Self-Assembly Properties of Cationic Gemini Surfactants with Biodegradable Groups in the Spacer
Abstract
:1. Introduction
2. Results and Discussion
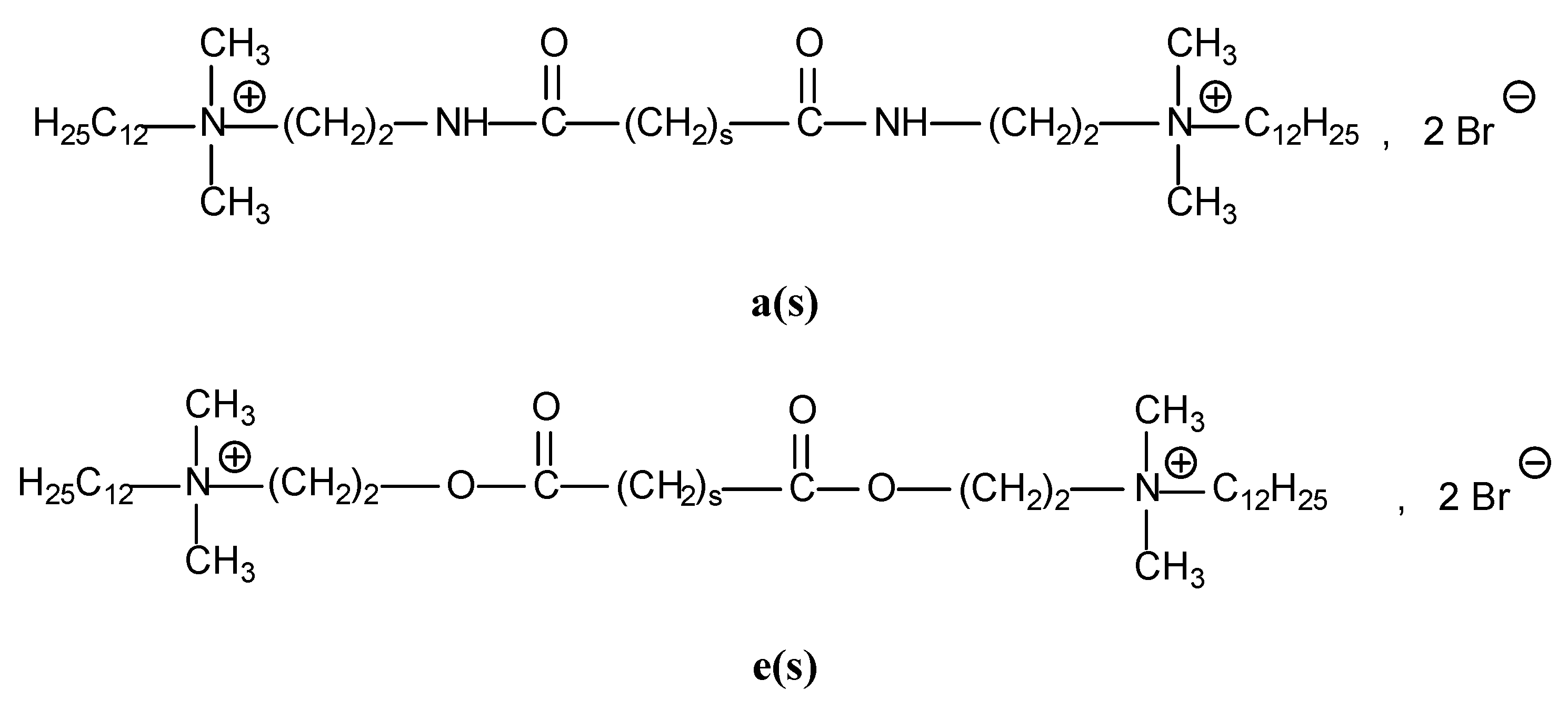
2.1. Diamide and Diester Gemini Surfactants
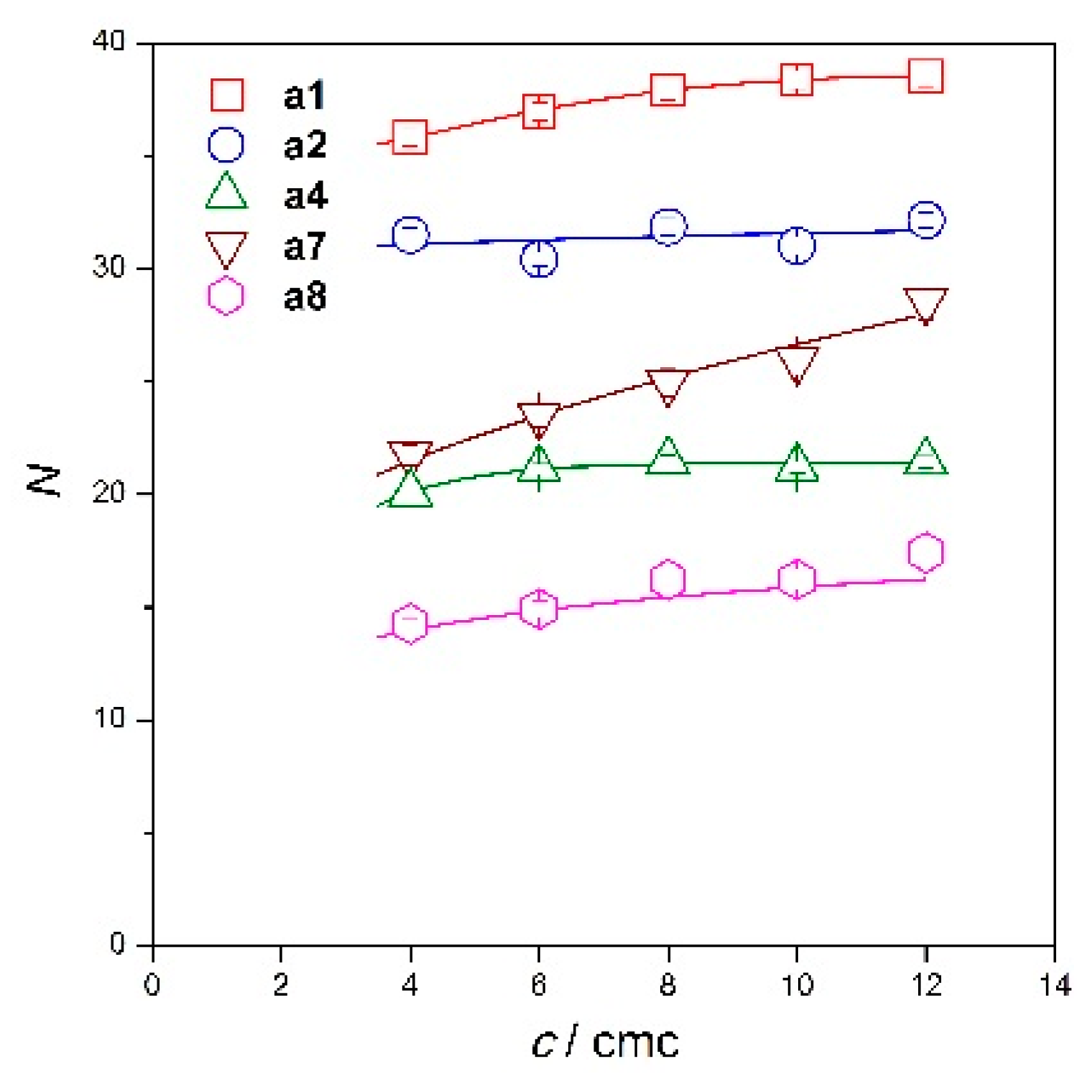
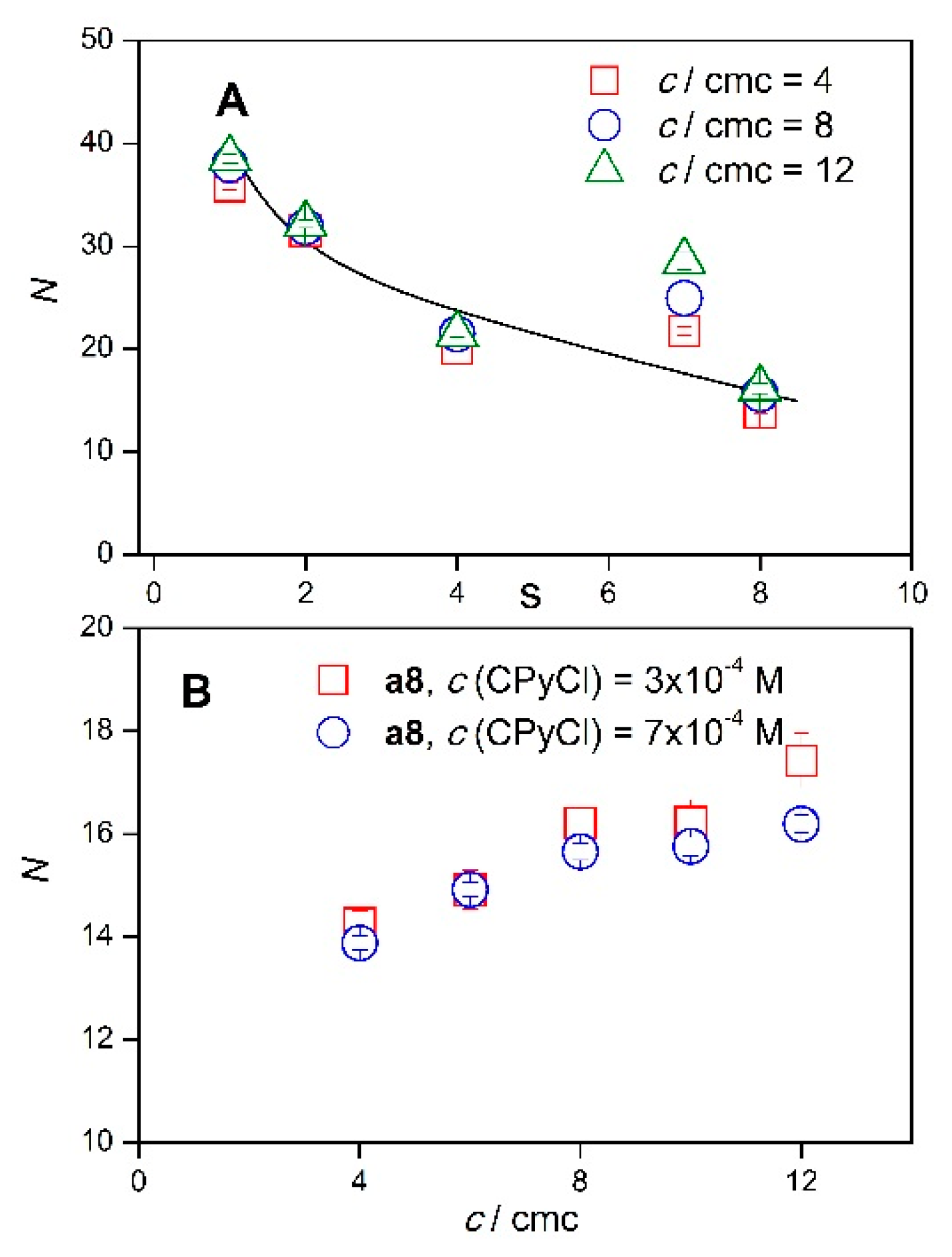
2.2. Micelle Aggregation Number of Diamide Gemini Surfactants
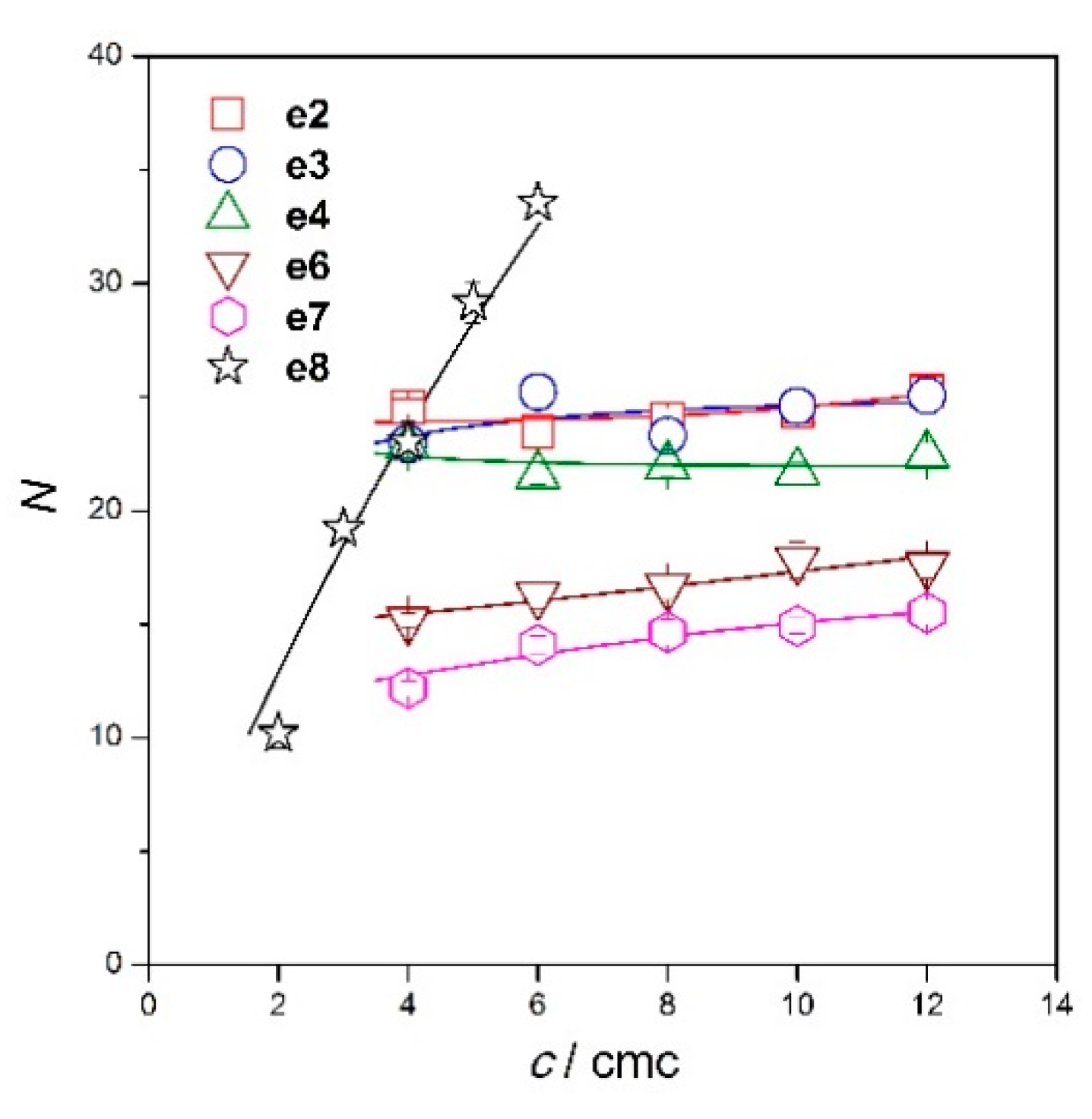
2.3. Micelle Aggregation Number of Diester Gemini Surfactants
2.4. Micelle Size, Zeta Potential Determination
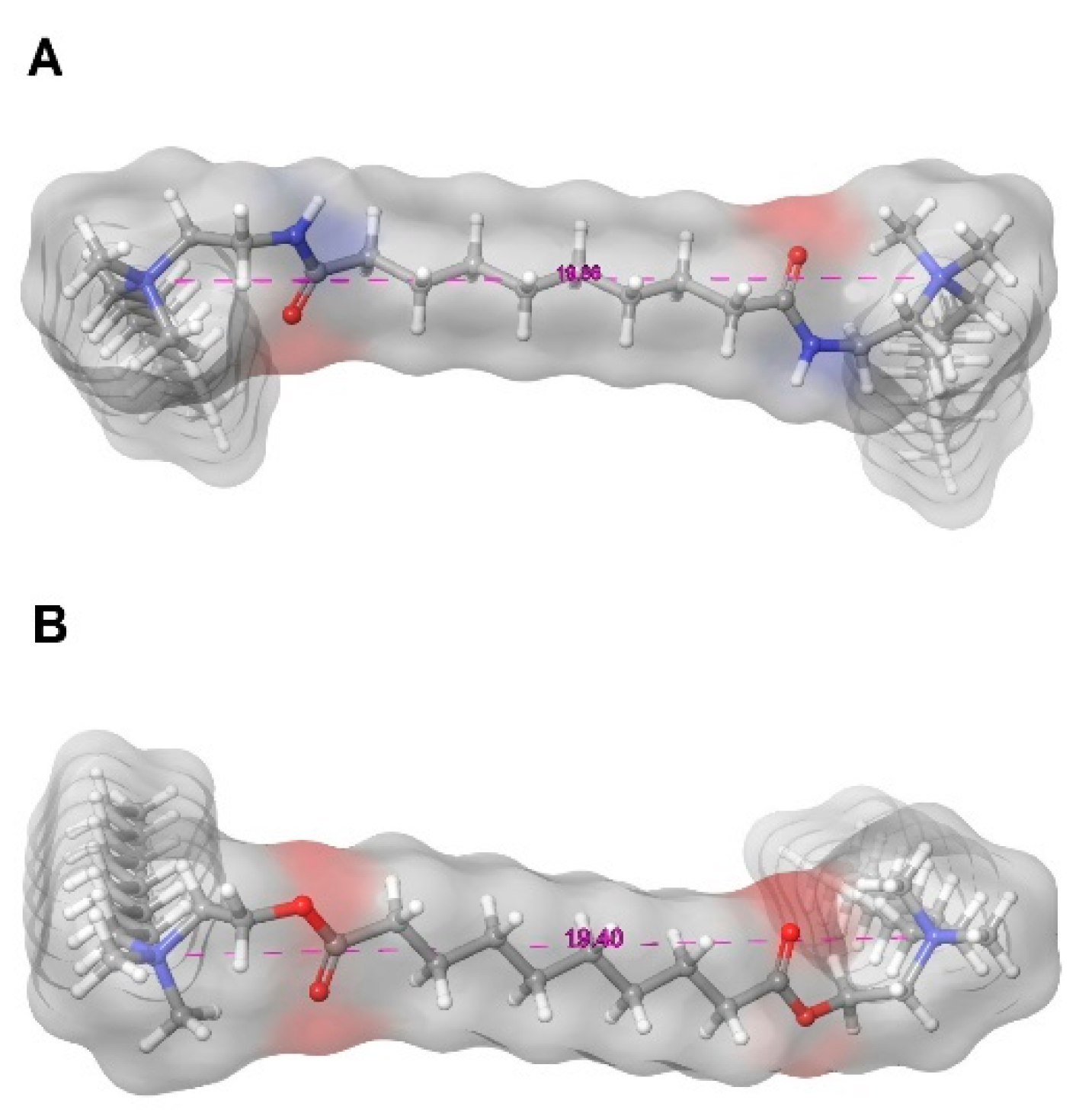
2.5. 3D Models of Optimised Gemini Molecules
3. Materials and Methods
3.1. Materials
3.2. Time-Resolved Fluorescence Quenching
3.3. Dynamic Light Scattering
3.4. Zeta Potential
3.5. 3D Modelling of Gemini Molecules
Author Contributions
Funding
Conflicts of Interest
References
- Rosen, M.J. Surfactants and Interfacial Phenomena, 3rd ed.; J. Wiley & Sons: New York, NY, USA, 2004. [Google Scholar]
- Zana, R. Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: A review. Adv. Colloid Interface Sci. 2002, 97, 205–253. [Google Scholar] [CrossRef]
- Holmberg, K. Novel Surfactants: Preparation Applications and Biodegradability, 2nd ed.; Marcel Dekker: New York, NY, USA, 2003; ISBN 978-0-8247-5621-5. [Google Scholar]
- Bunton, C.A.; Robinson, L.B.; Schaak, J.; Stam, M.F. Catalysis of nucleophilic substitutions by micelles of dicationic detergents. J. Org. Chem. 1971, 36, 2346–2350. [Google Scholar] [CrossRef]
- Schmitt, V.; Schosseler, F.; Lequeux, F. Structure of Salt-Free Wormlike Micelles: Signature by SANS at Rest and under Shear. Europhys. Lett. EPL 1995, 30, 31–36. [Google Scholar] [CrossRef]
- Dreja, M.; Tieke, B. Polymerization of Styrene in Ternary Microemulsion Using Cationic Gemini Surfactants. Langmuir 1998, 14, 800–807. [Google Scholar] [CrossRef]
- Dreja, M.; Pyckhout-Hintzen, W.; Mays, H.; Tieke, B. Cationic Gemini Surfactants with Oligo(oxyethylene) Spacer Groups and Their Use in the Polymerization of Styrene in Ternary Microemulsion. Langmuir 1999, 15, 391–399. [Google Scholar] [CrossRef]
- Devínsky, F.; Masárová, Ľ.; Lacko, I.; Mlynarčík, D. Structure-activity relationships of “soft” quaternary ammonium amphiphiles. J. Biopharm. Sci. 1991, 2, 1–10. [Google Scholar]
- Devínsky, F.; Masárová, Ľ.; Lacko, I. Surface activity and micelle formation of some new bisquaternary ammonium salts. J. Colloid Interface Sci. 1985, 105, 235–239. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Kärnbratt, J.; Löfroth, J.-E.; Holmberg, K. Cationic ester-containing gemini surfactants: Determination of aggregation numbers by time-resolved fluorescence quenching. J. Colloid Interface Sci. 2012, 376, 126–132. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Singh, R.G.; Holmberg, K. Solubilization of two organic dyes by cationic ester-containing gemini surfactants. J. Colloid Interface Sci. 2012, 376, 112–118. [Google Scholar] [CrossRef]
- Bergström, L.M.; Tehrani-Bagha, A.; Nagy, G. Growth Behavior, Geometrical Shape, and Second CMC of Micelles Formed by Cationic Gemini Esterquat Surfactants. Langmuir 2015, 31, 4644–4653. [Google Scholar] [CrossRef]
- Zhuang, L.-H.; Yu, K.-H.; Wang, G.-W.; Yao, C. Synthesis and properties of novel ester-containing gemini imidazolium surfactants. J. Colloid Interface Sci. 2013, 408, 94–100. [Google Scholar] [CrossRef]
- Bhadani, A.; Endo, T.; Koura, S.; Sakai, K.; Abe, M.; Sakai, H. Self-Aggregation and Liquid Crystalline Behavior of New Ester-Functionalized Quinuclidinolium Surfactants. Langmuir 2014, 30, 9036–9044. [Google Scholar] [CrossRef]
- Bhadani, A.; Endo, T.; Sakai, K.; Sakai, H.; Abe, M. Synthesis and dilute aqueous solution properties of ester functionalized cationic gemini surfactants having different ethylene oxide units as spacer. Colloid Polym. Sci. 2014, 292, 1685–1692. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K.; van Ginkel, C.G.; Kean, M. Cationic gemini surfactants with cleavable spacer: Chemical hydrolysis, biodegradation, and toxicity. J. Colloid Interface Sci. 2015, 449, 72–79. [Google Scholar] [CrossRef]
- Kabir-ud-Din; Yaseen, Z.; Aswal, V.K.; Dar, A.A. Rheological response and small-angle neutron-scattering study of diester-bonded cationic biodegradable gemini surfactants in presence of different additives. Colloid Polym. Sci. 2014, 292, 3113–3125. [Google Scholar] [CrossRef]
- Akram, M.; Bhat, I.A.; Yaseen, Z. Kabir-ud-Din Physicochemical investigation of novel biodegradable dicationic ester bonded m-E2-m gemini surfactants with bile salts: Insights from surface tension, dynamic light scattering and fluorescence. Colloids Surf. Physicochem. Eng. Asp. 2014, 444, 209–216. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, P.; Guo, Y.; Yang, Y.; Chen, Z.; Wang, X.; An, X.; Shen, W. The effect of the spacer rigidity on the aggregation behavior of two ester-containing Gemini surfactants. J. Colloid Interface Sci. 2012, 379, 64–71. [Google Scholar] [CrossRef]
- Parikh, K.; Mistry, B.; Jana, S.; Gupta, S.; Devkar, R.V.; Kumar, S. Physico-biochemical studies on cationic gemini surfactants: Role of spacer. J. Mol. Liq. 2015, 206, 19–28. [Google Scholar] [CrossRef]
- Fatma, N.; Panda, M.; Kabir-ud-Din; Beg, M. Ester-bonded cationic gemini surfactants: Assessment of their cytotoxicity and antimicrobial activity. J. Mol. Liq. 2016, 222, 390–394. [Google Scholar] [CrossRef]
- Xu, D.; Ni, X.; Zhang, C.; Mao, J.; Song, C. Synthesis and properties of biodegradable cationic gemini surfactants with diester and flexible spacers. J. Mol. Liq. 2017, 240, 542–548. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Z.; Xu, F.; Tai, S.; Liu, X.; Mo, S.; Niu, F. Surface Tension and Aggregation Properties of Novel Cationic Gemini Surfactants with Diethylammonium Headgroups and a Diamido Spacer. Langmuir 2012, 28, 11979–11987. [Google Scholar] [CrossRef]
- Zhong, S.; Wu, K.K.; Gong, L.J. Synthesis and Properties of a New Gemini Surfactant with Amide Group as Spacer. Adv. Mater. Res. 2011, 415–417, 1777–1780. [Google Scholar] [CrossRef]
- Hoque, J.; Kumar, P.; Aswal, V.K.; Haldar, J. Aggregation Properties of Amide Bearing Cleavable Gemini Surfactants by Small Angle Neutron Scattering and Conductivity Studies. J. Phys. Chem. B 2012, 116, 9718–9726. [Google Scholar] [CrossRef]
- Hoque, J.; Gonuguntla, S.; Yarlagadda, V.; Aswal, V.K.; Haldar, J. Effect of amide bonds on the self-assembly of gemini surfactants. Phys. Chem. Chem. Phys. 2014, 16, 11279–11288. [Google Scholar] [CrossRef]
- Hu, D.; Guo, X.; Jia, L. Synthesis, Surface Active Properties of Novel Gemini Surfactants with Amide Groups and Rigid Spacers. J. Surfactants Deterg. 2013, 16, 913–919. [Google Scholar] [CrossRef]
- Pisárčik, M.; Jampílek, J.; Devínsky, F.; Drábiková, J.; Tkacz, J.; Opravil, T. Gemini Surfactants with Polymethylene Spacer: Supramolecular Structures at Solid Surface and Aggregation in Aqueous Solution. J. Surfactants Deterg. 2016, 19, 477–486. [Google Scholar] [CrossRef]
- Alami, E.; Beinert, G.; Marie, P.; Zana, R. Alkanediyl-.alpha.,.omega.-bis(dimethylalkylammonium bromide) surfactants. 3. Behavior at the air-water interface. Langmuir 1993, 9, 1465–1467. [Google Scholar] [CrossRef]
- Danino, D.; Talmon, Y.; Zana, R. Alkanediyl-α,ω-Bis(Dimethylalkylammonium Bromide) Surfactants (Dimeric Surfactants). 5. Aggregation and Microstructure in Aqueous Solutions. Langmuir 1995, 11, 1448–1456. [Google Scholar] [CrossRef]
- Pisárčik, M.; Pupák, M.; Devínsky, F.; Almásy, L.; Tian, Q.; Bukovský, M. Urea-based gemini surfactants: Synthesis, aggregation behaviour and biological activity. Colloids Surf. Physicochem. Eng. Asp. 2016, 497, 385–396. [Google Scholar] [CrossRef]
- Cheon, H.-Y.; Jeong, N.-H.; Kim, H.-U. (first) Spontaneous Vesicle Formation in Aqueous Mixtures of Cationic Gemini Surfactant and Sodium Lauryl Ether Sulfate. Bull. Korean Chem. Soc. 2005, 26, 107–114. [Google Scholar] [CrossRef]
- Pisárčik, M.; Polakovičová, M.; Devínsky, F.; Lacko, I. Dynamic Light Scattering, Interfacial Properties, and Conformational Analysis of Biodegradable Quarternary Ammonium Surfactants. Langmuir 2006, 22, 9160–9168. [Google Scholar] [CrossRef]
- Tachiya, M. Application of a generating function to reaction kinetics in micelles. Kinetics of quenching of luminescent probes in micelles. Chem. Phys. Lett. 1975, 33, 289–292. [Google Scholar] [CrossRef]
- Alargova, R.G.; Kochijashky, I.I.; Sierra, M.L.; Kwetkat, K.; Zana, R. Mixed Micellization of Dimeric (Gemini) Surfactants and Conventional Surfactants: II. CMC and Micelle Aggregation Numbers for Various Mixtures. J. Colloid Interface Sci. 2001, 235, 119–129. [Google Scholar] [CrossRef]
Sample Availability: Samples of a(s) and e(s) series of gemini surfactants are available from the authors. |
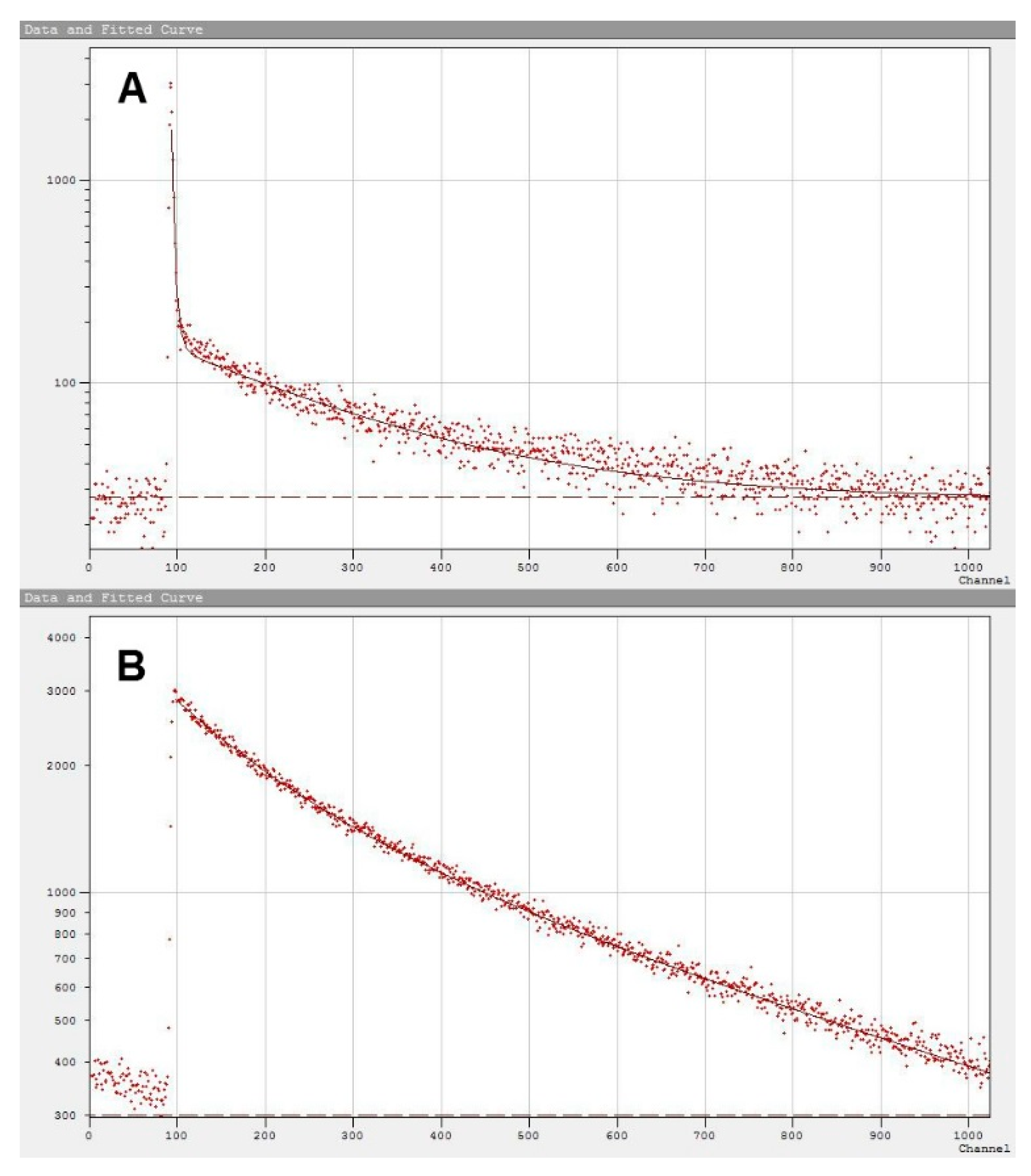
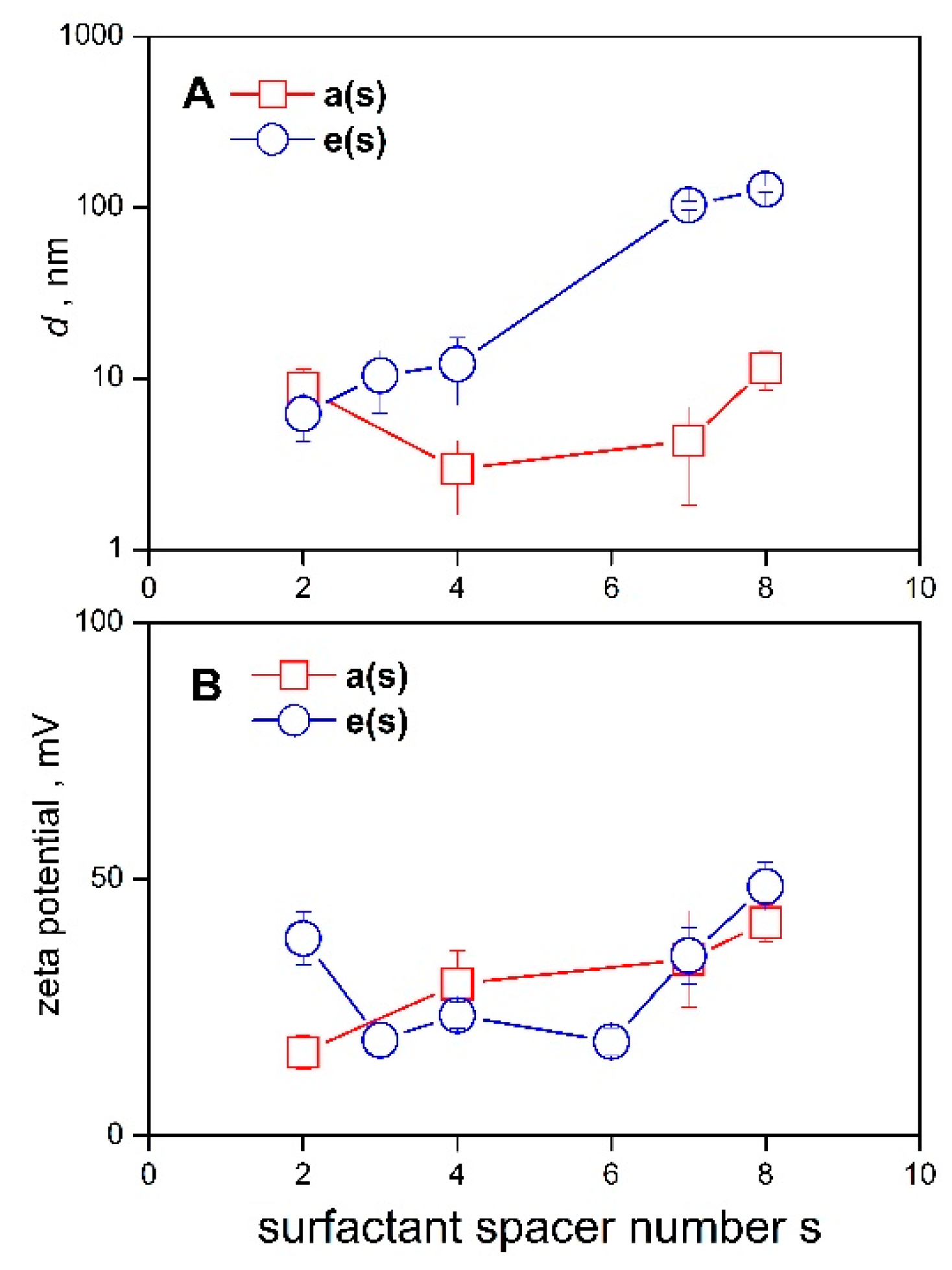
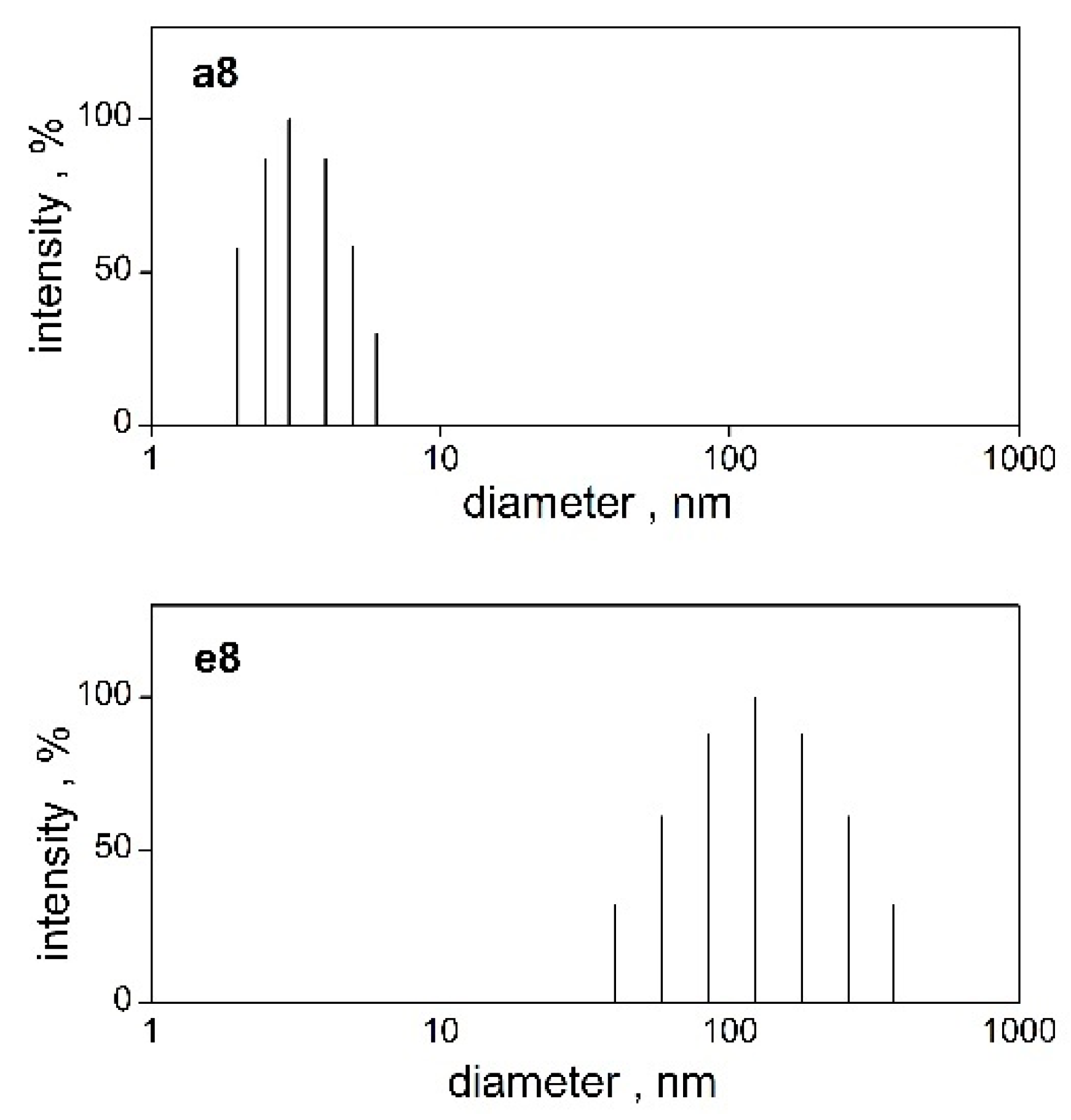
| Surfactant | Mr | cmc | Surfactant | Mr | cmc |
|---|---|---|---|---|---|
| 10−3M | 10−3M | ||||
| a1 | 742.80 | 1.50 ± 0.09 | e2 | 758.79 | 1.52 ± 0.12 |
| a2 | 756.82 | 1.26 ± 0.11 | e3 | 772.82 | 0.57 ± 0.11 |
| a4 | 784.88 | 1.36 ± 0.06 | e4 | 786.85 | 0.86 ± 0.08 |
| a7 | 826.96 | 1.01 ± 0.04 | e6 | 814.90 | 0.53 ± 0.09 |
| a8 | 840.98 | 0.74 ± 0.06 | e7 | 828.93 | 0.47 ± 0.07 |
| e8 | 842.95 | 0.29 ± 0.10 |
| c/cmc | a1 | a2 | a4 | a7 | a8 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | N | R | N | R | N | R | N | R | N | |
| 4 | 1.861 ± 0.020 | 35.8 ± 0.4 | 1.948 ± 0.019 | 31.5 ± 0.3 | 1.151 ± 0.013 | 20.1 ± 0.2 | 1.681 ± 0.035 | 21.7 ± 0.5 | 0.639 ± 0.011 | 14.3 ± 0.2 |
| 6 | 1.729 ± 0.018 | 37 ± 0.4 | 1.693 ± 0.017 | 30.4 ± 0.3 | 1.091 ± 0.012 | 21.1 ± 0.2 | 1.635 ± 0.037 | 23.5 ± 0.5 | 0.601 ± 0.015 | 14.9 ± 0.4 |
| 8 | 1.690 ± 0.021 | 38 ± 0.5 | 1.688 ± 0.020 | 31.9 ± 0.4 | 1.056 ±0.012 | 21.5 ±0.2 | 1.654 ± 0.040 | 25 ± 0.6 | 0.623 ± 0.011 | 16.2 ± 0.3 |
| 10 | 1.661 ± 0.018 | 38.4 ± 0.4 | 1.598 ± 0.020 | 31 ± 0.4 | 1.013 ± 0.012 | 21.2 ± 0.3 | 1.670 ± 0.041 | 25.9 ± 0.6 | 0.606 ± 0.016 | 16.2 ± 0.4 |
| 12 | 1.638 ± 0.019 | 38.5 ± 0.5 | 1.627 ± 0.018 | 32.2 ± 0.3 | 1.006 ± 0.012 | 21.4 ± 0.3 | 1.807 ± 0.051 | 28.6 ± 0.8 | 0.639 ± 0.019 | 17.4 ± 0.5 |
| c/cmc | e2 | e3 | e4 | e6 | e7 | c/cmc | e8 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | N | R | N | R | N | R | N | R | N | R | N | ||
| 4 | 1.077 ± 0.015 | 24.6 ± 0.3 | 0.901 ± 0.013 | 23 ± 0.3 | 1.177 ± 0.015 | 22.8 ± 0.3 | 0.636 ± 0.012 | 15.2 ± 0.3 | 0.576 ± 0.016 | 12.2 ± 0.3 | 2 | 1.177 ± 0.074 | 10.2 ± 0.6 |
| 6 | 0.927 ± 0.023 | 23.5 ± 0.6 | 0.892 ± 0.016 | 25.2 ± 0.4 | 0.999 ± 0.020 | 21.6 ± 0.4 | 0.614 ± 0.022 | 16.3 ± 0.6 | 0.600 ± 0.017 | 14.1 ± 0.4 | 3 | 1.661 ± 0.040 | 19.2 ± 0.5 |
| 8 | 0.903 ± 0.018 | 24 ± 0.5 | 0.785 ± 0.022 | 23.3 ± 0.7 | 0.971 ± 0.022 | 22 ± 0.5 | 0.598 ± 0.020 | 16.6 ± 0.5 | 0.594 ± 0.022 | 14.6 ± 0.5 | 4 | 1.770 ± 0.069 | 23 ± 0.9 |
| 10 | 0.891 ± 0.020 | 24.4 ± 0.5 | 0.805 ± 0.027 | 24.6 ± 0.8 | 0.932 ± 0.018 | 21.7 ± 0.4 | 0.623 ± 0.029 | 17.8 ± 0.8 | 0.588 ± 0.014 | 14.9 ± 0.3 | 5 | 2.102 ± 0.065 | 29.2 ± 0.9 |
| 12 | 0.909 ± 0.021 | 25.3 ± 0.6 | 0.805 ± 0.027 | 25.1 ± 0.8 | 0.947 ± 0.023 | 22.5 ± 0.6 | 0.604 ± 0.020 | 17.6 ± 0.6 | 0.600 ± 0.021 | 15.5 ± 0.5 | 6 | 2.322 ± 0.079 | 33.6 ± 1.1 |
| N-to-N distance | Surface | |
|---|---|---|
| Å | Å2 | |
| a8 | 19.86 | 849.83 |
| e8 | 19.40 | 839.65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisárčik, M.; Polakovičová, M.; Markuliak, M.; Lukáč, M.; Devínsky, F. Self-Assembly Properties of Cationic Gemini Surfactants with Biodegradable Groups in the Spacer. Molecules 2019, 24, 1481. https://doi.org/10.3390/molecules24081481
Pisárčik M, Polakovičová M, Markuliak M, Lukáč M, Devínsky F. Self-Assembly Properties of Cationic Gemini Surfactants with Biodegradable Groups in the Spacer. Molecules. 2019; 24(8):1481. https://doi.org/10.3390/molecules24081481
Chicago/Turabian StylePisárčik, Martin, Mája Polakovičová, Mário Markuliak, Miloš Lukáč, and Ferdinand Devínsky. 2019. "Self-Assembly Properties of Cationic Gemini Surfactants with Biodegradable Groups in the Spacer" Molecules 24, no. 8: 1481. https://doi.org/10.3390/molecules24081481






