temperature and Host Concentration on the Supramolecular Enantiodifferentiating [4 + 4] Photodimerization of 2-Anthracenecarboxylate through Triplet-Triplet Annihilation Catalyzed by Pt-Modified Cyclodextrins" Molecules 24, no. 8: 1502. https://doi.org/10.3390/molecules24081502






