Neuroprotective Effect of Dioscin on the Aging Brain
Abstract
:1. Introduction
2. Results
2.1. Dioscin Alleviated H2O2-Induced Injury in PC12 Cells
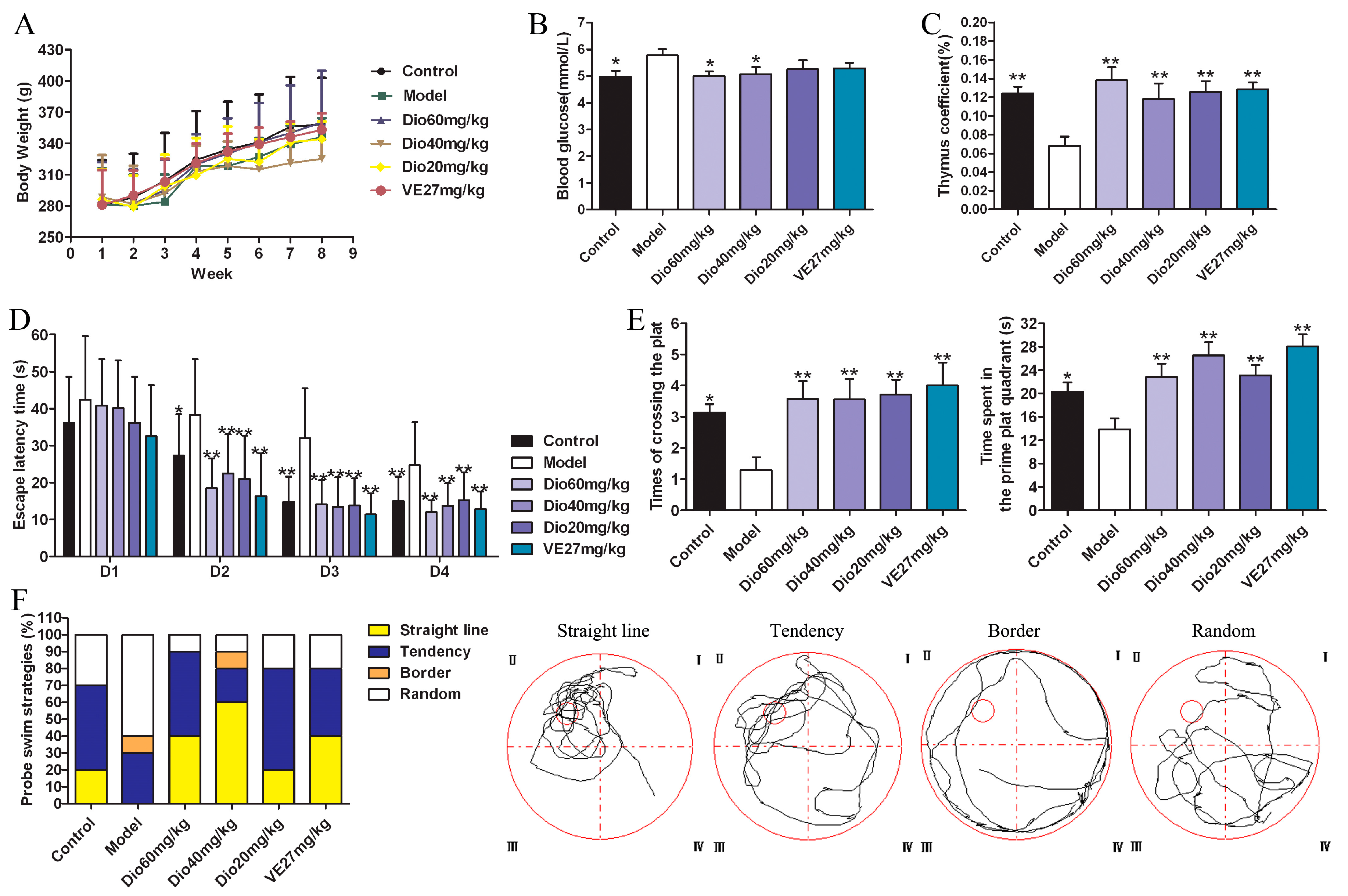
2.2. Effects of Dioscin on Body Weight, Blood Glucose and Thymus Coefficient in Rats
2.3. Dioscin Improved the Cognition Deficit of Aging Rats
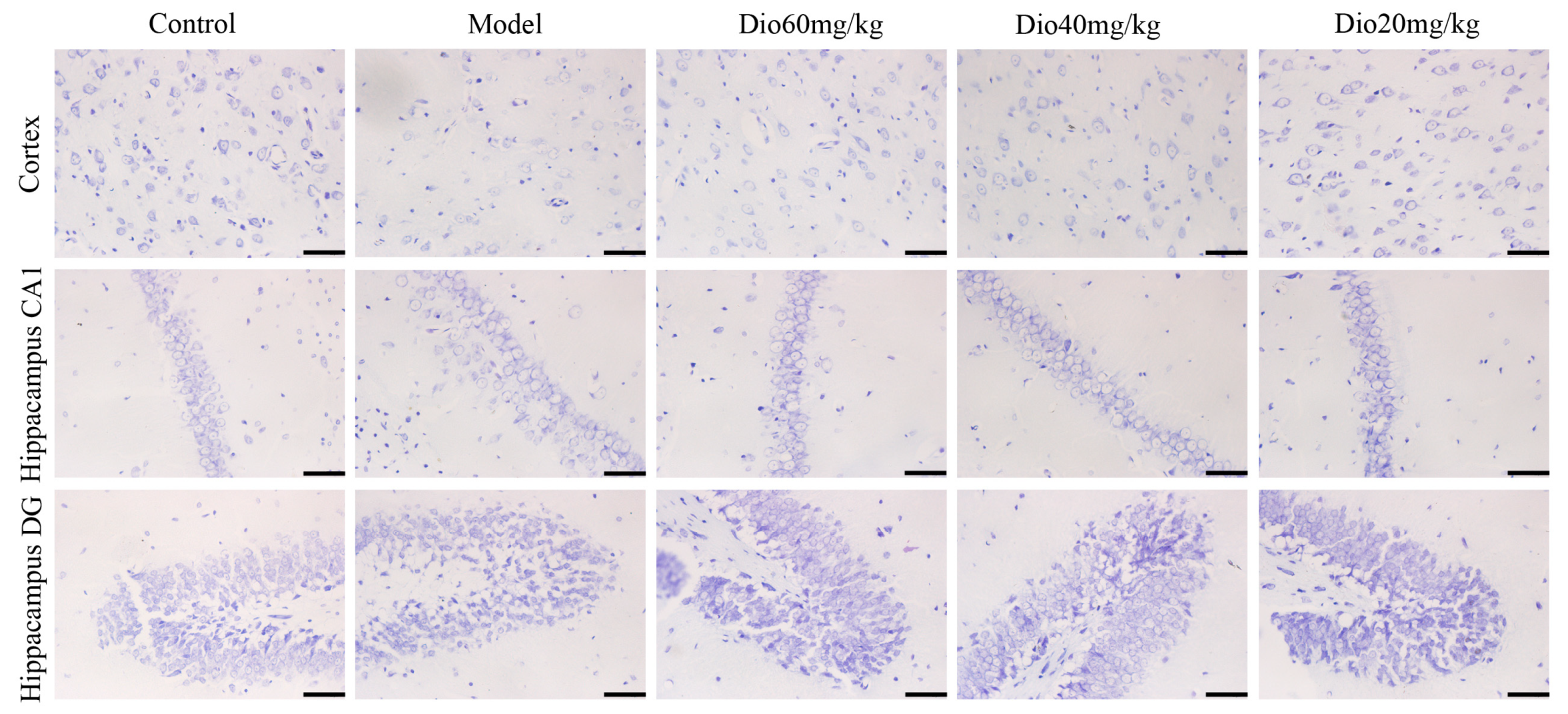
2.4. Dioscin Attenuated Neuropathological Changes in Aging Brain
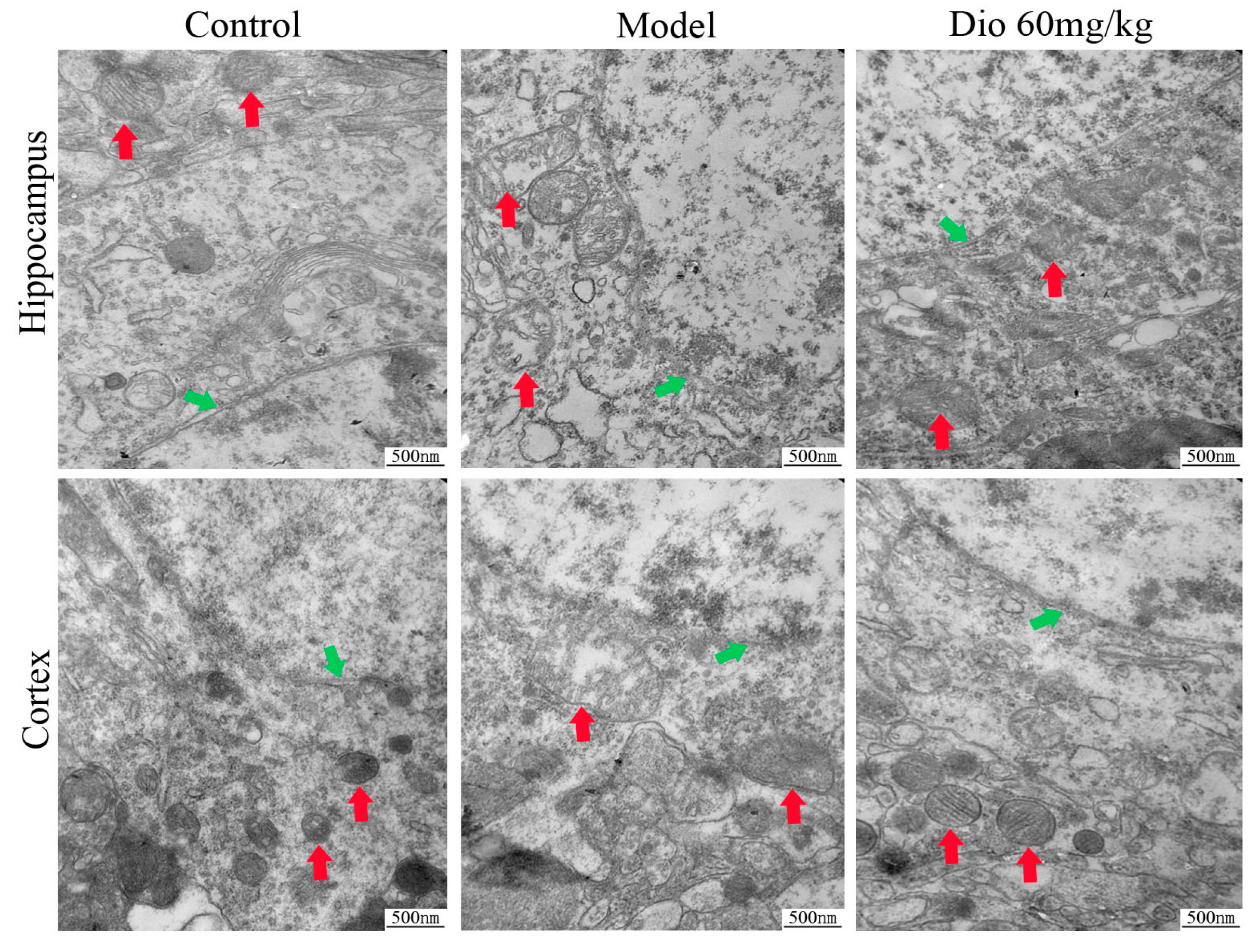
2.5. Dioscin Attenuated Ultrastructure Changes in Aging Brain
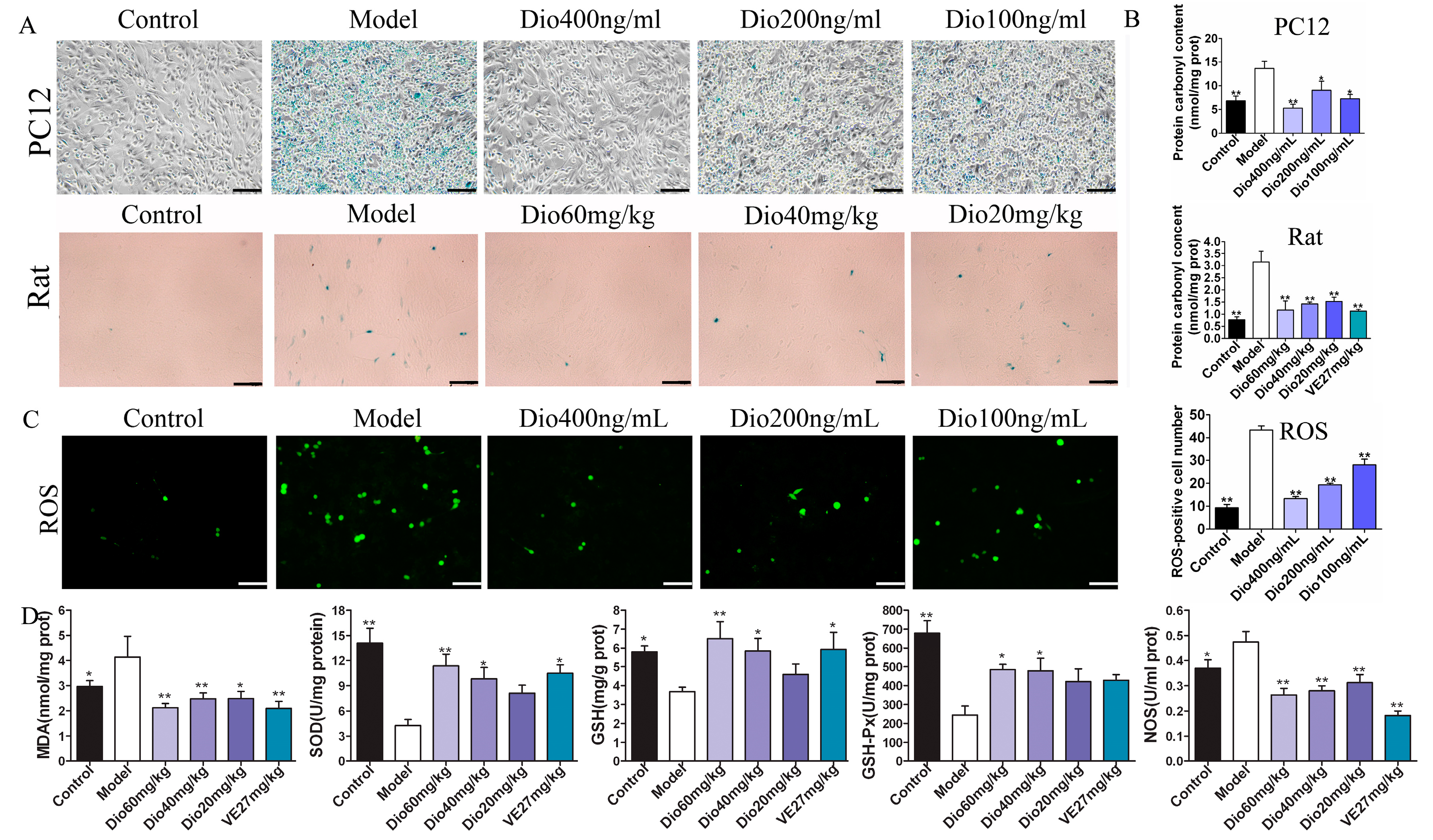
2.6. Dioscin Alleviated Oxidative Damage In Vitro and In Vivo
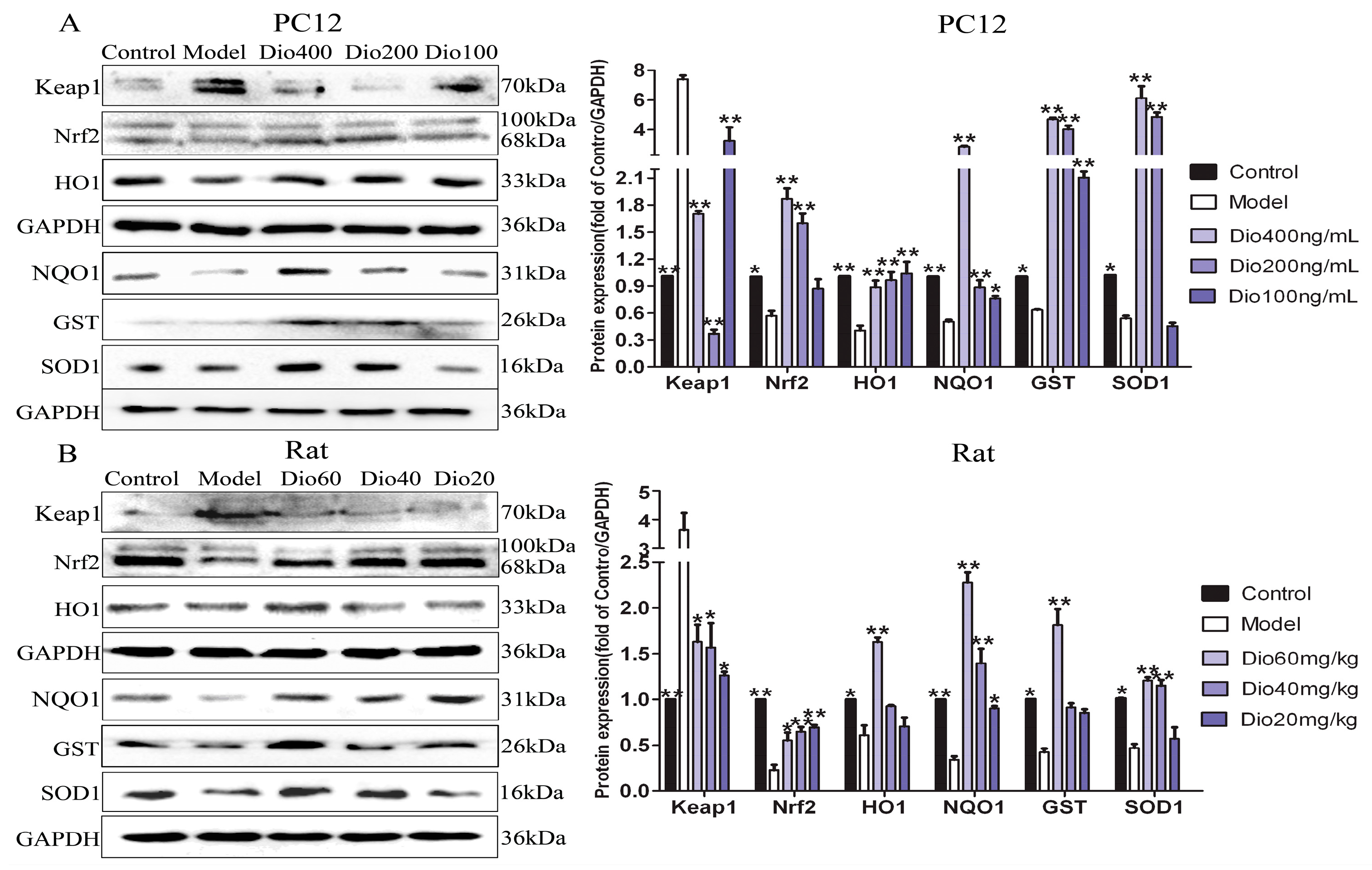
2.7. Dioscin Adjusted Nrf2/ARE Signal Pathway
2.8. Dioscin Attenuated MAPKs Phosphorylation
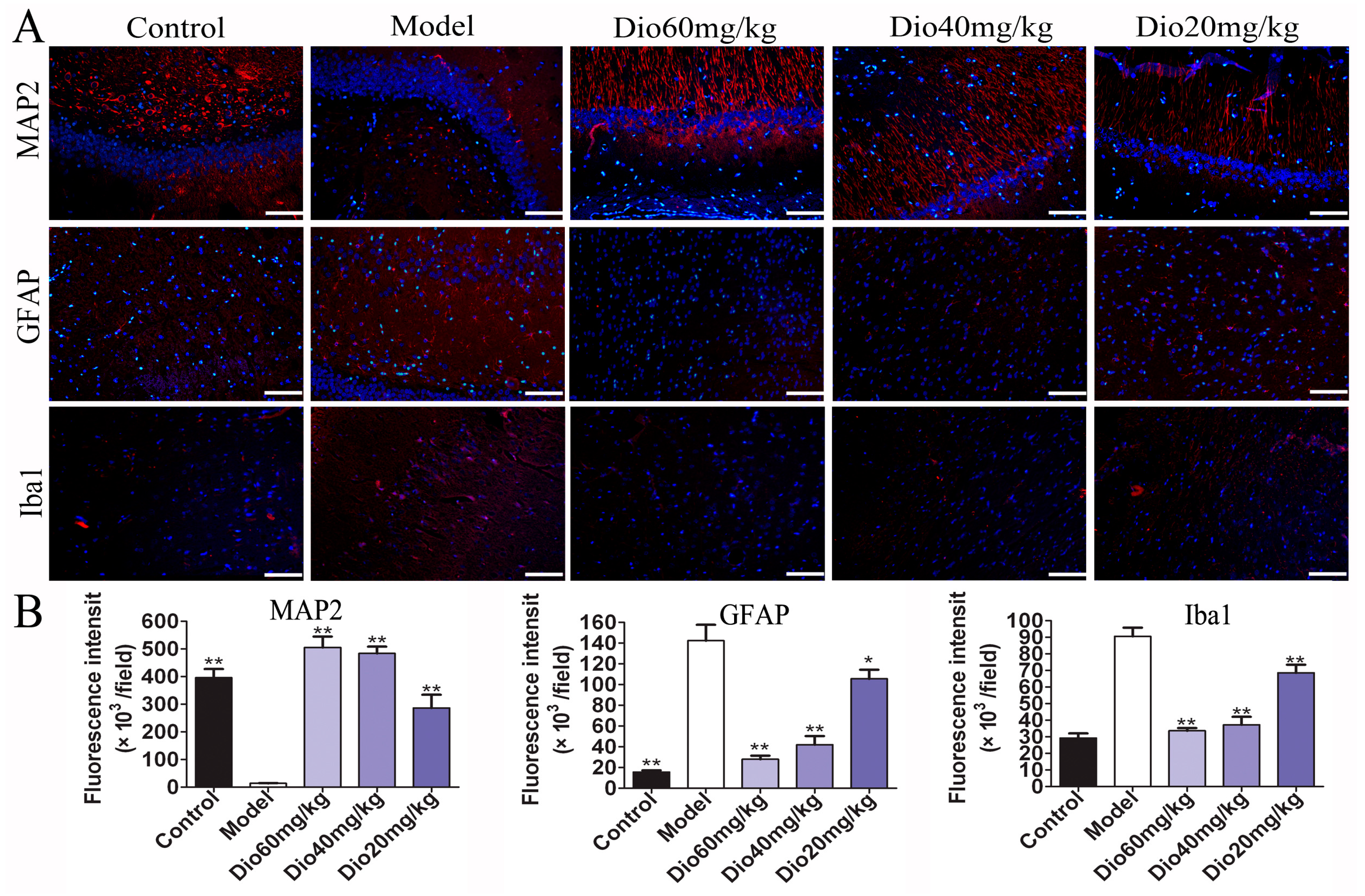
2.9. Dioscin Ameliorated Neuroinflammation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Cell Culture
5.3. Cytotoxicity of Dioscin
5.4. H2O2-Induced Cell Injury
5.5. Cell Viability Study
5.6. Detection of LDH Release and Intracellular Protein Carbonyl
5.7. Cellular Senescence β-galactosidase Staining
5.8. Determination of Intracellular ROS Level
5.9. Animals and Treatment
5.10. Behavioral Test by Morris Water Maze
5.11. Determination of Protein Carbonyl, MDA, NOS, SOD, GSH and GSH-Px Levels in Brain Tissue
5.12. Nissl Staining
5.13. Senescence Related β-galactosidase Staining In Vivo
5.14. Transmission Electron Microscopy Assay
5.15. Immunofluorescence Assay
5.16. Quantitative Real-Time PCR Assay
5.17. Western Blot Assay
5.18. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- United Nations. World Population Ageing. Available online: https://www.un.org/en/sections/issues-depth/ageing/ (accessed on 11 February 2019).
- Peters, R. Aging and the brain. Postgrad. Med. J. 2006, 82, 84–88. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Alzheimer’s Disease International. World Alzheimer Reports. Available online: https://www.alz.co.uk/research/world-report (accessed on 11 February 2019).
- Reiter, R.J. Oxidative processes and antioxidative defense mechanisms in the aging brain. FASEB J. 1995, 9, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. The free radical theory of aging. Antioxid. Redox Signal 2003, 5, 557–561. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Cannizzo, E.S.; Clement, C.C.; Sahu, R.; Follo, C.; Santambrogio, L. Oxidative stress, inflamm-aging and immunosenescence. J. Proteom. 2011, 74, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Corlier, F.; Hafzalla, G.; Faskowitz, J.; Kuller, L.H.; Becker, J.T.; Lopez, O.L.; Thompson, P.M.; Braskie, M.N. Systemic inflammation as a predictor of brain aging: Contributions of physical activity, metabolicrisk, and genetic risk. Neuroimage 2018, 172, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Yanckello, L.M.; Ma, D.; Hoffman, J.D.; Parikh, I.; Thalman, S.; Bauer, B.; Hartz, A.M.S.; Hyder, F.; Lin, A.L. Neuroimaging Biomarkers of mTOR Inhibition on Vascular and Metabolic Functions in Aging Brain and Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10. [Google Scholar] [CrossRef]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondy, S.C.; Lahiri, D.K.; Perreau, V.M.; Sharman, K.Z.; Campbell, A.; Zhou, J.; Sharman, E.H. Retardation of brain aging by chronic treatment with melatonin. Ann. N. Y. Acad. Sci. 2004, 1035, 197–215. [Google Scholar] [CrossRef]
- Bondy, S.C.; Sharman, E.H. Melatonin and the aging brain. Neurochem. Int. 2007, 50, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Lamming, D.W.; Ye, L.; Katajisto, P.; Goncalves, M.D.; Saitoh, M.; Stevens, D.M.; Davis, J.G.; Salmon, A.B.; Richardson, A.; Ahima, R.S.; et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 2012, 335, 1638–1643. [Google Scholar] [CrossRef] [Green Version]
- Kahan, B. Toxicity spectrum of inhibitors of mammalian target of rapamycin in organ transplantation: Etiology, pathogenesis and treatment. Expert Opin. Drug Saf. 2011, 10, 727–749. [Google Scholar] [CrossRef] [PubMed]
- Pallet, N.; Legendre, C. Adverse events associated with mTOR inhibitors. Expert Opin. Drug Saf. 2013, 12, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Sun, X.; Yin, L.; Han, X.; Xu, L.; Qi, Y.; Xu, Y.; Li, H.; Lin, Y.; Liu, K.; et al. Dioscin ameliorates cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via HMGB-1 inhibition. Free Radic. Biol. Med. 2015, 84, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Sun, Y.; Song, S.; Qi, Y.; Tao, X.; Xu, L.; Yin, L.; Han, X.; Xu, Y.; Li, H.; et al. Protective Effects of Dioscin against Lipopolysaccharide-Induced Acute Lung Injury through Inhibition of Oxidative Stress and Inflammation. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Qiao, Y.; Xu, L.; Tao, X.; Yin, L.; Qi, Y.; Xu, Y.; Han, X.; Tang, Z.; Ma, X.; Liu, K.; et al. Protective effects of dioscin against fructose-induced renal damage via adjusting Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and inflammation. Toxicol. Lett. 2018, 284, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Tao, X.; Qi, Y.; Xu, L.; Yin, L.; Peng, J. Protective effect of dioscin against doxorubicin-induced cardiotoxicity via adjusting microRNA-140-5p-mediated myocardial oxidative stress. Redox Biol. 2018, 16, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, S.; Zheng, L.; Yin, L.; Xu, L.; Peng, J. A 90-day subchronic toxicological assessment of dioscin, a natural steroid saponin, in Sprague-Dawley rats. Food Chem. Toxicol. 2012, 50, 1279–1287. [Google Scholar] [CrossRef]
- Bishop, N.A.; Lu, T.; Yankner, B.A. Neural mechanisms of ageing and cognitive decline. Nature 2010, 464, 529–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedden, T.; Gabrieli, J.D. Insights into the ageing mind: A view from cognitive neuroscience. Nat. Rev. Neurosci. 2004, 5, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Kemp, S.; Benito León, J.; Reuber, M. The influence of cognitive impairment on health-related quality of life in neurological disease. Acta Neuropsychiatr. 2010, 22, 2–13. [Google Scholar] [CrossRef]
- Bokov, A.; Chaudhuri, A.; Richardson, A. The role of oxidative damage and stress in aging. Mech. Ageing Dev. 2004, 125, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Shwe, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. Role of d-galactose-induced brain aging and its potential used for therapeutic interventions. Exp. Gerontol. 2018, 101, 13–36. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Majdi, A.; McCann, S.K.; Mahmoudi, J.; Vafaee, M.S.; Macleod, M.R. d-galactose-induced brain ageing model: A systematic review and meta-analysis on cognitive outcomes and oxidative stress indices. PLoS ONE 2017, 12, e0184122. [Google Scholar] [CrossRef]
- Buckner, R.L. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron 2004, 44, 195–208. [Google Scholar] [CrossRef]
- Lu, T.; Pan, Y.; Kao, S.Y.; Li, C.; Kohane, I.; Chan, J.; Yankner, B.A. Gene regulation and DNA damage in the ageing human brain. Nature 2004, 429, 883–891. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Juraska, J.M.; Lowry, N.C. Neuroanatomical changes associated with cognitive aging. Curr. Top Behav. Neurosci. 2012, 10, 137–162. [Google Scholar] [PubMed]
- Pannese, E. Morphological changes in nerve cells during normal aging. Brain Struct. Funct. 2011, 216, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Perry, V.H.; Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 2014, 10, 217–224. [Google Scholar] [CrossRef]
- Rodríguez-Arellano, J.J.; Parpura, V.; Zorec, R.; Verkhratsky, A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience 2016, 323, 170–182. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J. Chem. Biol. 2003, 278, 21592–21600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging Roles of Nrf2 and Phase II Antioxidant Enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef]
- Pajares, M.; Cuadrado, A.; Rojo, A.I. Modulation of proteostasis by transcription factor NRF2 and impact in neurodegenerative diseases. Redox Biol. 2017, 11, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Yen, G.C. Differential expressions of antioxidant status in aging rats: The role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology 2007, 8, 71–80. [Google Scholar] [CrossRef]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Kjellerup, R.B.; Kragballe, K.; Iversen, L.; Johansen, C. Pro-inflammatory cytokine release in keratinocytes is mediated through the MAPK signal-integrating kinases. Exp. Dermatol. 2008, 17, 498–504. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Shaikh, M.F.; Chakraborti, A.; Kumari, Y.; Aledo-Serrano, Á.; Aleksovska, K.; Alvim, M.K.M.; Othman, I. HMGB1: A Common Biomarker and Potential Target for TBI, Neuroinflammation, Epilepsy, and Cognitive Dysfunction. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Du, J.; Zhang, Y.; Yan, J.T.; Hu, X.M. Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation and neuronalapoptosis in MCAO/reperfusion rats. Exp. Brain Res. 2015, 233, 2753–2765. [Google Scholar] [CrossRef] [PubMed]
- García-Suástegui, W.A.; Ramos-Chávez, L.A.; Rubio-Osornio, M.; Calvillo-Velasco, M.; Atzin-Méndez, J.A.; Guevara, J.; Silva-Adaya, D. The Role of CYP2E1 in the Drug Metabolism or Bioactivation in the Brain. Oxid. Med. Cell Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Yin, L.; Xu, L.; Wang, X.; Lu, B.; Liu, Y.; Peng, J. An EconomicalMethod for isolation of dioscin from dioscorea nipponicaMakino by HSCCC coupled with ELSD, and a computer-Aided UNIFAC mathematical model. Chromatographia 2010, 71, 15–23. [Google Scholar] [CrossRef]
- Jia, Y.; Ji, L.; Zhang, S.; Xu, L.; Yin, L.; Li, L.; Zhao, Y.; Peng, J. Total flavonoids from Rosa Laevigata Michx fruit attenuates hydrogen peroxide induced injury in human umbilical vein endothelial cells. Food Chem. Toxicol. 2012, 50, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |



| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| TNF-α | TCAGTTCCATGGCCCAGAC | GTTGTCTTTGAGATCCATGCCATT |
| IL-1β | CCCTGAACTCAACTGTGAAATAGCA | CCCAAGTCAAGGGCTTGGAA |
| IL-6 | ATTGTATGAACAGCGATGATGCAC | CCAGGTAGAAACGGAACTCCAGA |
| GAPDH | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
| Antibody | Separation Gel Concentration (%) | Dilution | Company |
|---|---|---|---|
| GAPDH | 10 | 1:5000 | Proteintech Group, Chicago, IL, USA |
| p38 | 10 | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| p-p38 | 10 | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| ERK | 10 | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| p-ERK | 10 | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| JNK | 10 | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| p-JNK | 10 | 1:500 | Bioworld Technology, St. Louis Park, MN, USA |
| CYP2E1 | 10 | 1:1000 | Proteintech Group, Chicago, IL, USA |
| HMGB1 | 12 | 1:1000 | Proteintech Group, Chicago, IL, USA |
| Keap1 | 10 | 1:1000 | Proteintech Group, Chicago, IL, USA |
| Nrf2 | 10 | 1:1000 | Proteintech Group, Chicago, IL, USA |
| HO1 | 10 | 1:500 | Proteintech Group, Chicago, IL, USA |
| GST | 10 | 1:500 | Proteintech Group, Chicago, IL, USA |
| NQO1 | 10 | 1:1000 | Proteintech Group, Chicago, IL, USA |
| SOD1 | 15 | 1:1000 | Proteintech Group, Chicago, IL, USA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Li, R.; Xu, L.; Yin, L.; Xu, Y.; Han, X.; Peng, J. Neuroprotective Effect of Dioscin on the Aging Brain. Molecules 2019, 24, 1247. https://doi.org/10.3390/molecules24071247
Qi Y, Li R, Xu L, Yin L, Xu Y, Han X, Peng J. Neuroprotective Effect of Dioscin on the Aging Brain. Molecules. 2019; 24(7):1247. https://doi.org/10.3390/molecules24071247
Chicago/Turabian StyleQi, Yan, Ruomiao Li, Lina Xu, Lianhong Yin, Youwei Xu, Xu Han, and Jinyong Peng. 2019. "Neuroprotective Effect of Dioscin on the Aging Brain" Molecules 24, no. 7: 1247. https://doi.org/10.3390/molecules24071247






