Purification of Polyphenols from Distiller’s Grains by Macroporous Resin and Analysis of the Polyphenolic Components
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of Polyphenols by Macroporous Resin
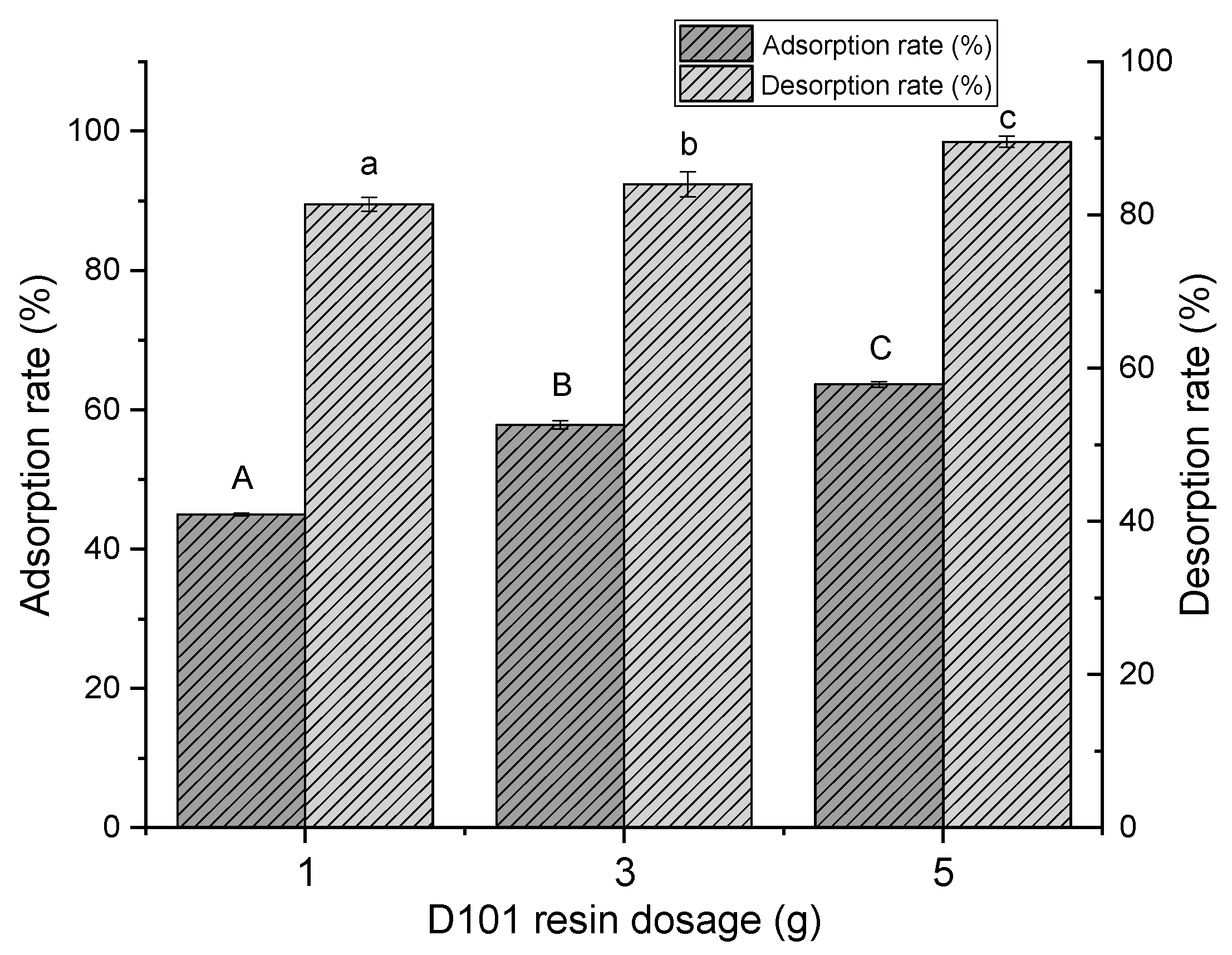
2.1.1. Screening of Macroporous Resin Type and Dosage
2.1.2. Static Adsorption Kinetic Curve
2.1.3. Static Desorption Kinetic Curve
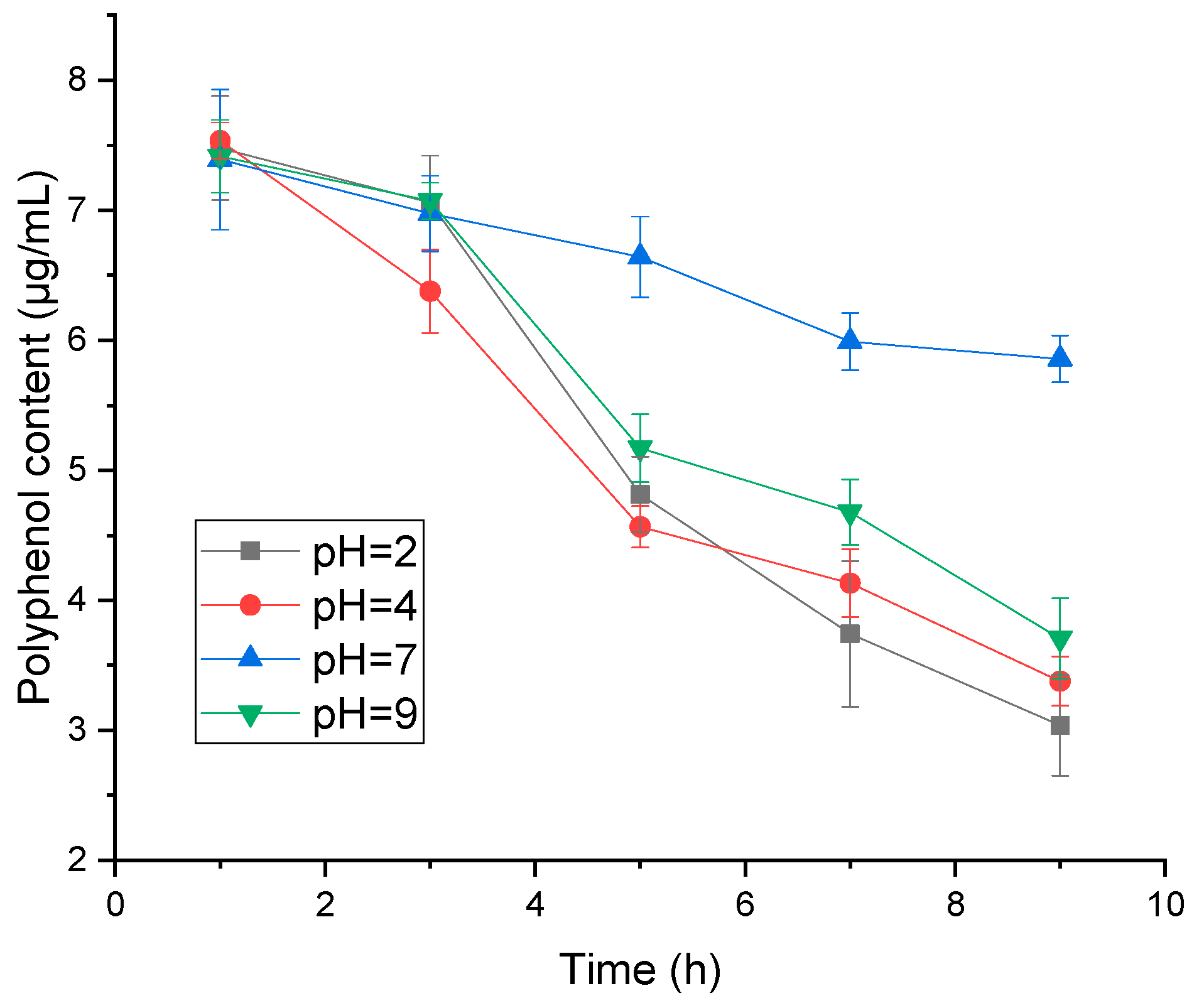
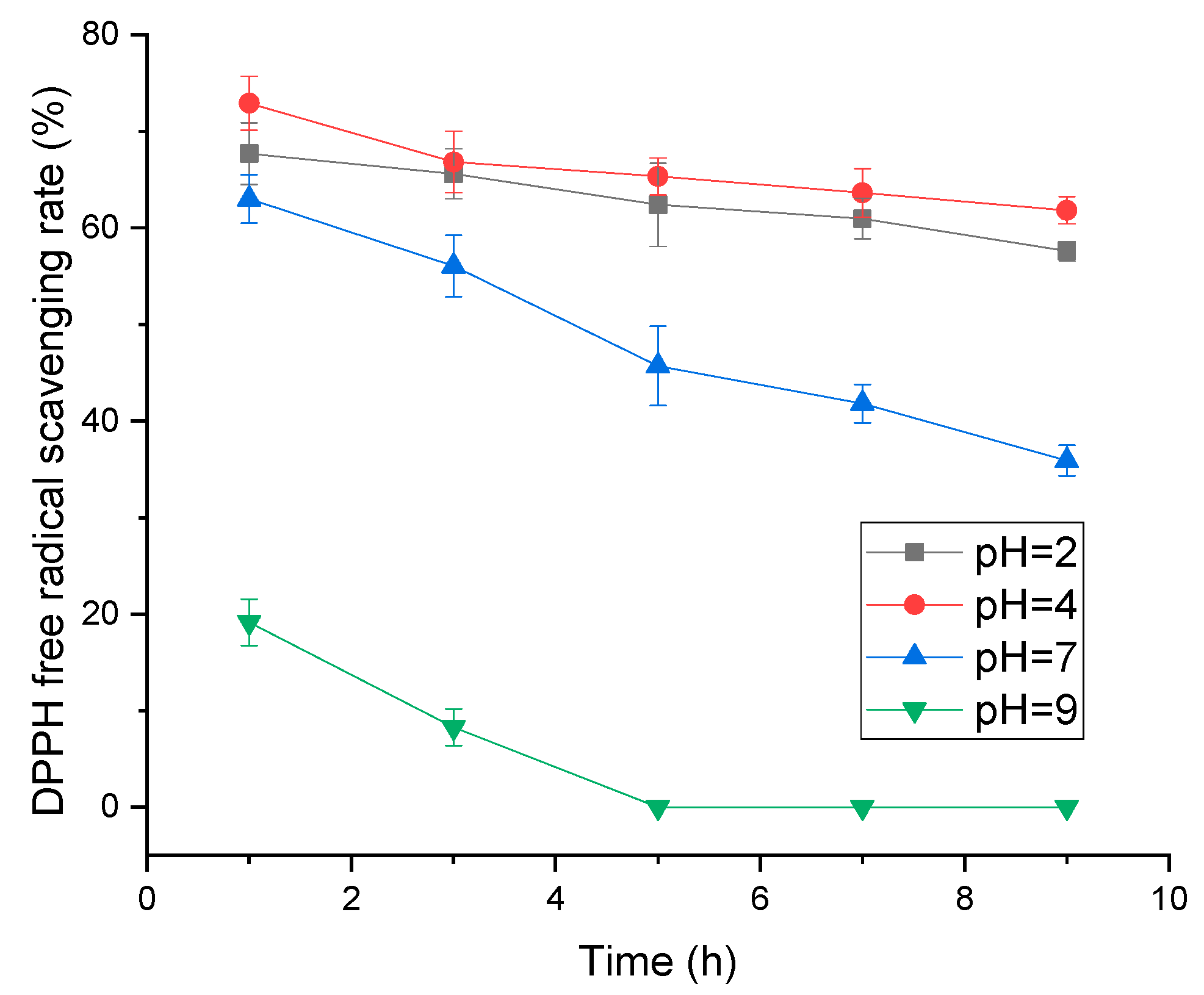
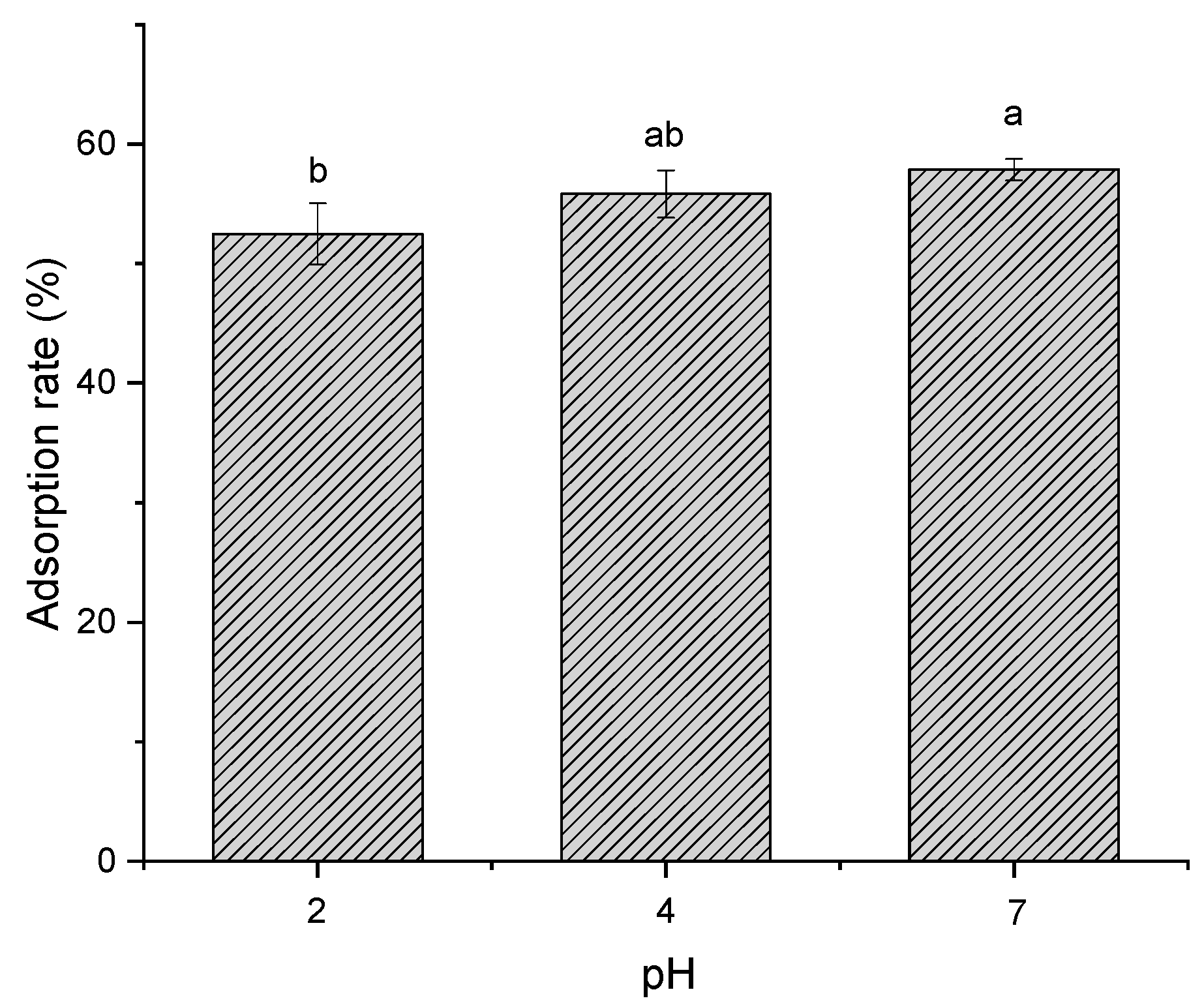
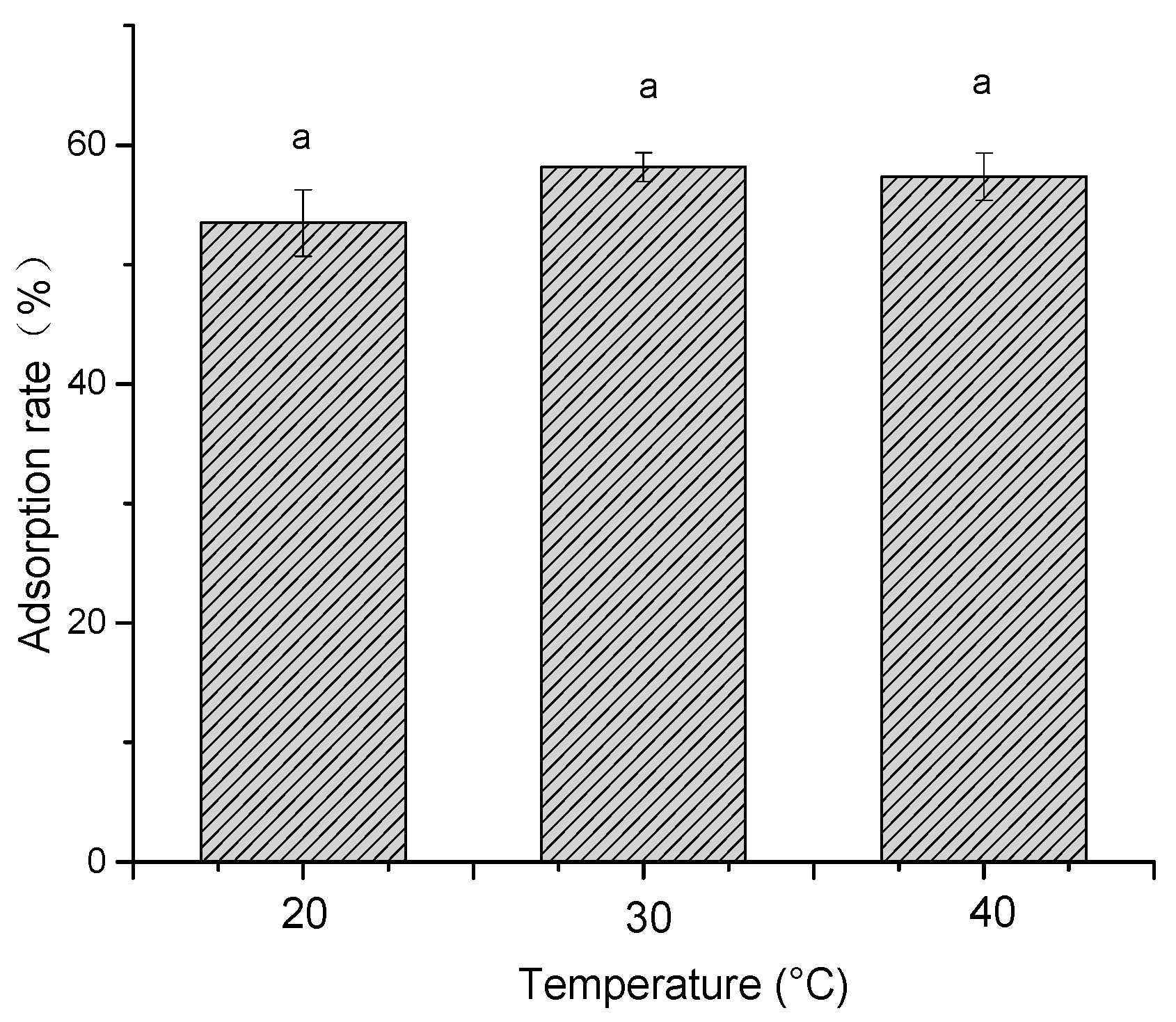
2.1.4. Effect of pH and Temperature on Adsorption Efficiency
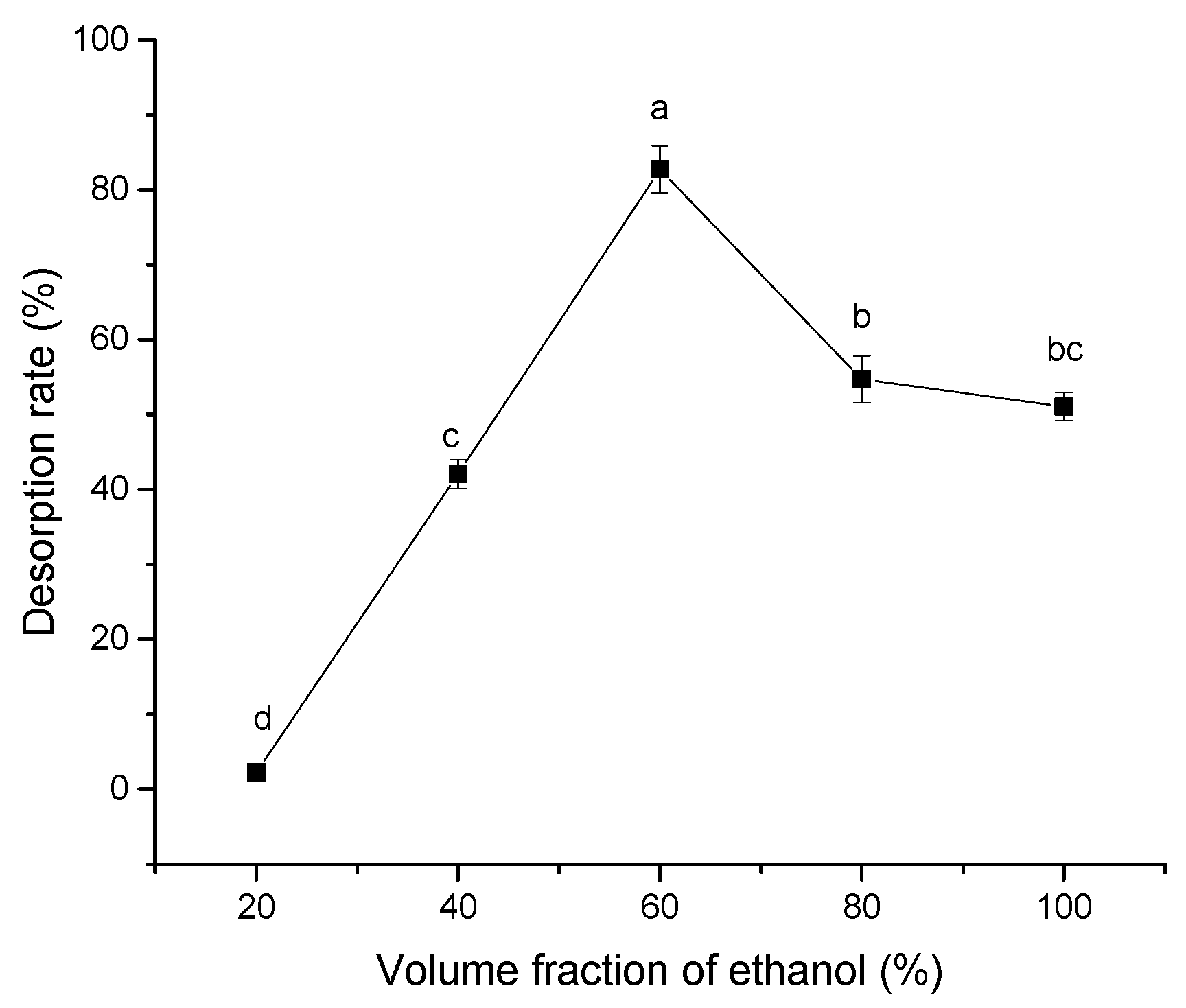
2.1.5. Effect of Ethanol Volume Fraction on Desorption Efficiency
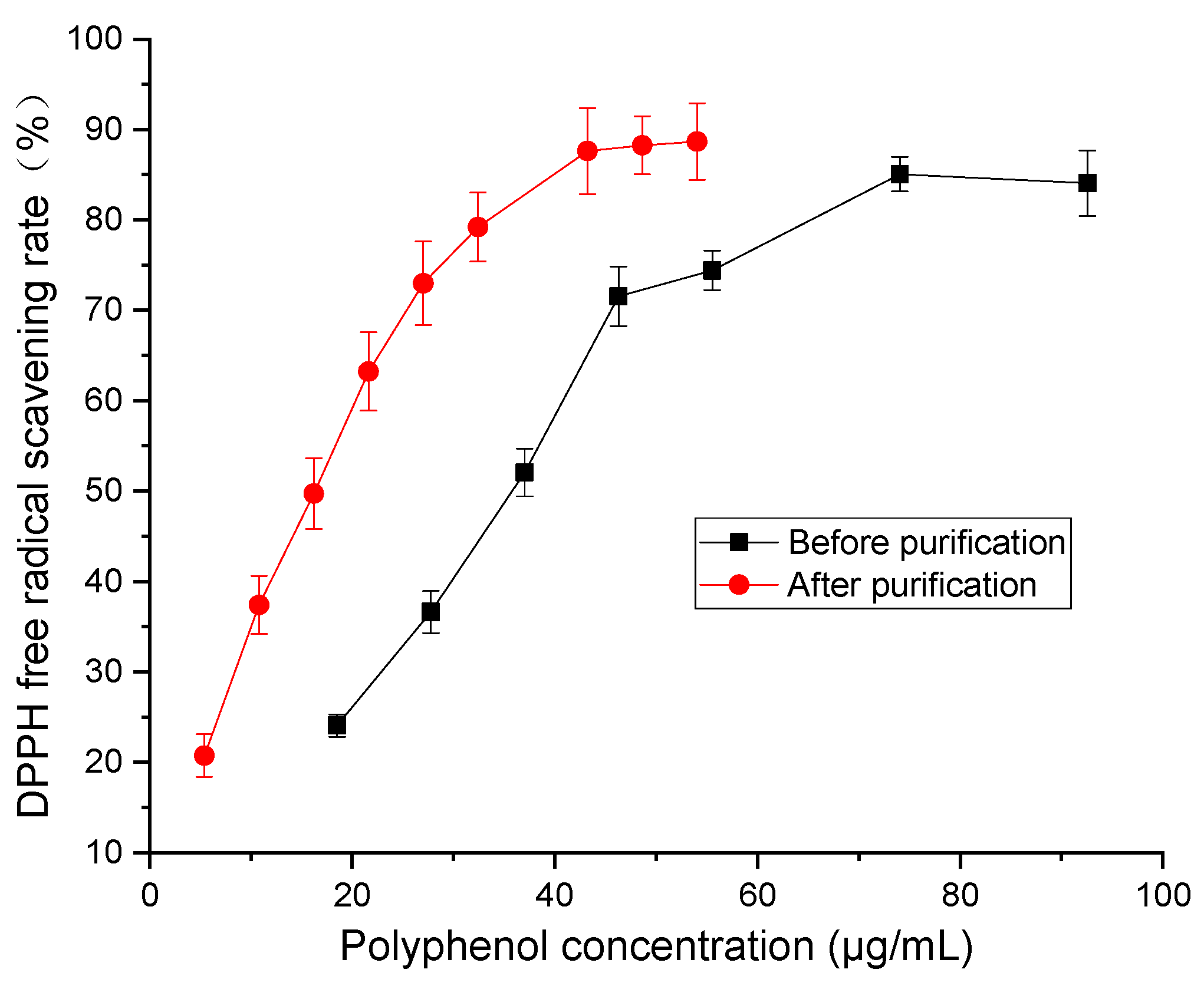
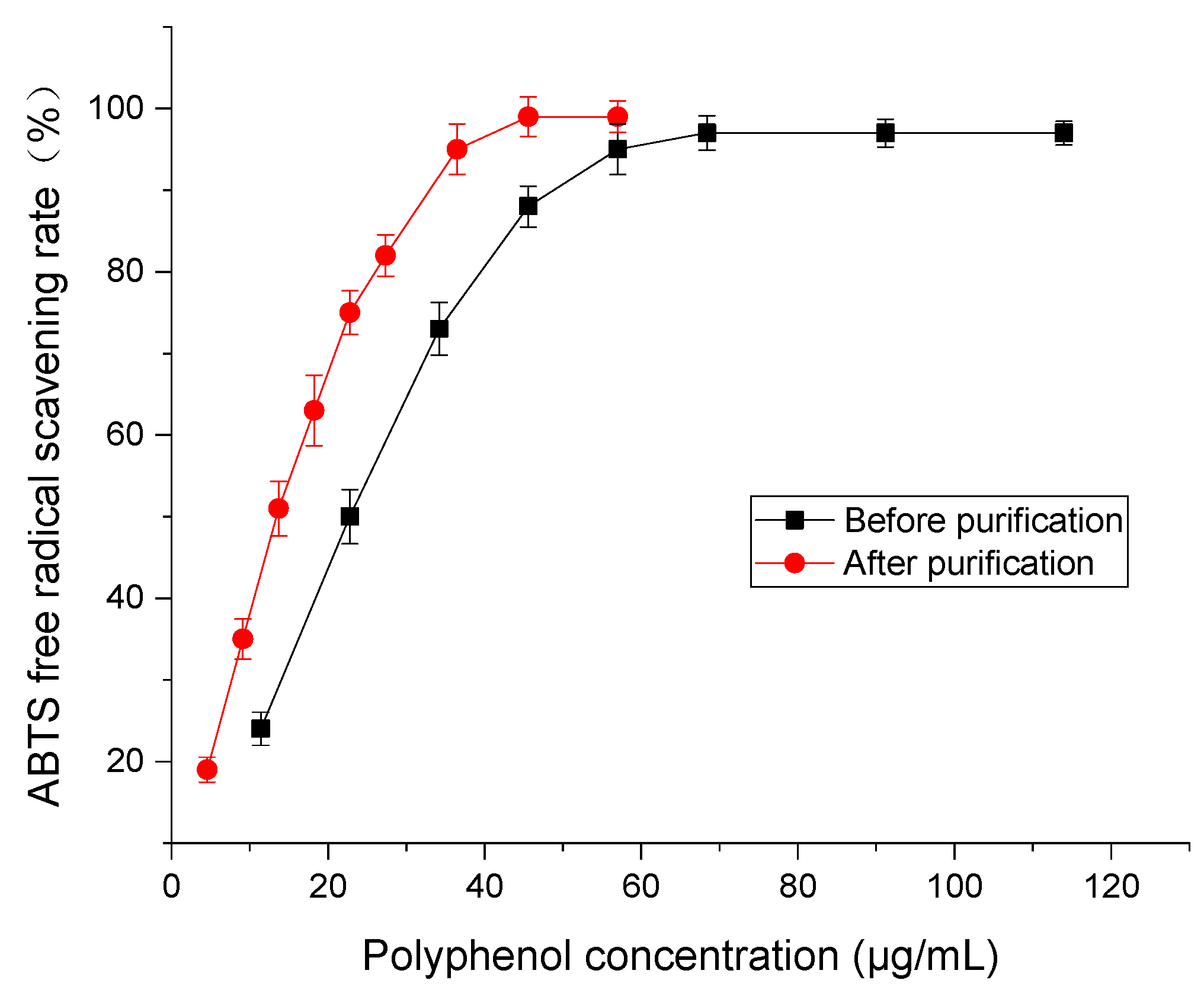
2.2. Antioxidant Activity Comparison
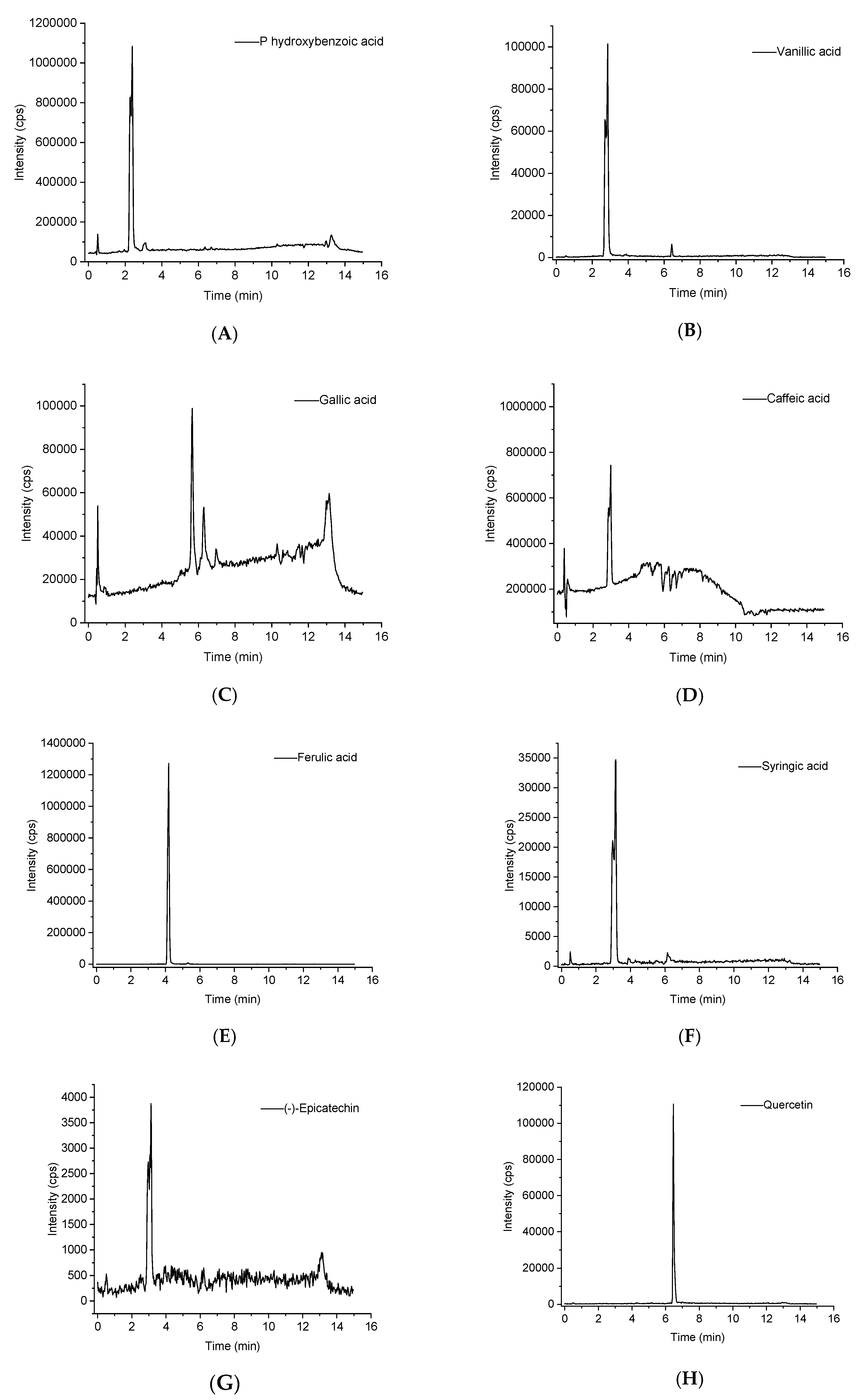
2.3. Identification of Phenolic Compounds in Distiller’s Grain Extracts
2.4. UPLC/MS Quantification of Identified Phenolic Compounds in Extracts of Distiller’s Grain
3. Materials and Methods
3.1. Materials
3.2. Extraction Procedure
3.3. Purification of Distiller’s Grain Polyphenols
3.3.1. Screening of Macroporous Resin Type and Added Amount
3.3.2. Static Adsorption Kinetic Curve
3.3.3. Static Desorption Kinetic Curve
3.3.4. Effect of pH on Polyphenol Stability, Activity and Resin Adsorption Efficiency
3.3.5. Effect of Temperature on Adsorption Efficiency
3.3.6. Effect of Eluent Concentration on Desorption Efficiency
3.4. Antioxidant Activity Detection
3.4.1. Free Radical Scavenging Rate of DPPH Detection
3.4.2. Free Radical Scavenging Rate of ABTS Detection
3.5. Qualitative and Quantitative Analysis of Composition of Distiller’s Grain Polyphenols
3.6. Statistical Analysis of Data
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, K. Chemical composition of distillers grains, a review. J. Agr. Food Chem. 2011, 59, 1508–1526. [Google Scholar] [CrossRef]
- Kim, Y.; Mosier, N.S.; Hendrickson, R.; Ezeji, T.; Blaschek, H.; Dien, B.; Cotta, M.; Dale, B.; Ladisch, M.R. Composition of corn dry-grind ethanol by-products: DDGS, wet cake, and thin stillage. Bioresource Technol. 2008, 99, 5165–5176. [Google Scholar] [CrossRef]
- Sha, S.; Chen, S.; Qian, M.; Wang, C.; Xu, Y. Characterization of the typical potent odorants in Chinese roasted sesame-like flavor type liquor by headspace solid phase microextraction–aroma extract dilution analysis, with special emphasis on sulfur-containing odorants. J. Agr. Food Chem. 2016, 65, 123–131. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energ. 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Renewable Fuels Association. Climate of Opportunity. Ethanol Industry Outlook. Available online: http://ethanolrfa.3cdn.net/32b7ed69bd36632cb_r/m626lb0.pdf (accessed on 1 April 2019).
- Böttger, C.; Südekum, K.H. Review: Protein value of distillers dried grains with solubles (DDGS) in animal nutrition as affected by the ethanol production process. Anim. Feed. Sci. Technol. 2018, 244, 11–17. [Google Scholar] [CrossRef]
- Cekmecelioglu, D.; Demirci, A. A statistical optimization study on dilute sulfuric acid pretreatment of distillers dried grains with solubles (DDGS) as a potential feedstock for fermentation applications. Waste Biomass Valori. 2018, 1–7. [Google Scholar] [CrossRef]
- Singha, P.; Singh, S.K.; Muthukumarappan, K.; Krishnan, P. Physicochemical and nutritional properties of extrudates from food grade distiller’s dried grains, garbanzo flour, and corn grits. Food Sci. Nutr. 2018, 6, 1914–1926. [Google Scholar] [CrossRef] [PubMed]
- Liu, K. Particle size distribution of distillers dried grains with solubles (DDGS) and relationships to compositional and color properties. Bioresour. Technol. 2008, 99, 8421–8428. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liu, K. Changes in composition and amino acid profile during dry grind ethanol processing from corn and estimation of yeast contribution toward DDGS proteins. J. Agric. Food Chem. 2010, 58, 3430–3437. [Google Scholar] [CrossRef]
- Liu, K.; Han, J. Changes in mineral concentrations and phosphorus profile during dry-grind processing of corn into ethanol. Bioresource Technol. 2011, 102, 3110–3118. [Google Scholar] [CrossRef]
- Belyea, R.L.; Clevenger, T.E.; Singh, V.; Tumbleson, M.; Rausch, K.D. Element concentrations of dry-grind corn-processing streams. Appl. Biochem. Biotechnol. 2006, 134, 113–128. [Google Scholar] [CrossRef]
- Spiehs, M.; Whitney, M.; Shurson, G. Nutrient database for distiller’s dried grains with solubles produced from new ethanol plants in Minnesota and South Dakota. J. Anim. Sci. 2002, 80, 2639–2645. [Google Scholar]
- Moreau, R.A.; Hicks, K.B.; Johnston, D.B.; Laun, N.P. The composition of crude corn oil recovered after fermentation via centrifugation from a commercial dry grind ethanol process. J. Am. Oil Chem. Soc. 2010, 87, 895–902. [Google Scholar] [CrossRef]
- Moreau, R.A.; Liu, K.; Winkler-Moser, J.K.; Singh, V. Changes in lipid composition during dry grind ethanol processing of corn. J. Am. Oil Chem. Soc. 2011, 88, 435–442. [Google Scholar] [CrossRef]
- Dia, V.P.; Wang, Z.; West, M.; Singh, V.; West, L.; De Mejia, E.G. Processing method and corn cultivar affected anthocyanin concentration from dried distillers grains with solubles. J. Agric. Food Chem. 2015, 63, 3205–3218. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Shufen, L.; Xiwen, W.; Xing, Z. Macroporous resin adsorption for purification of flavonoids in Houttuynia cordata Thunb. Chin. J. Chem. Eng. 2007, 15, 872–876. [Google Scholar] [CrossRef]
- Samah, R.A.; Zainol, N.; Yee, P.L.; Pawing, C.M.; Abd-Aziz, S. Adsorption of vanillin using macroporous resin H103. Adsorpt. Sci. Technol. 2013, 31, 599–610. [Google Scholar] [CrossRef]
- Chang, X.L.; Wang, D.; Chen, B.Y.; Feng, Y.M.; Wen, S.H.; Zhan, P.Y. Adsorption and desorption properties of macroporous resins for anthocyanins from the calyx extract of roselle (Hibiscus sabdariffa L.). J. Agric. Food Chem. 2012, 60, 2368–2376. [Google Scholar] [CrossRef]
- Lin, L.; Zhao, H.; Dong, Y.; Yang, B.; Zhao, M. Macroporous resin purification behavior of phenolics and rosmarinic acid from Rabdosia serra (MAXIM.) HARA leaf. Food Chem. 2012, 130, 417–424. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, G.; Shen, H.; Shen, J.X.; Aubry, A.-F.; Sivaraman, L.; Arnold, M.E. Bioanalysis of propylparaben and p-hydroxybenzoic acid, and their sulfate conjugates in rat plasma by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2014, 947, 68–74. [Google Scholar] [CrossRef]
- Grieman, M.; Greaves, J.; Saltzman, E. A method for analysis of vanillic acid in polar ice cores. Clim. Past 2014, 2, 227–232. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.; Baptista, P.; Santos-Buelga, C. Phenolic acids determination by HPLC–DAD–ESI/MS in sixteen different Portuguese wild mushrooms species. Food Chem. Toxicol. 2009, 47, 1076–1079. [Google Scholar] [CrossRef]
- Song, R.; Xu, L.; Zhang, Z.; Tian, Y.; Xu, F.; Dong, H. Determination of gallic acid in rat plasma by LC-MS-MS. Chromatographia 2010, 71, 1107–1111. [Google Scholar] [CrossRef]
- Sawant, L.; Prabhakar, B.; Pandita, N. Quantitative HPLC analysis of ascorbic acid and gallic acid in Phyllanthus emblica. J. Anal. Bioanal. Tech. 2010, 1, 111. [Google Scholar] [CrossRef]
- Bazylko, A.; Boruc, K.; Borzym, J.; Kiss, A.K. Aqueous and ethanolic extracts of Galinsoga parviflora and Galinsoga ciliata. Investigations of caffeic acid derivatives and flavonoids by HPTLC and HPLC-DAD-MS methods. Phytochem. Lett. 2015, 11, 394–398. [Google Scholar] [CrossRef]
- Karl, W.; Cathy, W.; Li, X.; Amy, G. Pharmacokinetic analyses of ferulic acid in rat plasma by liquid chromatography? tandem mass spectrometry: A synergistic action of an ancient herbal decoction fo shou san. Pharm. Anal. Acta 2015, 6, 2. [Google Scholar] [CrossRef]
- Wang, T.; Ding, L.; Jin, H.; Shi, R.; Li, Y.; Wu, J.; Li, Y.; Zhu, L.; Ma, Y. Simultaneous quantification of catechin, epicatechin, liquiritin, isoliquiritin, liquiritigenin, isoliquiritigenin, piperine and glycyrrhetinic acid in rat plasma by HPLC-MS/MS: Application to a pharmacokinetic study of Longhu Rendan pills. Biomed. Chromatogr. 2016, 30, 1166–1174. [Google Scholar] [CrossRef]
- Verma, P.; Singh, S.; Viswanathan, S. LC-ESI-MS/MS analysis of quercetin in rat plasma after oral administration of biodegradable nanoparticles. Biomed. Chromatogr. 2015, 29, 1731–1736. [Google Scholar]
- Luthria, D.; Liu, K.; Memon, A. Phenolic acids and antioxidant capacity of distillers dried grains with solubles (DDGS) as compared with corn. J. Am. Oil Chem. Soc. 2012, 89, 1297–1304. [Google Scholar] [CrossRef]
- Moreira, M.M.; Morais, S.; Barros, A.A.; Delerue-Matos, C.; Guido, L.F. A novel application of microwave-assisted extraction of polyphenols from brewer’s spent grain with HPLC-DAD-MS analysis. Anal. Bioanal. Chem. 2012, 403, 1019–1029. [Google Scholar] [CrossRef]
- Lempereur, I.; Rouau, X.; Abecassis, J. Genetic and agronomic variation in arabinoxylan and ferulic acid contents of durum wheat (Triticum durum L.) grain and its milling fractions. J. Cereal. Sci. 1997, 25, 103–110. [Google Scholar] [CrossRef]
- Claudine, M.; Augustin, S.; Christine, M.; Christian, R.; Jime’nez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar]
- Shao, Y.; Bao, J. Polyphenols in whole rice grain: Genetic diversity and health benefits. Food Chem. 2015, 180, 86–97. [Google Scholar] [CrossRef]
- Kaur, P.; Purewal, S.; Sandhu, K.; Kaur, M.; Salar, R. Millets: A cereal grain with potent antioxidants and health benefits. J. Food Meas. Charact. 2018, 13, 793–806. [Google Scholar] [CrossRef]
- Aikpokpodion, P.E.; Dongo, L.N. Effects of fermentation intensity on polyphenols and antioxidant capacity of cocoa beans. Int. J. Sustain. Crop Prod. 2010, 5, 66–70. [Google Scholar]
- Gan, R.Y.; Shah, N.P.; Wang, M.F.; Lui, W.Y.; Corke, H. Fermentation alters antioxidant capacity and polyphenol distribution in selected edible legumes. Int. J. Food Sci. Technol. 2016, 51, 875–884. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Zong, W. Comparison of the influence of dynamic high-pressure microfluidization and conventional homogenization on the quality of kiwi fruit juice. Appl. Eng. Agric. 2018, 34, 1039–1045. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R.; Inoue, H.; Suzuki, N. Preparation and antioxidant properties of water extract of propolis. Food Chem. 2003, 80, 29–33. [Google Scholar] [CrossRef]
- Ouattara, N.; Meda, R.N.T.; Hilou, A.; Guenné, S.; Konaté, K.; Coulibaly, A.Y.; Kiendrébeogo, M.; Millogo, J.F.; Nacoulma, O.G. Anti-acetylcholinesterase and antioxidant activities and HPLC-MS analysis of polyphenol from extracts of Nelsonia canescens (Lam.) Spreng. Asian Pac. J. Trop. Dis. 2013, 3, 382–388. [Google Scholar] [CrossRef]


| Resin Type | Polarity | Specific Surface Area/(m2/g) | Average Pore Size (Å) | Adsorption Rate (%) | Desorption Rate (%) |
|---|---|---|---|---|---|
| AB-8 | weakly polar | 480–520 | 130–140 | 35.56 ± 0.35b | 97.39 ± 0.23a |
| D101 | nonpolar | 600–700 | 100–110 | 44.99 ± 0.11a | 98.47 ± 0.19a |
| HPD400 | nonpolar | 500–550 | 75–80 | 45.71 ± 0.63a | 86.94 ± 0.56b |
| HPD750 | middle polar | 650–700 | 85–90 | 27.00 ± 0.16d | 94.19 ± 0.39ab |
| NAK-9 | polar | 250–290 | 155–165 | 44.29 ± 0.78a | 92.46 ± 0.13ab |
| S-8 | polar | 100–120 | 280–300 | 28.81 ± 0.40c | 90.33 ± 0.22ab |
| Peak | [M − H]− | Compounds | MS2 |
|---|---|---|---|
| A1 | 137 | p-Hydroxybenzoic acid | 93 |
| A2 | 167 | Vanillic acid | 151.9, 122.9, 107.9, 91 |
| A3 | 168.9 | Gallic acid | 125, 107, 97, 81, 78.9, 69 |
| A4 | 179 | Caffeic acid | 135 |
| A5 | 193.1 | Ferulic acid | 178.1, 149, 134, 117 |
| A6 | 197 | Syringic acid | 182, 152.9, 137.9, 121 |
| A7 | 289.1 | (−)-Epicatechin | 202.8, 197.1, 153, 151.1, 121, 108.7 |
| A8 | 301.2 | Quercetin | 255.2, 179, 150.8, 121.1, 107 |
| Polyphenols | Retention Time (min) | Concentrations (μg/g Extract) |
|---|---|---|
| p-Hydroxybenzoic acid | 2.39 | 417.7 |
| Vanillic acid | 2.86 | 66.5 |
| Gallic acid | 0.51 | 41.4 |
| Caffeic acid | 2.99 | 217.1 |
| Ferulic acid | 4.19 | 518.2 |
| Syringic acid | 3.14 | 158.0 |
| (−)-Epicatechin | 3.13 | 562.7 |
| Quercetin | 6.46 | 147.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, S.; Huang, S.; Zhang, L.; Ge, Z.; Sun, L.; Zong, W. Purification of Polyphenols from Distiller’s Grains by Macroporous Resin and Analysis of the Polyphenolic Components. Molecules 2019, 24, 1284. https://doi.org/10.3390/molecules24071284
Wang X, Wang S, Huang S, Zhang L, Ge Z, Sun L, Zong W. Purification of Polyphenols from Distiller’s Grains by Macroporous Resin and Analysis of the Polyphenolic Components. Molecules. 2019; 24(7):1284. https://doi.org/10.3390/molecules24071284
Chicago/Turabian StyleWang, Xiaoyuan, Shuangshuang Wang, Shasha Huang, Lihua Zhang, Zhenzhen Ge, Liping Sun, and Wei Zong. 2019. "Purification of Polyphenols from Distiller’s Grains by Macroporous Resin and Analysis of the Polyphenolic Components" Molecules 24, no. 7: 1284. https://doi.org/10.3390/molecules24071284





